Abstract
The introduction of cancer nanomedicine has substantially enhanced the effectiveness of cancer treatments. Nano-formulations are becoming more prevalent among other treatment methods due to their improved therapeutic efficacy and low systemic toxicity. The discovery of the enhanced permeability and retention (EPR) effect has led to the development of numerous nanodrugs that passively target tumours. Then researchers identified certain cancer cells overexpress certain receptors, targeting these over-expressing receptors using targeting moiety on the surface of the nanoparticles becomes promising and surface functionalization of nanoparticles has become an important area of cancer nanomedicine. This leads to the physiochemical modification of nanoparticles for strengthening the EPR effect and active targeting. This review comprehensively outlines the origins of cancer nanomedicine, the role of the EPR effect, the tools of nanotechnology and their specifications, and the nature of passive and active targeting, which gives important direction for the progress of cancer therapy using nanomedicine. The review briefly enlists the available nano formulations for different cancers and attempts were made to account for the barriers to clinical translation. The review also briefly describes the transition of research from nanomedicine to nano-immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer nanomedicine is at a point of debate when we collectively analyze the past 15 years of its growth and contribution to medicine. Recent discussions have conveyed that the influence of nanotechnology is supreme in the research world, but its societal impact on health and translation from research to product is very minimal. Dr Kinam Park remarked in the cover story of the Journal of control release that the nanomedicine hype is close to an unfortunate end by quoting the announcement of the US National Cancer Institute (NCI) about the stoppage of funding for its Centres of Cancer Nanotechnology Excellence (CCNEs) [1]. However, the revolutionary 60-year journey and the shift from Feynman’s nanotechnology [2] to Metchnikov and Ehrlich’s nanomedicine should not go unnoticed. The shift in using nanomedicine for cancer therapy is promoted by the introduction of Professor Maeda’s enhanced permeability and retention (EPR) effect. It was a pioneering concept in the 1980s [3] and found its way to clinical use over the last three decades. Most of the commercialized nanodrugs for cancer treatment entirely use the special transport capabilities made possible by the EPR effect to enter the tumour and exert an anticancer impact. From the very primitive Doxil (1995) to the modified multi-drug Vyxeos (2017) and Hensify (2019), nanomedicine substantiates its significance over conventional therapies for cancer.
The meritorious benefits of nanodrugs can only be revealed when compared to traditional tumour treatment methods. Since the most widely used chemotherapy agents fail to differentiate between normal and cancerous cells [4], patients are more likely to experience treatment failures as well as undesirable side effects. Initially, the nanomedicine used for cancer treatment was based on its enhanced accumulation in the tumour site because of the leaky vasculature, poor lymphatic drainage, and tumour microenvironment factors that promote cellular permeability. Apart from passive choosing, the exclusive and active targeting of tumour cells by nanodrugs [5] was initiated in the noughties with the utilization of targeting moieties like antibodies, aptamers, ligands, peptides, etc. Modern antibody technology joined hands with nanomedicine to use monoclonal antibodies [6] and small antibody constructs for targeting specific cell surface markers in tumour cells. Concurrently, ligand-based targeting has also progressed well. The overexpression of transferrin [7, 8], folate [7, 9], and integrin [10, 11] receptors served as tumour cell surface markers, and ligands for the same were employed to synthesize surface-functionalized nanoformulations [7,8,9,10, 12,13,14]. All these biggest expansions in nano-based cancer therapies for the past 20 years are remarkable and designated as the “golden era” of cancer clinical trials.
The evolution of cancer nanomedicine is like an ocean being filled drop-by-drop. Its genesis is grateful to the contributions of chemistry, polymer science, biophysics, and molecular biology. The power and popularity of nanodrugs in cancer treatments have been revealed by the 20,300 publications in the last year and the 192 active clinical trials around the world. The sixth-most significant growth technology to monitor over the next 10 years, according to Forbes in 2021, is nanotechnology. In this review, we will showcase the entry of nanodrugs in cancer treatment, their superiority over conventional therapies, available nano-formulations, ongoing developments, and the drawbacks of existing systems while exploring different types of nanoparticles and their features.
2 Setbacks in conventional cancer treatments
Conventional cancer therapies involve chemotherapeutic agents that disrupt regular cellular functions, causing apoptosis or limiting the proliferation rate. Primary chemotherapeutic agents are Doxorubicin, Daunorubicin, Paclitaxel, Docetaxel, and Cisplatin. The Anthracyclines drugs, Daunorubicin and Doxorubicin are considered effective drugs that stimulate high toxicity towards various aggressive tumours like breast cancer, myeloblastic leukaemia, and lymphoma [15]. Even though these drugs are therapeutically effective, their inability to distinguish between healthy and tumour tissues followed by the unselective targeting leads to severe side effects [4]. The vigorous growth and metastasis of tumour make them unavailable for getting targeted by antitumour drugs.
3 Convergence of nanomedicine to conventional cancer therapy
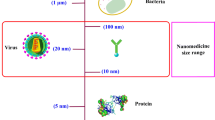
Nanomedicine is not a discrete treatment strategy for cancer. It is a focused unit of traditional treatment strategies to improve drug delivery and overall therapeutic efficacy. Nanotechnology has become the limelight of drug delivery research due to the advantages of reduced drug toxicity and increased drug bioavailability. By utilizing nanotechnology, drugs may be dissolved, adsorbed, and covalently attached to the surface of nanocarriers, as well as encapsulated and embedded within nanocarriers [16]. Through cell-specific targeting, molecular transport to specific organelles, and other techniques, nanotechnology might help overcome the constraints of traditional delivery, which range from large-scale challenges like bio-distribution to smaller-scale obstacles like intracellular trafficking [17]. This reduces drug wastage by preventing its transfer to normal tissues and increases treatment safety. Nanoparticles are moieties in the nano range (10−9 m); which are particularly effective due to their small size, stability, and varied compositions. This provides greater access to the tumour tissue resulting in higher drug accumulation at the tumour site [18].
Tumour physiology and its differences from normal tissue is also a vital aspect of drug delivery. The sprouting of new blood vessels from the existing capillaries followed by the neovascularization of malignant growth transforms the tumour vasculature into complex and indistinct. In contrast to the normal cells, the morphology and positioning of the tumour vasculature are random with the lack of a definite endothelial lining. This irregular margin of endothelial cells impairs the cellular tight junctions [19]. The pericytes which wrap the capillaries and the associated basement membrane are also weakly attached to the tumour endothelial cells [20]. The defective and excessive vascular network in the malignant cells permits high vascular permeability, irregular blood flow, and faulty lymphatic drainage [21].
The exploration of anatomical differences of tumour tissues to the normal ones along with the analysis of physical and chemical properties of anticancer drugs for selective drug delivery was first done by Maeda et al. [22,23,24]. They chemically conjugated the poly(styrene-co-maleic acid) to an anticancer antibiotic protein (Neocarzinostatin) to extend the molecular size and produced a new drug formulation, SMANCS (polystyrene-maleic acid conjugated neocarzinostatin) [24]. Later, the physio-chemical properties and biological activity of SMANCS were tested. The 16 kDA, the anticancer proteinaceous drug proved its enhanced hydrophobicity, tumortropism, lymphotropism, and poor immunogenicity [3, 22, 23]. Compiling the formerly stated architectural imbalance of tumour tissues to the properties of a new drug derivative, the EPR effect was coined [3].
4 EPR effect: the driving force of cancer nanomedicine
Most of the traditional anti-cancer drugs have molecular weights between 300 and 1500 Da, but their size and tumour-targeting ability were not linked till the EPR effect came into the picture. From 1979 to 1986, Maeda and colleagues worked on SMANCS and other macromolecules to test their preferential capability to accumulate within tumour cells. They have used other macromolecular plasma proteins and found that the proteins which are larger than 40 kDA have selective accumulation and more retention within the tumour site. The role of aberrant vasculature of blood and lymph vessels in the tumour for this preferential drug accumulation was detailed by Albumin-Evans blue complex experiment. The 66 kDA—bovine serum albumin (BSA) was complexed with Evans Blue dye and injected into the centre of the tumour (mice). The qualitative and quantitative analysis deduced the longer retention time of dye within the tumour than in regular tissues [3]. The generalization of the EPR effect within the tumour was then stated. The simple definition of this special effect can be stated as “the mechanism of passive targeting that enables the enhanced permeability and retention of drugs with certain molecular weights (> 40 kDA) in rapidly growing solid tumours due to their aberrant pathophysiology” (Fig. 1).
Even though Maeda’s EPR was ground-breaking, it cannot be considered as a universal rule. Because the heterogeneity of the effect was spotted in tumours that differ in size, site, stage, and type. Tiny tumours at their initial stage have comparatively high vascular density and smooth blood flow. However, the increase in the size of more than 2 cm in diameter, and the appearance of more necrotic areas within tumours make them hypovascular in nature [25, 26]. The necrosis induced by hypoxia highly depends on the tumour volume and metastasis [26,27,28,29]. The advanced tumour growth is also correlated with solid stress followed by the shrinkage of tumour capillaries and less perfusion rate. Also, the interstitial pressures towards the tumour core are exceedingly high due to impaired lymphatic drainage and high vascular permeability. But the extremities of the tumour have lower pressure. Tumour interstitial pressure is also quite variable and region-dependent [30,31,32]. The nanoparticles need to overcome the efflux of interstitial fluids to diffuse into the tumour, where it is retained due to the EPR effect. The reticuloendothelial system (RES) or mononuclear phagocytic system (MPS) is a significant obstacle for the drug to reach the tumour site. The macrophages present in the RES can remove foreign nanoparticles from the bloodstream. Their opsonin proteins can bind to the unprotected nanoparticles, which get detected by liver macrophages [33]. The internal loop created by these microenvironment factors marks each tumour as unique and thereby rejects the generalization of the EPR effect.
5 Progress of cancer nanomedicine
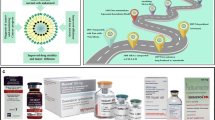
The theorization of Maeda’s EPR effect familiarized the nanomaterials to play a prominent role in cancer therapy. Recently, many nanomedicines got licenced and, a few were suggested as first-line treatments. New nanoparticle designs have exploited the advancements in controlled synthesis processes to combine complex structures, bio-responsive moieties, and targeting agents to enhance delivery. Therefore, these NPs can be used as combination therapies to target certain cell surface macromolecules, such as proteins, carbohydrates, and efflux transporters, as well as specific phases of the cell cycle to modify various oncogenic pathways and enhance therapeutic efficacy [34]. Nano-formulations approved by FDA/EMA/ under clinical trials for cancer treatment are listed (Table 1).
5.1 Types and traits of cancer nanotherapeutics
Nanoparticles are particularly appealing for drug delivery due to their size as well as structure and surface properties. Easy modification of nanoparticle composition and structure are the main factors that can be altered for specific applications. This section will emphasize the various forms, characteristics, and applications of nanocarriers.
5.1.1 Organic nanocarriers
Organic polymer-based nanocarriers are the most used drug delivery vehicles due to their significant characteristics like low toxicity, biodegradability, biocompatibility, small size, and prolonged circulation [35]. Upon degradation, there will be a release of non-toxic components that get cleared by the RES. These properties aided researchers in utilizing organic nanocarriers as drug delivery vehicles for controlled and target-specific drug release. There are many types of organic nanocarriers like nanospheres (Fig. 2a), nanocapsules (Fig. 2b), and dendrimers (Fig. 2c. Nanospheres are spherical-shaped polymer matrices encapsulating the drug spread uniformly throughout the matrix, releasing the drug by diffusion. The release rate of the drug depends on the composition of the matrix and its capacity to imbibe fluids [36, 37]. A study of 5-fluorouracil release from molecularly imprinted hydrogel nanospheres exhibited enhanced binding sites in the polymeric matrix, along with a controlled release of the drug [38]. Nanocapsules are referred to as the reservoir of the drug delivery system since the drug is loaded into the core and protected by a polymeric membrane [39]. The decomposition of the polymeric membrane results in the release of the active ingredients from the core [40]. Dendrimers are nanoscale multi-branched surface-functionalized molecules with an inner core. The unique properties of dendrimers, like surface functionality, spherical shape, and branches can be utilized as a drug delivery carrier [41]. The multi-branch surface helps bind and load different hydrophobic/hydrophilic anticancer drugs, where drugs are either complex or conjugated. Docosahexaenoic acid (DHA) and Paclitaxel conjugated to polyamide amine-based dendrimer (PAMAM-PTX) were formulated to improve anticancer efficacy and reduce toxicity. The study reported that conjugating DHA to PAMAM-PTX further increased cellular toxicity towards cancer cells compared to PAMAM-PTX [42]. Polylactic-co-glycolic acid (PLGA) is another extensively studied polymer for NP drug delivery and biological applications. Its biodegradability and biocompatibility are responsible for PLGA’s phenomenal success in drug delivery applications. This synthetic copolymer which is authorized by FDA and EMA is comprized of lactic acid and glycolic acid monomers in varying proportions. All PLGA by-products are non-toxic and readily tolerated by the human body. The matrix degradation of PLGA nanoparticles (PLGA-NPs) is regulated by molecular weight, the ratio of lactic to glycolic acid, and the number of acid terminal groups. In addition to their utility for controlled drug release, PLGA-NPs have been extensively studied for their potential in targeted drug delivery due to their availability of vast surface area for functionalization, which permits active targeting. PLGA-NPs are composed of a polymer matrix containing their drug payload and have surfaces that may be coated with diverse functions to impart distinct properties [43].
5.1.2 Lipid-based nanocarriers and polymersomes
Lipid-based nanocarriers (Fig. 3a, b) and polymersomes (Fig. 3c) have been used in clinical and preclinical models for many years [44]. Most of the chemotherapeutic agents are hydrophobic. Lipid-based vehicles are used to improve their solubility and to reduce the toxicity of the drug. On the other hand, a few limitations like rapid clearance, non-specific uptake, and instability affect the therapeutic efficacy [45]. Many studies have been conducted to overcome the limitations. Liposome surface-functionalized with Polyethylene glycol (PEG) showed increased bioavailability due to reduced RES uptake [46, 47]. The formulation of liposomes with novel lipid polymers increased the bioavailability and membrane stability [48]. To increase the target specificity in cancer therapy, liposomes, and micelles were conjugated with tumour-specific antibodies for effective treatment [49]. Recent research on lipid-based nanocarriers focuses on smart liposomes with multifunctional properties, enzyme-sensitive liposomes, redox-stimulated liposomes, and magnetic liposomes [50,51,52,53]. Like liposomes, synthetic spherical polymer vesicles known as polymersomes are used to deliver the drug moieties. Polymersomes are constructed of a polymer-based bilayer membrane with a liquid core containing the therapeutic agent [54]. A study of the synthesis of self-porating polymersomes of PEG conjugated to polycaprolactone or polylactic acid and hydrolysis triggered time-controlled release of the drug has been reported [55].
5.1.3 Inorganic nanocarriers
Inorganic nanocarriers like metallic nanocarriers (Fig. 4a), carbon nanotubes (Fig. 4b), and quantum dots (Fig. 4c) play an essential role in the recent advances in drug delivery systems due to their multifunctional behaviours, meticulous surface characteristics, tunable physicochemical properties, and size control. A series of inorganic nanocarriers have been synthesized in the past few years with exceptional properties which can be used for cancer therapy. Gold nanoparticles (AuNPs) being one of them are widely studied in a variety of shapes, including nanospheres, nanorods, nanostars, nanoshells, and nanocages possessing distinct physical, electrical, magnetic, and optical capabilities. AuNPs were employed as delivery vehicles for numerous cytotoxic drugs, unstable nucleic acid drugs, and hydrophobic/hydrophilic photosensitizers for photodynamic therapy (PDT). In addition, AuNPs have been utilized in cancer phototherapy, such as photo thermotherapy (PTT), by using their light-absorption characteristics. In preclinical research, AuNPs have also been employed as contrast agents for biological imaging, notably computed tomography (CT) imaging, which assists doctors in recognizing tumour states and selecting appropriate therapy options [34, 56]. In addition to AuNPs, zinc oxide nanoparticles (ZnONPs) are explored extensively due to their biocompatibility and pH-dependent cytotoxicity via their dissolution into Zn2+ ions, which exhibit cancer cell-specific cytotoxicity. In addition, ZnONPs demonstrate a variety of biomedical applications in the fields of tissue engineering, targeted drug delivery systems, and bioimaging, and may be readily synthesized from affordable precursors [57]. Carbon-based nanomaterials are used significantly in drug delivery, imaging, and diagnosis of cancer. The physicochemical properties like modifiable surfaces, increased drug loading, and high surface area are a few excellent properties of carbon-based nanocarriers [58,59,60,61,62,63]. The development of multi-walled carbon nanotubes (MWCNTs) as carriers exhibited increased uptake, accumulation, and release of drugs at the tumour site [64]. The use of quantum dots in recent years has increased due to their unique optical properties. These semiconductor nanocrystals have extensive use in the diagnosis, and treatment of cancer [65, 66]. Quantum dots consist of an inorganic elemental core with metallic exterior shells protected by coordinating ligands and amphiphilic polymer with good fluorescence emission spectra. The surface of the polymer-coated quantum dots can be PEGylated to increase circulation time and reduce immunogenicity [67]. The optical properties of quantum dots help to build resistance towards photobleaching [68]. Fluorescent quantum dots injected in the Xenopus embryo resulted in sharp contrast with no change in signal intensity even after 80 min of constant illumination. In contrast, a wide range of photobleaching was observed with the control [69].
5.2 Physical traits of nanoparticles
The potential to target the tumour cells and exert a therapeutic effect depend on the various physical features of nanoparticles [70]. The most accountable features and their benefits in the aspect of cancer therapy are tabulated as follows (Table 2).
6 The shift from passive to active targeting
First, the general confusion between passive targeting and EPR-operated drug delivery can be clarified. Passive targeting is the diffusion and selective accumulation of low-molecular-weight drugs in tumours, followed by quick clearance from the site without any prolonged retention. In contrast, EPR-driven targeting is the preferential accumulation and extended tumour retention of high molecular weight nanodrugs for several weeks [82]. Both the pathophysiology of the tumour and the physiochemical characteristics of the nanoformulations have a significant impact on the effectiveness of EPR-driven tumour targeting. Each nanodrug’s size, shape, charge, surface area, and permeability can be tuned based on the type of cancer [70]. However, due to the hypovascularity of some malignancies, particularly pancreatic and prostate tumours, the EPR effect does not apply to all tumour types [83]. Additionally, certain restrictions can affect the effectiveness of the passive treatment, such as poor drug loading that results in insufficient medication to achieve therapeutically effective concentration or delivering a larger amount of carrier materials that result in unfavourable side effects. Before the medicine reaches the target site, it frequently releases a sizable amount during burst release. It was still difficult to create an efficient nanocarrier system with low immunogenic characteristics, low toxicity, and increased accumulation of the nanocarrier at the target cells because of non-controlling physicochemical qualities and non-targeted NPs [84]. Many commercially available nanomedicines are first-generation medications that target cancer cells indirectly through the EPR effect. Approximately 24 nanodrugs have been developed from Doxil (1995) to Pazenir (2019) and are currently being used to treat various malignancies. Second-generation nanodrugs are active tumour-targeting agents through receptor-ligand mechanisms. More than 20 such nanodrugs are in phase 1 and 2 clinical trials but have not yet been approved for treatment [85].
6.1 Designing of targeted nanodrugs
Active targeting is the usage of molecular recognition like antigen–antibody or ligand–receptor interaction to deliver the drug to a specific site. Ligands can be conjugated to the NPs, which interact with a receptor at the target site. An antibody can be associated with the NP to make it bind to a specific antigen. For example, a ligand-bound, drug-carrying NP can enter the cell due to receptor-mediated endocytosis and initiate cytotoxic action [39]. This specificity allows us to identify overexpressed receptors such as CD44, folate, transferrin, etc., thereby minimizing side effects. Actively targeted NPs have been found to have performed significantly more than the non-targeted ones with reduced side effects and increased therapeutic efficiency [86]. Important active targeting moieties are discussed below.
6.1.1 Antibodies
Antibodies are Y-shaped proteins that play a vital role in neutralizing foreign substances in the body. Antitumour activity of unconjugated antibodies is seen in colorectal cancers, breast cancers, lymphomas, chronic lymphocytic leukaemia, and non-Hodgkin’s lymphomas [87, 88]. In the past, there have been problems associated with using mouse antibodies that have led to an immunological response against the antibodies themselves. But with advances in technology, numerous types of antibodies and antibody–drug conjugates take advantage of receptors like CD 20, Alpha-v integrin, EGFR, etc., which have been developed and approved for treatment by the FDA [87, 89]. Antibodies coupled with drug-NPs attach to antigens of the tumours and help achieve the desired outcome. Monoclonal antibodies are widely used to target numerous receptors, including the epidermal growth factor receptor (EGFR), which is overexpressed in a wide range of tumours, with the human epidermal receptor-2 (HER-2) being over-expressed in most breast cancer cases. Anti-HER-2 or anti-EGFR monoclonal antibody-conjugated NPs have also been comprehensively studied as potential therapeutics [87, 90]. Bispecific antibodies that can bind to two different epitopes have also garnered the limelight in the recent decade, with a couple of them already approved by the FDA. Antibodies could be engineered to possess affinity toward two different antigens, thereby increasing their therapeutic efficacy [87, 91].
6.1.2 Transferrin
Transferrin receptor 1 (TfR 1) encodes a 95-kDa homodimeric transmembrane glycoprotein necessary for cellular iron uptake. Roughly tenfold overexpression of TfR on the cell surface of various cancers makes it an attractive molecule for targeted cancer therapy [86, 92]. A cytotoxic response could be achieved by blocking the receptors’ natural function or by delivering the drug molecules into the tumour cells [93]. Initially, more studies were conducted on Doxorubicin-transferrin (Dox-TfR) conjugate [94]. Doxorubicin was a widely accepted drug for various tumours like bone sarcomas, acute lymphoblastic leukaemia (ALL), mammary carcinoma, and ovarian cancer. But the tumour-killing potential of the drug was overshadowed by its cardiotoxicity [95]. So, a range of Dox-TfR conjugates was tested for selective drug delivery to the tumour site [96,97,98,99,100]. Dexamethasone conjugated solid lipid nanoparticles (SLN) surface modified with Transferrin-PEG-Phosphatidylethanolamine enhanced the tumour-targeted transfection [93]. Recently, Transferrin-conjugated lipid-based polymer micelles encapsulated with curcumin could produce a greater accumulation of drugs inside the tumour and enhanced cytotoxicity [101]. Wu et al. [102] tested the role of TfR in the H1299-lung cancer cell line by blocking the receptor and showed reduced proliferation followed by tumour destruction. The co-encapsulation of Curcumin and Paclitaxel in TfR-anchored polymeric micelles proved their selective cytotoxic effect in both Paclitaxel-sensitive and -resistant SK-OV-3 cell lines [103].
6.1.3 Folic acid
There are several accounts of using folic acid (FA) conjugated NPs to target the folate receptor (FR) to make use of receptor-mediated endocytosis [104]. As FRs are commonly overexpressed in many cancer cells, they are widely employed as a drug delivery target. Folate-conjugated liposomes have been repeatedly used [105]. Early work by Goren et al. showed tenfold higher toxicity on M109R cells when doxorubicin-loaded liposomes were conjugated with FA [106]. Similarly, drug-loaded PLA-PEG block copolymers conjugated with FA ligand displayed significantly higher uptake and toxicity when compared to non-targeting carriers [107,108,109]. Recently, there have been developments in utilizing a folate-modified lipoplex to deliver a plasmid to target lung cancer cells [110]. Increased cell uptake and better inhibitory effects have also been observed on different cell lines with varying carriers of drug conjugated to FA [111,112,113,114,115]. Targeting tumours via FR have continued to gain significant attention in recent years as new techniques to conjugate FA have been developed [116, 117].
6.1.4 Hyaluronic acid
The cluster of differentiation-44 (CD44) is a glycoprotein expressed on a wide range of epithelial cells. CD44 plays a vital role in varied cellular events like proliferation, migration, and differentiation through the interactions with hyaluronic acid (HA) molecules [118, 119]. CD44 receptors are overexpressed in various malignant tumour cells in the brain, head-neck, breast, prostate, and lung cancers [120,121,122]. Various groups have studied specific targeting of CD44 receptors using HA to enhance the efficacy of anticancer therapeutics at the tumour sites [123, 124]. Due to its non-immunogenicity and biocompatibility, HA is widely employed in various formulations, including conjugate-based nanomedicines, self-assembled NPs, and liposomes [125,126,127]. The specific binding of HA to CD44 receptor has become a prevalent method to target and efficiently deliver nano-therapeutics to CD44 overexpressed cancer cells.
6.1.5 Peptides
Peptides are a sequence of amino acids that are small ligands with immense targeting potential. There are numerous possible structures for these moieties. Tumour cells generally overexpress many receptors with a peptide as their target molecule. They are especially useful as peptide-conjugated NP that are expected to yield fewer immunological problems [128].
Integrin αvβ3, considered a crucial element for angiogenesis, exists at higher levels in tumour cells. Arginine-glycine-aspartic acid (RGD) peptide sequence recognizes this integrin, and thus the affinity of the RGD sequence towards αvβ3 integrin can be utilized for drug delivery devices [86]. An iRGD peptide represents a specific sequence of cyclic RGD (cRGD) peptides that can bind to αv integrins expressed on tumour endothelial cells [129]. When proteases cleave the iRGD peptide, its resultant molecules show lesser affinity towards αv integrin and higher affinity towards neuropilin-1 (NRP-1), promoting tumour-specific penetration of the molecules. Due to these properties, iRGD peptides can be used extensively for enhanced drug delivery research [130]. Peptides have considerable gains over other ligands for their good stability and low cost of production. They can be easily conjugated by chemical modifications on the surface of NPs [131]. Numerous receptors are overexpressed by many cancers, to which specific peptides can be engineered as targeting molecules. Integrin α6 is a very promising target, with results showing a specially designed peptide conjugated NP having better targeting and cytotoxicity compared to anti-integrin α6 antibody NPs [132]. These conjugated peptides can inhibit the proliferation of the cancer cells by acting as inhibitors of vital receptors such as VEGFR, coupled with drug-loaded NPs, they showed long-term circulation and accurate targeting in in vivo studies [133].
Another approach has been to create self-assembled peptide NPs conjugated to a drug molecule to enhance cellular uptake of the drug by the tumour cells. While this approach has led to better targeting of the drug, it was shown that the cytotoxicity of the NPs was lower than the free drug at lower concentrations [134]. Cell-penetrating peptides (CPP) such as the trans-activator of transcription have been employed to form NPs. With the added advantage of having significantly more drug-loading capacity, these NPs were able to show increased uptake and cytotoxicity towards cancer stem cells [135]. Similarly, CPPs were also used to develop NPs for tumour theranostics. It was shown that it is possible to create NPs that aid in imaging the tumour and effectively deliver the drug to the cancer cells [136].
6.1.6 Aptamers
Aptamers are highly sensitive short molecules comprising several nucleotides (DNA or RNA) that can change their conformation to engage in ligand binding. These nucleotides have the added advantage of being able to be developed to target molecules of varying sizes or even whole cells [137]. Their size and ability to penetrate tumour cells make them perfect for delivering payloads to the tumour cells [138]. The aptamers are three-dimensionally folded to form a specific affinity for the chosen protein [139]. In most cases, soluble, purified cell surface proteins are effectively used as targets for aptamer selection in vitro (protein-SELEX) [140]. This would enable us to modify the cell internalization pathway of the drug, from passive diffusion to receptor-mediated endocytosis [141]. Several results have shown that aptamer-based NPs can significantly enhance the cytotoxicity of several drugs and target specific receptors of interest [142]. Aptamers may have the ability to selectively identify cancer stem cells (CSCs), which would pave the way for more effective treatments [143].
6.2 Limitations of active targeting
Cost is a limiting factor in active targeting. Antibodies are highly specific and can target a diverse range of structures, but at the same time, their production and NP formulation costs are high [144]. Some NPs show promise at the lab scale but cannot be replicated in clinical trials. Lack of standardization is also a significant limitation in the design of new targeted drug delivery systems [145]. A major drawback in the conventional method of drug delivery is that only a small part of the administered dose reaches the tumour, and the rest tends to get localized elsewhere in the body. While active targeting increases the efficiency of this process, there may be other side effects due to the presence of the conjugated ligand in drug internalization [146]. Tumour heterogeneity is also a factor as not all receptors may be found in high levels of overexpression. Rapid clearance from the bloodstream and accumulation of the NPs in the spleen and liver before they can reach the tumour is also a major factor. While active targeting solves many of the issues associated with passive targeting, there is a large scope of research to be done to increase the efficacy and localization of NPs in the tumour tissue [86].
7 Barriers: bench-to-bedside research
Despite these rigorous studies, the number of nanomedicines available to patients is far lower than anticipated due to a translational gap between animal models and human trials [147]. This knowledge gap results from a lack of understanding of the physiology and pathology variations between animal model species and humans, especially how these differences affect the behaviour and functionality of nanomedicines within the body. Not only do species differences restrict clinical translation, but other factors such as the heterogeneity of patients and limited research on the interactions between nanomedicines and stratified patient groups limit the treatment’s success [148, 149]. In cancer research, xenogeneic and syngeneic mice models are frequently employed. Since they are smaller than humans, obstacles to successful clinical translation include differences in tumour size, blood volume, heart rate, and other relevant physiological factors [150]. When compared to humans, the higher heart rate (600/min) helps the body to quickly get rid of any chemicals and develop a tolerance to high doses of medications [151]. In addition to these biological barriers, technological difficulties in the creation, improvement, and scalability of nanomedicines are a cause for concern [147, 149]. The reproducibility and transparency of nanomedicines are the major determinants of clinical translation and success rate [152]. In 2016, a controversy involving the disparity between the therapeutic efficacy of Doxil and LipoDox, the generic equivalent, was reported, and the discrepancy in outcomes was only hazily tied to the dependability of scale-up production [149, 153, 154]. Another crucial point to consider is that the development of nanomedicine has tended to be formulation-oriented rather than tumour pathophysiology-oriented, which is vital to note.
8 Recent emerging direction of cancer research: nanomedicine to nano-immunotherapy
Initially, nanomedicine and immunotherapy were moving simultaneously to provide a complete remission from the cancer. Recently, nano-immunotherapy has emerged as a focal point of cancer research. Most of the immunotherapy agents are less soluble and unstable, with a short half-life [155]. The low immunogenicity of the tumour and the presence of immune suppressive factors in the tumour microenvironment (TME) will inhibit the smooth delivery of these agents to the target site [156, 157]. Moreover, immunotherapeutic approaches cause severe allergic and inflammation reactions in patients due to the use of immune cells or immunomodulatory agents at higher doses [158]. Since the nano-drug delivery system provides higher stability, enhanced tumour penetration and accumulation, and protection from immune suppressive factors, it perfectly compensates for the said drawbacks and exerts a tumour-specific immune response [159]. A carbon nanodot incorporated in a mesoporous silica nanoparticle framework (CD@MSN) has been formulated and used for photothermal immunotherapy. Through the activation of macrophages and natural killer cells, these nanoparticles have altered the cancer microenvironment, triggering an adaptive immune response. A reduction in tumour-promoting M2-macrophages and an increase in tumour-killing M1 macrophages have been linked to overall tumour regression after CD@MSN treatment [160]. Many established nanocarriers were tested for optimized results for immune checkpoint immunotherapy. Different nano-based delivery systems have combined anti-programmed cell death protein 1 (anti-PD1) antibodies with other immunomodulatory agents or adjuvants [161, 162]. To activate natural killer (NK) cells in situ, Zheng et al. created immunomodulatory core–shell constructed nanoparticles. The pH-responsive with glucose-modified poly(2-methacryloyloxyethyl phosphorylcholine)-b-poly(N-(3-aminopropyl)-methacrylamine) (PMPC-b-PApm/Glu) shell can separate from the immunomodulatory nanoparticle when it is exposed to a tumour’s acidic environment. The conjugating phenylboronic acid (PBA) and immunoglobulin G (IgG) onto the nBSA surface (nBSA-PBA-IgG) naked bifunctional core binds sialic acid expressed on the tumour cell membrane right away, enabling IgG to activate NK cells [163]. All these methods show that nanoparticles can be used in a variety of nano-immunotherapies to enhance the specific targeting and killing of tumour cells.
9 Conclusion
The evolutionary growth of cancer nanomedicine was exponential. The long 40-year journey has provided various opportunities to redefine existing anticancer treatments. Like the compound word ‘cancer-nano-medicine’, the review kept the same flow in discussing the factors of cancer, technology, and medicine. The first section introduces cancer physiology, existing treatment strategies, and their drawbacks. The complexity and heterogeneity of tumour tissues restrict the entry and effect of conventional drugs. Current chemotherapy treatments involve injecting anticancer drugs into the bloodstream, which reach tumours and healthy tissues simultaneously, leading to several detrimental effects on healthy tissues. Anticancer drugs usually fail to reach therapeutic concentrations at the tumour site to treat cancerous cells effectively. In some cases, the drug loses its biological activity even before it reaches the core part of the tumour. Nanomaterials utilized as vehicles for drug loading have the potential to overcome the limitations of conventional medicines. The role of physical and chemical properties in nanodrug delivery systems has been extensively discussed to state their dominance over other carrier platforms. These passive and active targeted NPs will help to deliver the drug molecule at the specific site, making sure that the effect of the drug is largely observed at the tumour, thereby reducing the side effects for those who undergo the treatment. The exclusive overexpression of some cell surface receptors like CD44, integrins, and folates in many tumours led to the design of targeted nanoparticles with ligand-antibody conjugation. Even though nanomedicine research has been widespread and has generated promising results in in vitro and small animal models, the clinical translation rate is insignificant due to various biological and technological barriers. A brief discussion of recent trends in nano-immunotherapy, a newly emerged area, is added to complete the overview of cancer nanomedicine and its path-breaking intervention in immune-oncology.
10 Future perspectives
The complexity of a tumour is mysterious at all physiological, biochemical, and molecular levels. The interdisciplinary approach is effective in treating cancer but, an in-depth understanding and functional merging of technology and medicine is crucial. The targeting of tumour microenvironment (TME) is more promising when it utilizes TME factors like pH, hypoxia, and infiltrating immune cells [164, 165]. The size-tunable nanoparticles are at the developmental stages to increase the penetration and retention capability [71, 166]. Since the role of mitochondrial regulation in tumour cells has been explored now, a few nanodrugs targeting mitochondria have been produced to trigger apoptosis in cancer cells. Other nanocarrier systems have also been investigated in the metabolic reprogramming of tumour and associated environment to extend the antitumour response [167, 168]. Even though this research is in the primitive phase, we will hope to see new nanodrugs with greater modifications will come to market to give a complete remission from deadly tumours.
References
Park K (2019) The beginning of the end of the nanomedicine hype. J Control Release 305:221–222
Feynman RP (1959). There’s plenty of room at the bottom. Eng Sci 23(5)
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46:6387–6392
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul 41:189–207. https://doi.org/10.1016/S0065-2571(00)00013-3
Wang AZ, Gu F, Zhang L, Chan JM (2008) Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin Biol Ther 8:1063–1070. https://doi.org/10.1517/14712590802181330
Béduneau A, Saulnier P, Benoit JP (2007) Active targeting of brain tumors using nanocarriers. Biomaterials 28:4947–4967
Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60:1615–1626
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148:135–146
Yu B, Tai HC, Xue W et al (2010) Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol 27:286–298
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22
Ruoslahti E (2002) Specialization of tumour vasculature. Nat Rev Cancer 2:83–90
Eavarone DA, Yu X, Bellamkonda RV (2000) Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. J Biomed Mater Res 51:10–14. https://doi.org/10.1002/(SICI)1097-4636(200007)51:1%3c10:AID-JBM2%3e3.0.CO;2-R
Kobayashi T, Ishida T, Okada Y et al (2007) Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int J Pharm 329:94–102. https://doi.org/10.1016/j.ijpharm.2006.08.039
Li XM, Ding LY, Xu Y et al (2009) Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm 373:116–123. https://doi.org/10.1016/j.ijpharm.2009.01.023
Minotti G, Menna P, Salvatorelli E et al (2004) Anthracyclines: molecular advances and pharmacologie developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229
Fang X, Cao J, Shen A (2020) Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J Drug Deliv Sci Technol 57:101662
Kou L, Bhutia YD, Yao Q et al (2018) Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00027
Park K, Lee S, Kang E et al (2009) New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater 19:1553–1566. https://doi.org/10.1002/adfm.200801655
Nakamura Y, Mochida A, Choyke PL, Kobayashi H (2016) Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 27:2225–2238
Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Fang J, Islam W, Maeda H (2020) Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev 157:142–160
Maeda H, Matsumoto T, Konno T et al (1984) Tailor-making of protein drugs by polymer conjugation for tumor targeting: A brief review on smancs. J Protein Chem 3:181–193 https://doi.org/10.1007/BF01040499
Maeda H, Ueda M, Morinaga T, Matsumotog T (1985) Conjugation of poly (styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem 28(4):455–461 https://doi.org/10.1021/jm00382a012
Maedal H, Takeshita J, Kanamaru R (1979) A lipophilic derivative of neocarzinostatin a polymer conjugation of an antitumor protein antibiotic. Int J Pept Protein Res 14(2):81–87 https://doi.org/10.1111/j.1399-3011.1979.tb01730.x
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Maeda H (2017) Polymer therapeutics and the EPR effect. J Drug Target 25:781–785
Baker GM, Goddard HL, Clarke MB, Whimster WF (1990) Proportion of necrosis in transplanted murine adenocarcinomas and its relationship to tumor growth. Growth Dev Aging 54:85–93
DE Jaeger K, Moreno Merlo F, Kavanagh M et al (1998) Heterogeneity of tumor oxygenation: relationship to tumor necrosis, tumor size, and metastasis. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/S0360-3016(98)00323-X
Milross CG, Tucker SL, Mason KA et al (1997) The effect of tumor size on necrosis and polarographically measured pO2. Acta Oncol (Madr) 36:183–189. https://doi.org/10.3109/02841869709109228
Fukumura D, Jain RK (2007) Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem 101:937–949
Junttila MR, De Sauvage FJ (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501:346–354
Sriraman SK, Aryasomayajula B, Torchilin VP (2014) Barriers to drug delivery in solid tumors. Tissue Barriers 2:e29528-1-e29528-10
Owens DE, Peppas NA (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307:93–102
Mitchell MJ, Billingsley MM, Haley RM et al (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/s41573-020-0090-8
Daima HK, Shankar S, Anderson A et al (2018) Complexation of plasmid DNA and poly(ethylene oxide)/poly (propylene oxide) polymers for safe gene delivery. Environ Chem Lett 16:1457–1462. https://doi.org/10.1007/s10311-018-0756-1
Brigger I, Dubernet C, Couvreur P (2012) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 64:24–36
Ratner BD (2004) Biomaterials science: an introduction to materials in medicine. Elsevier Academic Press
Cirillo G, Iemma F, Puoci F et al (2009) Imprinted hydrophilic nanospheres as drug delivery systems for 5-fluorouracil sustained release. J Drug Target 17:72–77. https://doi.org/10.1080/10611860802455813
Haley B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol Semin Orig Investig 26:57–64
Mayer C (2005) Nanocapsules as drug delivery systems. Int J Artif Organs 28:1163–1171. https://doi.org/10.1177/039139880502801114
Mignani S, Rodrigues J, Tomas H et al (2018) Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem Soc Rev 47:514–532
Dichwalkar T, Patel S, Bapat S et al (2017) Omega-3 fatty acid grafted PAMAM-paclitaxel conjugate exhibits enhanced anticancer activity in upper gastrointestinal cancer cells. Macromol Biosci. https://doi.org/10.1002/mabi.201600457
Zeb A, Gul M, Nguyen TTL, Maeng HJ (2022) Controlled release and targeted drug delivery with poly(lactic-co-glycolic acid) nanoparticles: reviewing two decades of research. J Pharm Investig 52:683–724
Sahoo SK, Labhasetwar V (2003) Nanotech approaches to drug delivery and imaging. Drug Discov Today 8:1112–1120. https://doi.org/10.1016/S1359-6446(03)02903-9
Torchilin VP (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–160
Li B, Shao H, Gao L et al (2022) Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv 29:2130–2161
Portney NG, Ozkan M (2006) Nano-oncology: drug delivery, imaging, and sensing. Anal Bioanal Chem 384:620–630
Xu JP, Ji J, Chen WD, Shen JC (2005) Novel biomimetic polymersomes as polymer therapeutics for drug delivery. J Control Release 107:502–512. https://doi.org/10.1016/j.jconrel.2005.06.013
Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B (2003) Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. PNAS 100(10):6039–6044 https://doi.org/10.1073/pnas.0931428100
Chen X, Zhang Y, Tang C et al (2017) Co-delivery of paclitaxel and anti-survivin siRNA via redox-sensitive oligopeptide liposomes for the synergistic treatment of breast cancer and metastasis. Int J Pharm 529:102–115. https://doi.org/10.1016/j.ijpharm.2017.06.071
Chi Y, Yin X, Sun K et al (2017) Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J Control Release 261:113–125. https://doi.org/10.1016/j.jconrel.2017.06.027
Heidarli E, Dadashzadeh S, Haeri A (2017) State of the art of stimuli-responsive liposomes for cancer therapy. Iran J Pharm Res: IJPR 16(4):1273 https://doi.org/10.22037/ijpr.2017.2164
Mock JN, Costyn LJ, Wilding SL et al (2013) Evidence for distinct mechanisms of uptake and antitumor activity of secretory phospholipase A2 responsive liposome in prostate cancer. Integr Biol (United Kingdom) 5:172–182. https://doi.org/10.1039/c2ib20108a
Rideau E, Dimova R, Schwille P et al (2018) Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev 47:8572–8610. https://doi.org/10.1039/c8cs00162f
Ahmed F, Discher DE (2004) Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles. J Control Release 96:37–53. https://doi.org/10.1016/j.jconrel.2003.12.021
Yang Y, Zheng X, Chen L et al (2022) Multifunctional gold nanoparticles in cancer diagnosis and treatment. Int J Nanomed 17:2041–2067
Singh TA, Das J, Sil PC (2020) Zinc oxide nanoparticles: a comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci 286:102317
Akhavan O, Ghaderi E, Aghayee S et al (2012) The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J Mater Chem 22:13773–13781. https://doi.org/10.1039/c2jm31396k
Bhirde AA, Patel V, Gavard J et al (2009) Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3:307–316. https://doi.org/10.1021/nn800551s
Ji S, Liu C, Zhang B et al (2010) Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta Rev Cancer 1806:29–35
Robinson JT, Welsher K, Tabakman SM et al (2010) High performance in vivo near-IR (> 1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res 3:779–793. https://doi.org/10.1007/s12274-010-0045-1
Wang Y, Wang H, Liu D et al (2013) Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 34:7715–7724. https://doi.org/10.1016/j.biomaterials.2013.06.045
Yang K, Feng L, Liu Z (2016) Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv Drug Deliv Rev 105:228–241
Kumar M, Sharma G, Misra C et al (2018) N-Desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: a synergistic approach to overcome MDR in cancer cells. Mater Sci Eng C 89:274–282. https://doi.org/10.1016/j.msec.2018.03.033
Mohammadi R, Naderi-Manesh H, Farzin L et al (2022) Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: a review. J Pharm Biomed Anal 212:114628
Seydel C (2003) Quantum dots get wet. Science 300:80–81
Gao X, Cui Y, Levenson RM et al (2004) In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 22:969–976. https://doi.org/10.1038/nbt994
Qu L, Peng X (2002) Control of photoluminescence properties of CdSe nanocrystals in growth. J Am Chem Soc 124:2049–2055. https://doi.org/10.1021/ja017002j
Dubertret B, Skourides P, Norris DJ et al (2002) In vivo imaging of quantum dots encapsulated in phospholipid micelles. Sci 298(5599): 1759–1762 https://doi.org/10.1126/science.1077194
Zein R, Sharrouf W, Selting K (2020) Physical properties of nanoparticles that result in improved cancer targeting. J Oncol 2020:1–16
Yu W, Liu R, Zhou Y, Gao H (2020) Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent Sci 6:100–116. https://doi.org/10.1021/acscentsci.9b01139
Toy R, Peiris PM, Ghaghada KB, Karathanasis E (2014) Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 9:121–134
Gavze E, Shapiro M (1998) Motion of inertial spheroidal particles in a shear flow near a solid wall with special application to aerosol transport in microgravity. Cambridge University Press, Cambridge
Lee SY, Ferrari M, Decuzzi P (2009) Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. https://doi.org/10.1088/0957-4484/20/49/495101
Champion JA, Mitragotri S (2006) Role of target geometry in phagocytosis. PNAS 103(13):4930–4934 https://doi.org/10.1073/pnas.0600997103
Chithrani BD, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668. https://doi.org/10.1021/nl052396o
Geng Y, Dalhaimer P, Cai S et al (2007) Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2:249–255. https://doi.org/10.1038/nnano.2007.70
Sharma G, Valenta DT, Altman Y et al (2010) Polymer particle shape independently influences binding and internalization by macrophages. J Control Release 147:408–412. https://doi.org/10.1016/j.jconrel.2010.07.116
Chirio D, Gallarate M, Peira E et al (2014) Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment. Eur J Pharm Biopharm 88:746–758 https://doi.org/10.1016/j.ejpb.2014.10.017
Fröhlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed 7:5577–5591
Gonda A, Zhao N, Shah JV et al (2019) Engineering tumor-targeting nanoparticles as vehicles for precision nanomedicine. Med One. https://doi.org/10.20900/mo.20190021
Maeda H (2021) The 35th anniversary of the discovery of EPR effect: a new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects. J Pers Med 11:229
Maeda H, Bharate GY, Daruwalla J (2009) Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm 71:409–419
Attia MF, Anton N, Wallyn J et al (2019) An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 71:1185–1198
Nirmala MJ, Kizhuveetil U, Johnson A et al (2023) Cancer nanomedicine: a review of nano-therapeutics and challenges ahead. RSC Adv 13:8606–8629. https://doi.org/10.1039/d2ra07863e
Steichen SD, Caldorera-Moore M, Peppas NA (2013) A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci 48:416–427
Lu RM, Hwang YC, Liu IJ et al (2020) Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27(1): 1-30
Von Mehren M, Adams GP, Weiner LM (2003) Monoclonal antibody therapy for cancer. Annu Rev Med 54:343–369
Teicher BA (2009) Antibody-drug conjugate targets. Curr Cancer Drug Targets 9:982–1004. https://doi.org/10.2174/156800909790192365
Kumari P, Ghosh B, Biswas S (2016) Nanocarriers for cancer-targeted drug delivery. J Drug Target 24:179–191
Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA (2019) A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther 201:103–119
Sahoo SK, Ma W, Labhasetwar V (2004) Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer 112:335–340. https://doi.org/10.1002/ijc.20405
Wang W, Zhou F, Ge L et al (2012) Transferrin-PEG-PE modified dexamethasone conjugated cationic lipid carrier mediated gene delivery system for tumor-targeted transfection. Int J Nanomed 7:2513–2522. https://doi.org/10.2147/IJN.S31915
Tortorella S, Karagiannis TC (2014) Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol 247:291–307
Carvalho FS, Burgeiro A, Garcia R et al (2014) Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 34:106–135. https://doi.org/10.1002/med.21280
Berczi A, Barabas K, Sizensky JA, Faulk WP (1993) Adriamycin conjugates of human transferrin bind transferrin receptors and kill K562 and HL60 cells. Arch Biochem Biophys 300:356–363. https://doi.org/10.1006/abbi.1993.1048
Faulk WP, Barabas K, Sun IL, Crane FL (1991) Transferrin-adriamycin conjugates which inhibit tumor cell proliferation without interaction with DNA inhibit plasma membrane oxidoreductase and proton release in K562 cells. Biochem Int 25:815–822
Faulk WP, Taylor CG, Yeh CJ, McIntyre JA (1990) Preliminary clinical study of transferrin-adriamycin conjugate for drug delivery to acute leukemia patients. Mol Biother 2:57–60
Munns J, Yaxley J, Coomer J et al (1998) Evaluation of the potential of transferrinadriamycin conjugates in the treatment of bladder cancer. Br J Urol 82(2):284-289. https://doi.org/10.1046/j.1464-410X.1998.00736.x
Singh M, Atwal H, Micetich R (1998) Transferrin directed delivery of adriamycin to human cells. Anticancer Res 18:1423–1427
Muddineti OS, Kumari P, Ghosh B, Biswas S (2018) Transferrin-modified vitamin-E/lipid based polymeric micelles for improved tumor targeting and anticancer effect of curcumin. Pharm Res. https://doi.org/10.1007/s11095-018-2382-9
Wu Y, Xu J, Chen J et al (2018) Blocking transferrin receptor inhibits the growth of lung adenocarcinoma cells in vitro. Thorac Cancer 9:253–261. https://doi.org/10.1111/1759-7714.12572
Abouzeid AH, Patel NR, Sarisozen C, Torchilin VP (2014) Transferrin-targeted polymeric micelles Co-loaded with curcumin and paclitaxel: efficient killing of paclitaxel-resistant cancer cells. Pharm Res 31:1938–1945. https://doi.org/10.1007/s11095-013-1295-x
Fernández M, Javaid F, Chudasama V (2018) Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci 9:790–810
Watanabe K, Kaneko M, Maitani Y (2012) Functional coating of liposomes using a folate-polymer conjugate to target folate receptors. Int J Nanomed 7:3679–3688. https://doi.org/10.2147/IJN.S32853
Goren D, Horowitz AT, Tzemach D et al (2000) Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res 6(5):1949–1957
Hami Z, Amini M, Ghazi-Khansari M et al (2014) Doxorubicin-conjugated PLA-PEG-Folate based polymeric micelle for tumor-targeted delivery: synthesis and in vitro evaluation. Daru. https://doi.org/10.1186/2008-2231-22-30
Wang S, Luo Y, Zeng S et al (2013) Dodecanol-poly(d,l-lactic acid)-b-poly (ethylene glycol)-folate (Dol-PLA-PEG-FA) nanoparticles: evaluation of cell cytotoxicity and selecting capability in vitro. Colloids Surf B 102:130–135. https://doi.org/10.1016/j.colsurfb.2012.07.030
Xiong J, Meng F, Wang C et al (2011) Folate-conjugated crosslinked biodegradable micelles for receptor-mediated delivery of paclitaxel. J Mater Chem 21:5786–5794. https://doi.org/10.1039/c0jm04410e
Tie Y, Zheng H, He Z et al (2020) Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct Target Ther. https://doi.org/10.1038/s41392-020-0115-0
Acharya S, Praveena J, Guru BR (2021) In vitro studies of prednisolone loaded PLGA nanoparticles-surface functionalized with folic acid on glioma and macrophage cell lines. Pharm Sci 27:407–417. https://doi.org/10.34172/PS.2020.94
Chen YC, Chiang CF, Chen LF et al (2014) Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials 35:4066–4081. https://doi.org/10.1016/j.biomaterials.2014.01.042
Pan J, Feng SS (2008) Targeted delivery of paclitaxel using folate-decorated poly(lactide)-vitamin E TPGS nanoparticles. Biomaterials 29:2663–2672. https://doi.org/10.1016/j.biomaterials.2008.02.020
Vortherms AR, Doyle RP, Gao D et al (2008) Synthesis, characterization, and in vitro assay of folic acid conjugates of 3′-azido-3′-deoxythymidine (AZT): toward targeted AZT based anticancer therapeutics. Nucleosides Nucleotides Nucleic Acids 27:173–185. https://doi.org/10.1080/1525777070179594
Zhang Z, Huey Lee S, Feng SS (2007) Folate-decorated poly(lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials 28:1889–1899. https://doi.org/10.1016/j.biomaterials.2006.12.018
Gilbert L, Oaknin A, Matulonis UA et al (2023) Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol 170:241–247. https://doi.org/10.1016/j.ygyno.2023.01.020
Yu Y, Zhang G, Li Z et al (2023) Designed fabrication of active tumor targeting covalent organic framework nanotherapeutics via a simple post-synthetic strategy. Nano Res 16:7085–7094. https://doi.org/10.1007/s12274-022-5265-7
Platt VM, Szoka FC (2008) Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm 5(4):474–486. https://doi.org/10.1021/mp800024g
Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4:33–45
Klingbeil P, Natrajan R, Everitt G et al (2010) CD44 is overexpressed in basal-like breast cancers but is not a driver of 11p13 amplification. Breast Cancer Res Treat 120:95–109. https://doi.org/10.1007/s10549-009-0380-7
Mansoori-Kermani A, Khalighi S, Akbarzadeh I et al (2022) Engineered hyaluronic acid-decorated niosomal nanoparticles for controlled and targeted delivery of epirubicin to treat breast cancer. Mater Today Bio. https://doi.org/10.1016/j.mtbio.2022.100349
Ranuncolo SM, Ladeda V, Specterman S et al (2002) Cd44 expression in human gliomas. J Surg Oncol 79:30–35. https://doi.org/10.1002/jso.10045
Cadete A, Alonso MJ (2016) Targeting cancer with hyaluronic acid-based nanocarriers: recent advances and translational perspectives. Nanomedicine 11:2341–2357
Mattheolabakis G, Milane L, Singh A, Amiji MM (2015) Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target 23:605–618. https://doi.org/10.3109/1061186X.2015.1052072
Jiang T, Zhang Z, Zhang Y et al (2012) Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials 33:9246–9258. https://doi.org/10.1016/j.biomaterials.2012.09.027
Lee H, Lee K, Tae GP (2008) Hyaluronic acid-paclitaxel conjugate micelles: synthesis, characterization, and antitumor activity. Bioconjug Chem 19:1319–1325. https://doi.org/10.1021/bc8000485
Li J, Huo M, Wang J et al (2012) Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 33:2310–2320. https://doi.org/10.1016/j.biomaterials.2011.11.022
Accardo A, Aloj L, Aurilio M et al (2014) Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int J Nanomed 9:1537–1557
Toti US, Guru BR, Grill AE, Panyam J (2010) Interfacial activity assisted surface functionalization: a novel approach to incorporate maleimide functional groups and cRGD peptide on polymeric nanoparticles for targeted drug delivery. Mol Pharm 7:1108–1117. https://doi.org/10.1021/mp900284c
Yoo J, Park C, Yi G et al (2019) Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel). https://doi.org/10.3390/cancers11050640
Chen K, Conti PS (2010) Target-specific delivery of peptide-based probes for PET imaging. Adv Drug Deliv Rev 62:1005–1022
Feng GK, Zhang MQ, Wang HX et al (2019) Identification of an integrin α6-targeted peptide for nasopharyngeal carcinoma-specific nanotherapeutics. Adv Ther (Weinh). https://doi.org/10.1002/adtp.201900018
Yu DH, Lu Q, Xie J et al (2010) Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials 31:2278–2292. https://doi.org/10.1016/j.biomaterials.2009.11.047
Zakeri-Milani P, Shirani A, Nokhodchi A et al (2020) Self-assembled peptide nanoparticles for efficient delivery of methotrexate into cancer cells. Drug Dev Ind Pharm 46:521–530. https://doi.org/10.1080/03639045.2020.1734017
Moku G, Layek B, Trautman L et al (2019) Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using tat peptide functionalized polymeric nanoparticles. Cancers (Basel). https://doi.org/10.3390/cancers11040491
Gao L, Yu J, Liu Y et al (2018) Tumor-penetrating peptide conjugated and doxorubicin loaded T1–T2 dual mode MRI contrast agents nanoparticles for tumor theranostics. Theranostics 8:92–108. https://doi.org/10.7150/thno.21074
Zhou J, Rossi JJ (2016) Evolution of cell-type-specific RNA aptamers via live cell-based SELEX. Methods Mol Biol 1421:191–214. https://doi.org/10.1007/978-1-4939-3591-8_16
Gu FX, Karnik R, Wang AZ et al (2007) Targeted nanoparticles for cancer therapy. Nano today 2(3):14–21. https://doi.org/10.1016/S1748-0132(07)70083-X
Kashida S, Inoue T, Saito H (2012) Three-dimensionally designed protein-responsive RNA devices for cell signaling regulation. Nucleic Acids Res 40:9369–9378. https://doi.org/10.1093/nar/gks668
Yang C, Jiang Y, Hao SH et al (2022) Aptamers: an emerging navigation tool of therapeutic agents for targeted cancer therapy. J Mater Chem B 10:20–33
Pi F, Zhang H, Li H et al (2017) RNA nanoparticles harboring annexin A2 aptamer can target ovarian cancer for tumor-specific doxorubicin delivery. Nanomedicine 13:1183–1193. https://doi.org/10.1016/j.nano.2016.11.015
Leach JC, Wang A, Ye K, Jin S (2016) A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int J Mol Sci. https://doi.org/10.3390/ijms17030380
Chen F, Zeng Y, Qi X et al (2018) Targeted salinomycin delivery with EGFR and CD133 aptamers based dual-ligand lipid-polymer nanoparticles to both osteosarcoma cells and cancer stem cells. Nanomedicine 14:2115–2127. https://doi.org/10.1016/j.nano.2018.05.015
Cheng Z, Al Zaki A, Hui JZ et al. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Sci 338(6109):903-910. https://doi.org/10.1126/science.1226338
Piktel E, Niemirowicz K, Watek M et al (2016) Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J Nanobiotechnol. https://doi.org/10.1186/s12951-016-0193-x
Phillips MA, Gran ML, Peppas NA (2010) Targeted nanodelivery of drugs and diagnostics. Nano Today 5:143–159
Bhatia SN, Chen X, Dobrovolskaia MA, Lammers T (2022) Cancer nanomedicine. Nat Rev Cancer 22:550–556. https://doi.org/10.1038/s41568-022-00496-9
Alshehri S, Imam SS, Rizwanullah M et al (2021) Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: preclinical promise and translational challenges. Pharmaceutics 13:1–35
Anselmo AC, Mitragotri S (2019) Nanoparticles in the clinic: an update. Bioeng Transl Med. https://doi.org/10.1002/btm2.10143
Park K (2019) Transcending nanomedicine to the next level: are we there yet? J Control Release 298:213
De Jong M, Maina T (2010) Of mice and humans: are they the same?—implications in cancer translational research. J Nucl Med 51:501–504
Leong HS, Butler KS, Brinker CJ et al (2019) On the issue of transparency and reproducibility in nanomedicine. Nat Nanotechnol 14:629–635
Barlas S (2013) FDA strategies to prevent and respond to drug shortages: finding a better way to predict and prevent company closures. Pharm Ther 38:261–263
Smith JA, Costales AB, Jaffari M et al (2016) Is it equivalent? Evaluation of the clinical activity of single agent Lipodox® compared to single agent Doxil® in ovarian cancer treatment. J Oncol Pharm Pract 22:599–604. https://doi.org/10.1177/1078155215594415
Waldmann TA (2018) Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a028472
Chen Z, Wang Z, Gu Z (2019) Bioinspired and biomimetic nanomedicines. Acc Chem Res 52:1255–1264. https://doi.org/10.1021/acs.accounts.9b00079
Vasan N, Baselga J, Hyman DM (2019) A view on drug resistance in cancer. Nature 575:299–309
Yong SB, Chung JY, Song Y et al (2019) Non-viral nano-immunotherapeutics targeting tumor microenvironmental immune cells. Biomaterials 219:119401
Zou MZ, Liu WL, Li CX et al (2018) A multifunctional biomimetic nanoplatform for relieving hypoxia to enhance chemotherapy and inhibit the PD-1/PD-L1 axis. Small. https://doi.org/10.1002/smll.201801120
Qian M, Chen L, Du Y et al (2019) Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett 19:8409–8417. https://doi.org/10.1021/acs.nanolett.9b02448
Bu J, Nair A, Iida M et al (2020) An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett 20:4901–4909. https://doi.org/10.1021/acs.nanolett.0c00953
Zhang Z, Wang Q, Liu Q et al (2019) Dual-locking nanoparticles disrupt the PD-1/PD-L1 pathway for efficient cancer immunotherapy. Adv Mater. https://doi.org/10.1002/adma.201905751
Zheng C, Wang Q, Wang Y et al (2019) In situ modification of the tumor cell surface with immunomodulating nanoparticles for effective suppression of tumor growth in mice. Adv Mater. https://doi.org/10.1002/adma.201902542
Raju GSR, Pavitra E, Varaprasad GL et al (2022) Nanoparticles mediated tumor microenvironment modulation: current advances and applications. J Nanobiotechnol 20:274
Wu P, Han J, Gong Y et al (2022) Nanoparticle-based drug delivery systems targeting tumor microenvironment for cancer immunotherapy resistance: current advances and applications. Pharmaceutics 14:1990
Kumar A, Das N, Rayavarapu RG (2023) Role of tunable gold nanostructures in cancer nanotheranostics: implications on synthesis, toxicity, clinical applications and their associated opportunities and challenges. J Nanotheranostics 4:1–34. https://doi.org/10.3390/jnt4010001
Gao Y, Tong H, Li J et al (2021) Mitochondria-targeted nanomedicine for enhanced efficacy of cancer therapy. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.720508
Musicco C, Signorile A, Pesce V et al (2023) Mitochondria deregulations in cancer offer several potential targets of therapeutic interventions. Int J Mol Sci 24:10420
Acknowledgements
The authors thank the Department of Biotechnology and Department of Chemical Engineering, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal for their constant support and encouragement. The images were created with BioRender.com.
Funding
This study was supported by Vision Group on Science and Technology (Grant No. K-FIST LEVEL 1 GRD 267).
Author information
Authors and Affiliations
Contributions
KKS: Data collection, investigation, writing—original draft and editing. YR: Data collection and investigation. DJP: Data collection and help in writing. SA: reviewed the writing and helped in editing. BRG: Conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kizhakkanoodan, K.S., Rallapalli, Y., Praveena, J. et al. Cancer nanomedicine: emergence, expansion, and expectations. SN Appl. Sci. 5, 385 (2023). https://doi.org/10.1007/s42452-023-05593-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05593-4