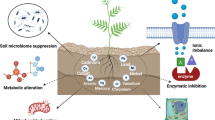
Abstract
Nigeria's most consumed potable water plastic wastes are indiscriminately dumped into agricultural soil despite their ability to become microplastics. The study evaluates the potential impacts of these microplastics on soil physico-chemical parameters, soil bacterial diversity and functions as well as antibiotic resistance. Soil sample was collected using a sterile hand-held auger and its physico-chemical parameters evaluated. Baseline microplastic concentration was determined via the flotation method while microbial isolates were obtained from the test (enriched with microplastics) and control samples using cultural technique and metagenomics. Metagenomic next-generation sequencing (mNGS) was done using the Illumina Miseq platform. The cluster of orthologous genes (COG) tool was used in the prediction of bacterial functional roles. Replicate readings were analysed using analysis of variance (ANOVA) and means compared using the student’s t test. Observed baseline microplastic concentration was 0.08 particles/g of soil. The addition of the microplastics to the soil sample decreased the concentrations of some metals (iron, zinc, lead and nickel) while cobalt concentration, pH level and microbial counts increased. Microbial count and pH clustered together while iron, magnesium, nitrate, nitrite, chromium, cobalt, total organic carbon, zinc, lead, and nickel showed positive loading values suggesting that the addition of microplastics could alter them. Dominant taxa were proteobacteria, unknown, firmicutes at the phyla level. At the level of species, Pseudomonas species dominated microplastics incubated soil while potential pathogenic species such as Klebsiella dominated the control sample. A higher level of multi-drug resistance and altered metabolisms was observed in the test sample. Sachet water microplastics could have serious implications for public health and food security.
Article Highlights
-
Sachet water microplastics altered the physicochemistry of the soil.
-
Sachet water microplastics enhance typical degraders while deselecting pathogens
-
Microplastics degraders showed higher multi-drug resistance than the non-degraders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plastics have outgrown majority of man-made materials globally [1]. According to Geyer et al. [1], cumulatively up to 2015, only 6300 metric tons of plastic had been produced out of which, 79% ended up in landfill or natural environments while the rest were either recycled or incinerated [1]. Furthermore, they estimated that 8300 million metric tons of virgin plastic have been produced as of 2017. Across Nigeria and most West African countries like Ghana and Liberia, a unique plastic waste is on the increase and this is the popular sachet water packaging plastic that is used in packaging the widely consumed potable water called “pure water” [2]. It is the water of choice for Nigerian middle-class populace due to its wide spread availability, ease of handling, gross inadequacy of pipe-borne water in homes and affordability. It is the preferred drinking water at schools, churches, and social events. In Liberia, an estimate showed that a total of four sachet water is consumed per individual per day, and the resulting plastic wastes are indiscriminately disposed into the environment [3, 4]. Its packaging material is made up of polyethene plastic [2]. Polyethene as a packaging material has numerous attractive properties such as toughness, near-zero moisture absorption, excellent chemical inertness, and ease of processing [3, 5]. Sadly, these sachet water wastes like other plastic wastes have the capacity to end up as microplastics in the environment. Microplastics are well known contaminants with diameter of 5 mm or below in various ecosystems and are widespread, recalcitrant and persistent in the environment [6]. This is made worse by the fact in Nigeria, these wastes are not recycled at all and at best, they are burnt in open dumpsites, farmlands, streets and in gardens causing air pollution [7].
The accumulation of plastics in terrestrial ecosystems via indiscriminate dumping serves as large pool and major route of entry of plastics into aquatic (fresh and marine) ecosystems [8, 9]. Evidence is beginning to emerge that indicates that microplastics irrespective of the source are becoming very abundant and are carriers of the microorganisms, other pollutants and antibiotics resistant genes [6, 10]. Whether in aquatic or terrestrial habitats, there is a high tendency for these microplastics to eventually bioaccumulate in apex consumers like humans and lower animals [11,12,13,14,15,16]. Studies have shown that microplastics exert a lot of biophysical effects on agricultural soil such as interaction with soil aggregates, increased soil holding capacity thus reducing bulk density, can migrate down the soil and affect soil organic matter and soil health negatively in general, and can culminate in reduction of plant performance [9, 17,18,19,20,21]. Other studies have shown that soil biota mediated processes such as biogeochemical cycling in the presence of microplastics are adversely altered. Furthermore, soil enzymes such as catalase and urease which act as an indicator of soil fertility, and also play important roles in biogeochemical cycling of nutrients such as carbon, nitrogen and phosphate are significantly altered in the presence of microplastics. These eventually bring about short-term negative changes in soil quality with potential implication in food security [9]. Microplastics have been shown to affect the bioavailability of heavy metals, can migrate deep down into the soil and bioaccumulate in plants [20]. Furthermore, there are concerns of plastic wastes including microplastics acting as hubs for the enrichment of antibiotic resistant bacteria and their genes, and in the process contribute to the pool of antibiotic resistance genes and resistant species in the environment [6].
It is therefore not surprising that antibiotic resistance genes are also abundant in soil environment [22, 23]. The distribution of antibiotics resistance genes in soil appears to be dependent on soil type [22] and widespread. These include resistance genes to antibiotics such as the sulfonamide, fluoroquinolone, penicillin, erythromycin and tetracycline [23]. This seems to be compounded by the emergence of microplastics in all kinds of environments which have been shown to be capable of harbouring these antibiotics resistance genes by acting as hotspot or creating a favourable environment for biofilm which helps to spread and select resistance genes [6, 20, 24,25,26]. Antibiotics resistance is now a global public health challenge with no end in sight despite advances in various drug discovery technologies [27]. It appears as though those microbes with plasmid-borne antibiotics genes are microplastic degraders [28]. These resistance genes can cross from environmental into clinical settings and vice versa [29]; thus, compounding and worsening clinical outcomes of diseases caused by microorganisms that harbour or have acquired these genes. Microplastics and antibiotics resistance genes have received enormous attention recently due to their potential and overlapping environmental and public health concerns. More worrisome is the fact that recent studies have shown potential linkage between microplastic degraders and antibiotics resistance in various environments [6, 8, 30]. These genes that confer resistance to microbes against antibiotics are called antibiotics-resistant genes and have been recognized as an emerging environmental pollutant as they are pervasive in various environments and can be transmitted amongst microbes with relative ease [6, 28].
From the foregoing, it is clear that sachet water plastic waste, a polyethene based plastic waste is capable of producing polyethene microplastics like other plastic wastes. To the best of our knowledge, there is a dearth of information on the impact sachet water microplastics could have on soil physicochemistry, microbial diversity and antibiotics resistance genes. Thus, the study evaluates the impact of this unique microplastics on soil physical and chemical parameters, soil microbial community and function, as well as antibiotic resistance.
In Sect. 2, the methods (sampling, physiochemical, microbiological and metagenomics analyses, etc.) utilized in the study are presented. Sections 3 captures the results of the study while Sect. 4 dwells on the results of the study and the last Section is the conclusions drawn from the results and discussion.
2 Methods
2.1 Study area
The study was carried out in Arthur Jarvis University, the first privately owned university in Cross River State, South-South Nigeria, located in Akpabuyo. The study site is well known for its fertile soil, luxuriant forest, characteristic rainy (April to September) and dry (October–March) seasons, and an annual rainfall that peaks at 3000–4000 mm per annum during wet season. The main occupations of the inhabitants as expected are peasant and/or commercial farming, and petty trading. However, with the rapid urbanization of Calabar, the capital of Cross River State, Akpabuyo has become the destination of choice for more middle-class families who consume sachet water.
2.2 Soil and sachet water plastic collection
Agricultural (loamy) soil was collected from garden of the University. Collection was done using a stainless sterile hand-held soil auger from three (3) locations at depths of 0–20 cm layer where a total of nine (9) samples were collected and made into a composite sample (4 kg in total). Soil sample was immediately transported to the laboratory in an ice pack held at 4 °C till further use. Before further use, the soil sample was allowed to assume room temperature first. Sachet water or “pure water” was purchased from a reputable company and the contents (potable water) emptied aseptically, the resulting plastic waste air-dried, and stored at 25 ± 2 °C for future use. The plastics type was identified as unsaturated polythene plastic at the Chemical Science Department, Faculty of Natural and Applied Sciences, Arthur Jarvis University.
2.3 Preparation of sachet water microplastics
Microplastics from our sachet water sample was generated mechanically to avoid addition or removal of its carbon content. Briefly, the air-dried sachet water polythene plastics were mechanically grated using a sterile grater that was first cleaned with 90% analytical grade ethanol (Merck). The pore sizes of the stainless grater were > 2 mm. To achieve this, the air-dried sachet bags were lumped together and mechanically grated gently until fine polythene powder was obtained. The collected fine microplastic powder were stored using moisture-free sterile petri dishes until required for further use.
2.4 Determination of microplastics in the study site
The microplastic concentration was estimated as previously reported using flotation method [8, 9, 30]. The composite soil sample was first air-dried and sieved through a sterilized steel mesh (< 5 mm). After sieving, the soil sample was weighed and recorded (100 g) and then added to 500 ml of distilled water and kept overnight to allow floatation of microplastics and impurities to take place. The suspended particles were then decanted gently and then filtered using a Whatman no 1 of pore diameter < 3 mm into a glass beaker. Given the small carrying capacity of the filter paper, the procedure was carried out using multiple filter papers and the procedure repeated until all the floating particles were trapped to the side of the glass funnel containing the filter papers. The procedure was repeated thrice to ensure that all potential particles floating were filtered off to the side of the filter papers. The filtrates were first weighed then dried using an electric oven pre-set at 60 °C for 3–5 min and weighed again for the potential weight of the microplastics. Finally, the debris were examined using a × 20 stereo light microscope to sort out the microplastics from other particles. After all the evaluations, the concentration of microplastics was established as 0.009 g from 8 items which is equivalent to 0.08 particles per gram.
2.5 Analysis of physical and chemical parameters
The total organic carbon, phosphorus, and nitrogen levels of the soil were evaluated as previously reported [31, 32]. However, pH, temperature (°C), and electrical conductivity (µs/cm) were measured in-situ before incubation with the microplastic using soil pH meter (PCE-228S, Japan), gardener’s thermometer (280 × 58 mm, 150 g), and soil conductivity meter (HI 993310-PCE, Germany), respectively. In addition to the aforementioned parameters, heavy metals were also evaluated. The metals limits of detection (mg/kg) were 0.052 for Pb, 0.018 for Cd, 0.015 for Mn, 0.025 for Ni, 1.790 for Zn, 0.079 for Cr, 0.02 for Fe and 0.01 mg/kg for Co. Concentrations of heavy metals were expressed in mg/kg dry weight and all determinations of the heavy metals done as reported [33, 34]. These determinations were done for the control and test samples.
2.6 Microbiological analysis
From the control and test samples (with microplastics), total heterotrophic bacteria counts were evaluated following a ten-fold serial dilution. Serial dilutions were carried out on days 8, 16, 24 and 32, respectively and then plated out on nutrient agar using spread plate technique. The freshly prepared nutrient agar was supplemented with 50 μg/ml of nystatin to inhibit fungal growth and incubated at 35 °C for 24 h after which the counts were recorded. Distinct colonies were selected and sub-cultured twice onto freshly prepared nutrient agar plates for purification. Pure isolates were characterized using Gram reaction and biochemical tests as reported previously [34, 35].
2.7 Preparation of mineral salt medium (MSM) and microplastic agar
This was done using the methods reported previously but with little modifications [36, 37]. Briefly, salts were weighed out using a Superior Mini Digital platform scale (Model I-2000, China). The salts and their amounts were 0.2 g MgSO4, 1.0 g KH2PO4, 1.0 g K2HPO4, 1.0 g NH4NO, 0.02 g CaCl2, and 0.05 g FeCl2. These salts were then dissolved in 1000 ml of sterile distilled water with rigorous mixing using a sterile glass rod until all the salt particles were completely dissolved. After dissolution, the pH was adjusted to 7.2 and the MSM autoclaved at 121 °C for 15 min at 15 psi. The MSM was then stored at 4 °C until further use. The microplastic agar was prepared by adding Agar Agar (14 g/L) and 10% of microplastics as sole carbon source. The mixture was then sterilized using autoclave at 121 °C for 15 min and 15 psi.
2.8 Incubation of the microplastics with soil sample and isolation of microplastic degraders
The microplastics obtained were first sterilized using a hot-air oven for 3 min preset at 60 °C in order to reduce or eliminate microbial contamination. After sterilization, the microplastics were stored in a sterile disposable petri-dish and weighed. To exactly 1000 g of soil held in an aluminum pot, 10 g of the microplastics was added (ratio of 1:100) and stirred using a sterile glass stirrer to ensure even distribution of the microplastics. This was done in triplicate and then allowed to stand for 32 days. Alongside the test experiments, control experiment devoid of microplastics was also set-up to allow for comparison. At intervals of 8, 16, 24, and 32 days, total heterotrophic count was evaluated as aforementioned. To completely mimic the natural environment, the experimental set-up was placed back where the soil samples were collected but using aluminum pots and under a shade in order to ensure that light intensity, temperature and moisture were not those of the laboratory but in-situ. Discrete colonies from the microplastic incubated sample were inoculated onto the microplastic agar and the plates were incubated at 37 °C for 96 h. Resulting distinct colonies were first purified, identified, and stored for future use as previously described [33, 34].
2.9 Antibiotic susceptibility test
The microbial isolates obtained from the control and test soil samples were subjected to antibiotic sensitivity test. This was done using the disc diffusion technique on freshly prepared Mueller Hinton Agar. The test antibiotics used in the study were Ciprofloxacin (30 μg), Streptomycin (30 μg), Septrin (15 μg), Gentamycin (15 μg), Amoxicillin (30 μg), Ceftriazone (10 μg), Ciprofloxacin (30 μg), Pefloxacin (10 μg), Gentamycin (30 μg), and Augmentin (10 μg) (Becton Dickinson, USA). The diameters of the zones of inhibition were interpreted as resistant and susceptible according to the NCCLS guideline [38].
2.10 Metagenomic DNA extraction, PCR amplification, and library construction
From the test and control microcosms, composite samples were obtained. Metagenomic DNA was extracted using Zymo DNA extraction kit (Zymo Research Model 6001 USA) and this was done by strictly following the instructions of the manufacturer. From both samples, one gram of soil sample each was weighed out after thorough mixing using a sterile glass rod. The choice of universal primer and polymerase chain reaction (PCR) conditions were all done as previously reported [39,40,41]. Briefly, we targeted the V3–V4 hyper-variable region of the bacterial 16S rRNA genes using the universal bacterial primer set TruSeq tailed 341F TGACTGGAGTTCAGACGTGTGCTCTTCCGATCT CCT ACGGGNGGCWGCAG and TruSeq tailed 785R CACTCTTTCCCCACACGACGCTCTTCCGATCT GACTACHVGGGTATCTAATCC. The reaction mixture was set up as follows: microbial DNA (5 ng/μl) 2.5 μl, PCR forward primer (1 μM) 5 μl, and PCR reverse primer (1 μM) 5 μl; 2 × KAPA HiFi Hot Start Ready Mix 12.5 μl (total 25 μl). The PCR was performed in a thermal cycler (Applied Biosystems 9700, USA) with 1 cycle of denaturing at 98 °C for 3 min, followed by 30 cycles of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and a final extension at 72 °C for 5 s. Construction of libraries, purification and validation, and other preprocessing were carried out as described previously [41].
2.11 Next generation DNA Sequencing and analysis of reads
Following successful extraction and amplification, the amplified extracted DNA were shipped to Inqaba, South Africa in dry-ice for metagenomic next generation sequencing (mNGS). The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. The library was sequenced on an Illumina MiSeq system (Illumina Inc, San Diego, California USA). Following mNGS, quality control of the raw sequence was carried out and sequences with q scores of > 20 were used for the analysis. Structural composition was inferred using NCBI-BLAST-2.2.24 and CLC Bio Genomics workbench v7.5.1. For the functional gene analysis, open reading frames (ORFs) were generated and then functionally annotated to Cluster of Orthologous Gene (COG) analysis tool [38]. The various classes of COG proteins were then visualized using CIRCOS visualization tool [42].
2.12 Statistical analysis
The microbial counts obtained from both the control and the test samples were first transformed into mean and Log10 and these plotted using a bar-chart. The replicate readings from the physico-chemical analysis were reported as mean values and standard deviations following analysis of variance. The mean values were then compared using the student t-test with significance set at 0.05. Principal component analysis was used to evaluate potential links between the microbial counts and physico-chemical parameters before and after incubation with the microplastics. This was done using SPSS version 21. Furthermore, alpha diversity indices (Shannon and Simpson) were evaluated using PAleontological Statistics (PAST) tool.
3 Results
3.1 Physical and chemical analysis
The result of the physico-chemical analyses of the soil samples with or without the microplastics is presented in Table 1. Comparison of the mean determinations did not reveal any significant difference in both samples; however, there were some observed differences in physical and chemical parameters examined in both samples. Conductivity and pH values were lower in the test soil sample compared to the control. Similarly, nitrate and nitrite levels were slightly lower than that of the control. In addition, similar trends were also observed for chromium, cobalt, sulphide, manganese, and magnesium. On the other hand, zinc, nickel, cadmium, lead, iron and total organic carbon were higher in the test than the control sample. The effect of the sachet water microplastics on the microbial load expressed as Log10 of viable cells using cultural technique is presented in Fig. 1. From the graph, there was a gradual increase in the log or number of viable cells as the days of incubation of the microplastic increased.
3.2 Impact of microplastics on antibiotics resistance
Table 2 shows the antibiotic sensitivity profile of the microplastics degraders. A total of twenty-five (25) microplastics degrading isolates were subjected to antibiotic sensitivity and these were Bacillus spp. (4), Aeromonas spp. (5), Micrococcus spp. (6), Enterococcus spp. (3) and Pseudomonas spp. (6). Apart from gentamycin and nobramycin, over half of the microplastic degrading Bacillus species were resistant. Similar pattern was also observed for other Aeromonas, Micrococcus, Enterococcus and Pseudomonas species as all the isolates showed multi-drug resistance. Table 3 on the other hand, shows the antibiotic sensitivity profile of the non- microplastic degraders from control sample. A total of 19 isolates namely Bacillus spp. (4), Klebsiella spp. (5), Micrococcus spp. (4), Enterococcus spp. (3) and Pseudomonas spp. (3) were obtained. Like the degraders, they also showed multiple drug resistance but not as high as those with microplastics degrading potential.
3.3 Diversity of the microbial community
Kingdom and phyla classifications are as presented in Table 4. In both samples, a total of three kingdoms were identified and these were bacteria, unknown and archae in decreasing order of abundance. However, there was a marked decrease in the abundance of the test sample at kingdom level. Similarly, phyla classification also revealed decreased phyla abundance and diversity in the sample incubated with microplastics. The top seven classes were dominated by members of the proteobacteria family and unknown but these varied in abundance (Fig. 2a). As observed in the phyla classification, the abundance of all the class of the proteobacteria were higher in the control than in the test sample, however, they were amongst the top five classes. Furthermore, the diversity dropped from 19 to 15 classes in the test sample. Similar trends were also observed in the order (Fig. 2b) and family taxa. At the genus and species levels, notable genus and species with degradation potentials were observed in the test sample. The top ten genus in the test samples were Unknown, Pseudomonas, Enterobacter, Sphingomonas, Aeromonas, Citrobacter, Comamonas, Kocuria, Luteimonas, and Paenibacillus. Compared to the control sample, Unknown, Pseudomonas, Enterobacter, Aeromonas, Citrobacter, and Comamonas did overlap while Klebsiella and Brevibacterium were unique to the control samples alone. Although the species- Unknown, Pseudomonas, Enterobacter, Aeromonas, Citrobacter, and Comamonas did overlap, their abundance were enhanced tremendously. In the soil sample incubated with the microplastics, the top ten species were Unknown, Sphingomonas asaccharolytica, Pseudomonas stutzeri, Pseudomonas aeruginosa, Desulfovibrio vulgaris, Enterobacter cloacae, Bacillus firmus, Micrococcus sp., Bacillus firmus, Micrococcus sp., Pseudomonas alcaligenes, and Sphingomonas sp. with abundances rates of 26.55, 17.01, 11.61, 7.90, 6.11, 4.85, 3.78, 2.95, 2.04 and 1.55%, respectively. The summary of the diversity indices at species level is also shown in Table 5. From the diversity analysis result, there was a reduction in the number of species in the test sample compared to the control. In addition, the diversity indices analysis gave values of 2.303 and 2.136 for Shannon and of 0.8344 and 0.8485 for Simpson, respectively for control and test soil samples.
3.4 Functional annotation using COG
Figure 3 shows the COG categories of proteins that were annotated in both samples as visualized using Circos. From the visualization, there were subtle differences in terms of abundance and type of the various classes of COG proteins annotated in the both samples. Control samples returned 13 classes while the test sample gave 6 classes of proteins. For the control sample, the classes of protein were J (Translation, ribosomal structure and biogenesis), K (Transcription), L (Replication, recombination and repair), D (Cell cycle control, cell division, chromosome partitioning), V (Defense mechanism), M (Cell wall/membrane/envelope biogenesis), U (Intracellular trafficking, secretion, and vesicular transport), C (Energy production and conversion), E (Amino acid transport and metabolism), H (Coenzyme transport and metabolism), Q (Secondary metabolites biosynthesis, transport and catabolism), R (General function prediction only) and S (Function unknown) with abundance (%) of 2, 5, 5,3, 5, 11, 8, 3, 18, 1, 1, 38 and 1 respectively. On the other hand, for the test group, the classes of protein were J (Translation, ribosomal structure and biogenesis), N (Cell motility), U (Intracellular trafficking, secretion, and vesicular transport), E (Amino acid transport and metabolism), Q (Secondary metabolites biosynthesis, transport and catabolism), and R (General function prediction only) with abundance of 79, 3,3, 12, 3 and 12, respectively. Comparison of the COG abundance using student t-test did not show any significance (p > 0.05).
Circos visualization of the COG categories of the various proteins in both types of soil. Key: Treated = Soil with MPs while non-treated = Soil without MPs. Classes S, O, H, P, L, J, Q, R, G, D and C = Function unknown; Posttranslational modification, protein turnover, chaperones; Coenzyme transport and metabolism; Inorganic ion transport and metabolism; Replication, recombination and repair; Translation, ribosomal structure and biogenesis; Secondary metabolites biosynthesis, transport and catabolism; General function prediction only; Carbohydrate transport and metabolism; Cell cycle control, cell division, chromosome partitioning and Energy production and conversion
3.5 Correlation between physical and chemical properties and microbial diversity
The summary of the principal component analysis (PCA) of physico-chemical parameters and microbial count in soil is presented in Fig. 4. Principal component 1 (PC 1) had high positive loading values of 0.94, 0.99, 0.89, 0.85, 0.99, 0.85, 0.97, and 0.83 for temperature, conductivity, iron (Fe), magnesium (Mg), nitrate, nitrite, chromium (Cr), and cobalt (Co), respectively, resulting in a total variance of 53.59%. The PC 2 had high positive loading values of 0.98, 0.99, 0.92, and 0.98 for total organic carbon (TOC), zinc (Zn), lead (Pb), and nickel (Ni), respectively, resulting in a total variance of 46.40%. The PCA biplot showed that Fe and temperature clustered together, while nitrate, conductivity, and Cr also formed a cluster. Furthermore, Mg, nitrate, Co, and sulphide clustered separately, while pH and microbial counts also formed a cluster (Fig. 4). The temperature, conductivity, Fe, Mg, nitrate, nitrite, Cr, Co, TOC, Zn, Pb, and Ni had high positive loading values, denoting that the microplastics introduced into the soil raised the levels of conductivity, Fe, Mg, nitrate, nitrite, Cr, Co, TOC, Zn, Pb, and Ni in the soil to levels that the soil becomes unhealthy. There was a cluster between Fe and temperature, indicating that both parameters are related, such that an increase in the temperature will also influence the Fe levels in the soil. Nitrate, conductivity, and Cr clustered together, denoting that these parameters are related, such than an increase in concentration of Cr will also raise the levels of nitrate and conductivity. Also, Mg, nitrate, Co, and sulphide clustered together, and this implies that they are related and the increase in Mg and Co will increase the nitrate and sulphide levels. Lastly, pH and microbial counts formed a cluster, indicating they are related, so that the microbial counts in the soil were influenced by the soil pH.
4 Discussion
In our study, the presence of sachet water microplastic in the soil did alter the physical and chemical parameters of the soil but this was not statistically significantly probably because of the duration of incubation of the microplastics. However, pH, conductivity, manganese, magnesium, sulphide, nitrite, nitrate, chromium, and lead were only slightly higher in the microplastic treated sample than in the control sample while other parameters were slightly higher in the control. Our findings corroborate those of other studies that have shown that microplastics can reduce certain soil physical properties culminating into reduced plant performance [8, 17,18,19,20,21]. The nitrite and nitrate levels in our study showed a reduction in the test sample and this correlates with a previous study that showed that microbiologically mediated processes such as biogeochemical cycling of nutrients are negatively altered by microplastics [18, 20, 26]. In another study, these microplastics have been shown to reduce important soil enzymes which are indicators of soil fertility, alter biogeochemical cycling of nutrients such as carbon, nitrogen, and phosphorus and produces short term changes in soil quality [9]. Furthermore, it has been shown that polythene microplastics may enhance the mobility of organic contaminants [43,44,45]. In Nigeria and particularly in the Niger Delta region, this is important due to incessant crude oil pollution [44] and the pervasive presence of sachet water polythene microplastics. Overall, our findings indicate that microplastics can also alter the physicochemistry of agricultural soil with implication in the food security of a country that is yet to attain food security. The concentration of microplastics in our study site was 0.009 g from 8 items or 0.08 particles per gram and this is lower than that reported by Corradini and colleagues [45] for Chile. In another earlier study, microplastics concentration was reported to be within the range of 7100–42,900 particles per kg in China. This high concentration of microplastics in both locations could be explained by the high population density as well as the high usage of plastics in agriculture in both countries [20, 26]. Alteration of soil physiochemistry by microplastics has been attributed to alteration in degradation of organic matter, evapotranspiration rate, water availability and general microbial metabolic activity [9].
Zhao et al. [46] showed that microplastics population increases soil pH in a fashion dependent on the type of the microplastics, time of incubation and size, albeit in a synergistic manner. In our study site, there was an over reduction in pH towards acidic range. These changes in pH have been shown to have a considerable effect of the microbial structure and functions such as biogeochemical cycling, plant growth and performance [19]. Microbial counts as revealed by metagenomics cluster with pH suggesting that the change is pH induced by the addition of the microplastics as one of the main driving forces in alteration of the microbial community structure. Heavy metals and microplastics individually are well known to be toxic to various organisms in a manner that is dependent on the type and concentration [47,48,49]. Their combined effect is still not well understood, however, Cao et al. [48] showed that their interaction is complex and their effects on one another could be synergistic or antagonistic. In our study, the presence of microplastics increased the concentrations of Fe, Zn, Pb and Ni but not that of Co which decreased, implying that their presence in soil could increase or decrease the concentration and availabilities of certain metals.
Cultural technique showed a reduction in the microbial load in the test samples. This reduction was further corroborated by culture-independent technique, metagenomics. Metagenomics evaluation of the test soil showed reduction in terms of abundance and diversity from kingdom to species. The higher Shannon index recorded in the control sample is an indication that the bacteria species were more diverse in the control sample than in the test soil sample. Similarly, the lower Simpson index recorded in the control sample indicates that the control soil sample had a higher bacterial abundance. The reduction in the test sample as indicated by the numerical abundance of bacteria species and diversity indices (Shannon and Simpson indices) is an indication of the effects of microplastics on bacteria diversity and abundance. The negative impact of the microplastics on the microbial diversity corroborated the earlier findings that suggested that they can significantly alter bacteria diversity [25, 50, 51]. Microbial structure in any habitat determines the functional role that they play in such an environment. This includes biogeochemical cycles and decomposition/degradation of organic and inorganic compounds. Reduction in the microbial diversity has the capacity to reduce or alter these functional roles. As seen in the phyla, class, order, families, genus, and species levels, there was a selection of taxa associated with degradation of organic pollutant such as the proteobacteria, unknown, and firmicutes while potential pathogenic species such as Klebsiella were deselected. The abundance of Pseudomonas species in the test sample is an indication that microplastics as carbon source could be toxic to species that cannot utilize same as carbon source. The amplification of unknown taxa suggests that more taxa than revealed in this study may be involved in the degradation process. The isolates incubated with microplastic agar grew very slowly and this confirms earlier findings that showed that the rate of biodegradation of microplastics is very slow [52] and can contribute to the carbon pool [53] and emission of greenhouse gases [54].
In our study, functional annotation revealed less functional categories in the test sample incubated with microplastic and this correlated with a reduction in taxa compared to the control sample without microplastic. The presence of the microplastics seems to alter key metabolisms such as those related to DNA, carbohydrate transport and metabolism, secondary metabolite biosynthesis, and proteins with functions unknown. The increase in the translation activity could be an indication that the presence of the microplastics makes the microbiome to shift their protein synthesis in line with the new carbon source. Given the high rate at which they are generated, microplastics from sachet water will become the leading microplastics source in both aquatic and terrestrial environments in Nigeria. In agricultural soils, polyethylene-based microplastics are known to alter microbiome structures and functions, biophysical properties of soil [6, 10, 17,18,19,20,21], and can act as carriers of unique microorganisms, antibiotics resistance genes and pollutant genes [6, 10, 19, 20]. Microplastics and antibiotics resistance genes have received enormous attention recently due to their potential and overlapping environmental and public health concerns. More worrisome is the fact that recent studies have shown potential linkage between microplastic degraders and antibiotics resistance in various environments [8, 30, 52]. These genes that confer resistance to microbes against antibiotics are called antibiotics-resistant genes. These genes have been recognized as an emerging environmental pollutant as they are pervasive in the environment and can be transmitted amongst microbes with relative ease [28, 38]. In our study, test sample isolates all showed multidrug resistance. Bacillus species showed resistance to all the antibiotics, more than half were resistant to erythromycin, levofloxacin, and ampliclox. Similarly, more than half of the Aeromonas species showed resistance to erythromycin, gentamycin, ampliclox, amoxicillin, streptomycin, and chloramphenicol. This resistance observed by the isolates could imply that the genes for resistance could be transmitted together where both pollutants co-exist. In our earlier study, we explored the abundance of antibiotic resistant genes in various ecosystem in the Niger Delta region of Nigeria and we observed a total of 12 antibiotic resistance genes and these were annotated dominantly to the Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, and the Firmicutes taxas [28]. Interestingly, these classes dominated our test soil samples incubated with the microplastics. These findings also corroborate with those of an earlier study that showed abundance of antibiotics resistant genes on biofilms on microplastic surfaces [29]. Although we did not explore the antibiotics resistant genes on microplastic surfaces, their absorption and accumulation on surfaces of microplastics could help explain why these microplastic degraders have the potential to also acquire antibiotics resistance that they can transfer via plasmids and also have the capacity to cross from environmental into clinical settings. Microplastic biofilm mediated antibiotic resistance has been attributed to conjugation (directed horizontal gene transfer); a process in which two bacteria in close contact can exchange genetic information via plasmid from a donor to a recipient cell [16, 55].
5 Conclusion
Our study was designed to evaluate the effect of sachet water microplastic on soil microbial diversity and function, soil physicochemistry and antibiotic resistance profiling of the isolated microplastic degraders. Although there was no statistical significance when we compared the physio-chemical parameters of the control and test soil samples, our results indicate that some physical and chemical parameters were altered and these included pH, conductivity, organic carbon, heavy metals, nitrite and nitrate among others. Soil sample incubated with the microplastic showed reduction in the microbial diversity and abundance across all taxa using cultural technique and metagenomics. Furthermore, there was marked reduction in the functional classes of proteins annotated, especially those related to carbohydrate, nucleic acid and biosynthesis of secondary metabolites in the test soil sample. The microplastics degraders showed higher multidrug resistance to the test antibiotics than the non-degraders obtained from the control soil. Amplification of multi-drug resistant organisms and their genes is worrisome since antibiotics resistant genes can cross into clinical settings. Given the public health and food security implications of the findings, there is a need for further studies as well as legislations that will control the indiscriminate dumping of sachet water plastic wastes into the environment.
Data availability
Dataset generated in this study is available from the corresponding author on request.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- NGS:
-
Next generation sequencing
- PCA:
-
Principal component analysis
- ANOVA:
-
Analysis of variance
- µg:
-
Microgram
References
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3(7):e1700782. https://doi.org/10.1126/sciadv.1700782
Sanni LO. Environmental impact of sachet water consumption in Saki Town, Oyo State, Nigeria. Accessed at https://www.theseus.fi/handle/10024/262945
Apeh CC (2018) Survey of sachet water waste disposal in Liberia. J Health Pollut 8(20):181211. https://doi.org/10.5696/2156-9614-8.20.181211
Dzodzomenyo M, Fink G, Dotse-Gborgbortsi W, Wardrop N, Aryeetey G, Coleman N, Hill A, Wright J (2018) Sachet water quality and product registration: a cross-sectional study in Accra, Ghana. J Water Health 16(4):646–656. https://doi.org/10.2166/wh.2018.055
Khanam PN, AlMaadeed MA (2015) Processing and characterization of polyethylene-based composites. Adv Manu Polym Compos Sci 1(2):63–79. https://doi.org/10.1179/2055035915Y.0000000002
Dong H, Chen Y, Wang J, Zhang Y, Zhang P, Li X, Zou J, Zhou A (2021) Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J Hazard Mater 5(403):123961. https://doi.org/10.1016/j.jhazmat.2020.123961
Bassey IU, Brooks AA, Asikong BE, Andy IE (2015) Environmental and public health aspects of solid waste management at the Lemna dumpsite in Calabar, Cross River State, Nigeria. J Trop Dis Health 10(3):1–3
Liu K, Wang X, Fang T, Xu P, Zhu L, Li D (2019Jul) Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci Total Environ 20(675):462–471. https://doi.org/10.1016/j.scitotenv.2019.04.110
Liu H, Yang X, Liu G, Liang C, Xue S, Chen H, Ritsema CJ, Geissen V (2017Oct) Response of soil dissolvedorganic matter to microplastic addition in Chinese loess soil. Chemosphere 185:907–917. https://doi.org/10.1016/j.chemosphere.2017.07.064
Qu SS, Zhu HJ, Liu FP, Zhu Y (2017) Adsorption behavior and effect on biont of microplastic. J Environ Hygiene 7(1):75–78
Adeogun AO, Ibor OR, Khan EA, Chukwuka AV, Omogbemi ED, Arukwe A (2020) Detection and occurrence of microplastics in the stomach of commercial fish species from a municipal water supply lake in southwestern Nigeria. Environ Sci Pollut Res Int 27(25):31035–31045. https://doi.org/10.1007/s11356-020-09031-5
Rillig MC, de Souza Machado AA, Lehmann A, Klümper U (2019) Evolutionary implications of microplastics for soil biota. Environ Chem 16(1):3–7. https://doi.org/10.1071/EN18118
Rillig MC, Ziersch L, Hempel S (2017) Microplastic transport in soil by earthworms. Sci Rep 7(1):1362. https://doi.org/10.1038/s41598-017-01594-7
Song Y, Cao C, Qiu R, Hu J, Liu M, Lu S, Shi H, Raley-Susman KM, He D (2019) Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ Pollut 250:447–455. https://doi.org/10.1016/j.envpol.2019.04.066
Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu Q, Ren H, Zhang Y (2019) Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 236:124334. https://doi.org/10.1016/j.chemosphere.2019.07.065
Arias-Andres M, Klümper U, Rojas-Jimenez K, Grossart HP (2018) Microplastic pollution increases gene exchange in aquatic ecosystems. Environ Pollut 237:253–261. https://doi.org/10.1016/j.envpol.2018.02.058
de Souza Machado AA, Lau CW, Kloas W, Bergmann J, Bachelier JB, Faltin E, Becker R, Görlich AS, Rillig MC (2019) Microplastics can change soil properties and affect plant performance. Environ Sci Technol 53(10):6044–6052. https://doi.org/10.1021/acs.est.9b01339
de Souza Machado AA, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC (2018) Impacts of microplastics on the soil biophysical environment. Environ Sci Technol 52(17):9656–9665. https://doi.org/10.1021/acs.est.8b02212
Azeem I, Adeel M, Ahmad MA, Shakoor N, Jiangcuo GD, Azeem K, Ishfaq M, Shakoor A, Ayaz M, Xu M, Rui Y (2021) Uptake and accumulation of nano/microplastics in plants: a critical review. Nanomaterials (Basel) 11(11):2935. https://doi.org/10.3390/nano11112935
Zhou J, Wen Y, Marshall MR, Zhao J, Gui H, Yang Y, Zeng Z, Jones DL, Zang H (2021) Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci Total Environ 15(787):147444. https://doi.org/10.1016/j.scitotenv.2021.147444
Boots B, Russell CW, Green DS (2019) Effects of microplastics in soil ecosystems: above and below ground. Environ Sci Technol 53(19):11496–11506. https://doi.org/10.1021/acs.est.9b03304
Seyoum MM, Obayomi O, Bernstein N, Williams CF, Gillor O (2021) Occurrence and distribution of antibiotics and corresponding antibiotic resistance genes in different soil types irrigated with treated wastewater. Sci Total Environ 15(782):146835
Seyoum MM, Lichtenberg R, Orlofsky E, Bernstein N, Gillor O (2022) Antibiotic resistance in soil and tomato crop irrigated with freshwater and two types of treated wastewater. Environ Res 1(211):113021
Zhou J, Gui H, Banfield CC, Wen Y, Zang H, Dippold MA, Charlton A, Jones DL (2021) The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol Biochem 1(156):108211. https://doi.org/10.1016/j.soilbio.2021.108211
Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X (2019) LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut 254(Pt A):112983. https://doi.org/10.1016/j.envpol.2019.112983
Wang F, Wang Q, Adams CA, Sun Y, Zhang S (2022) Effects of microplastics on soil properties: Current knowledge and future perspectives. J Hazard Mater 424(Pt C):127531. https://doi.org/10.1016/j.jhazmat.2021.127531
Ebana RU, Edet UO, Anosike IK, Etok CA (2019) Bdellovibrio and like organisms: the much-anticipated “magic bullet.” World News Nat Sci 23:1
Edet UO, Antai SP, Brooks AA, Asitok AD (2017) Metagenomic assessment of antibiotics resistance genes from four ecosystems in the Niger Delta Area of Nigeria. AJBGE 1(1):10–10. https://doi.org/10.9734/AJBGE/2017/38009
Zhang Y, Lu J, Wu J, Wang J, Luo Y (2020Jan) Potential risks of microplastics combined with superbugs: enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol Environ Saf 15(187):109852. https://doi.org/10.1016/j.ecoenv.2019.109852
American Public Health Association (APHA) (2008) Standard methods for the examination of water and wastewater, 21st edn, Washington, DC
Vincent-Akpu IF, Tyler AN, Wilson C, Mackinnon G (2015) Assessment of physico-chemical properties and metal contents of water and sediments of Bodo Creek, Niger Delta, Nigeria. Toxicol Environ Chem 97(2):135–144. https://doi.org/10.1080/02772248.2015.1041526
Alam MG, Tanaka A, Allinson G, Laurenson LJ, Stagnitti F, Snow ET (2002) A comparison of trace element concentrations in cultured and wild carp (Cyprinus carpio) of Lake Kasumigaura. Jpn Ecotoxicol Environ Saf 53(3):348–354. https://doi.org/10.1016/s0147-6513(02)00012-x
Collins CH, Lyne PM (1984) Microbiological methods, 5th edn. Butterworth&co Ltd, pp 137–40
Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST Bergey's Manual of determinate bacteriology
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 13(7):e0198446. https://doi.org/10.1371/journal.pone.0198446
Ogunbayo AO, Olanipekun OO, Adamu IA (2019) Preliminary studies on the microbial degradation of plastic waste using Aspergillus niger and Pseudomonas sp. J Environ Prot 10(5):625–631. https://doi.org/10.4236/jep.2019.105037
Wayne PA (2003) National Committee for clinical laboratory standards (NCCLS), methods for dilution antimicrobial susceptibility tests for bacterial that grow aerobically, 6th edn. Approved standard M7-A6
Mbim EN, Mboto CI, Edet UO (2016) Plasmid profileanalysis and curing of multidrug-resistant bacteria isolated from twohospital environments in Calabar Metropolis, Nigeria. Asian J Med Health 1(1):1–11
Bassey IU, Edet UO, Umoafia NG, Nwachi AC, Ebenge IA, Odokuma L (2021) Microbial structure and function diversity of open dumpsite compost used as fertilizer by peasant farmers. Sci Afr 11:e00699. https://doi.org/10.1016/j.sciaf.2021.e00699
Salam LB, Obayori SO, Nwaokorie FO, Suleiman A, Mustapha R (2017) Metagenomic insights into effects of spent engine oil perturbation on the microbial community composition and function in a tropical agricultural soil. Environ Sci Pollut Res 24(8):7139–7159. https://doi.org/10.1007/s11356-017-8364-3
Wu S, Zhu Z, Fu L, Niu B, Li W (2011) WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics 7(12):444. https://doi.org/10.1186/1471-2164-12-444
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19(9):1639–1645. https://doi.org/10.1101/gr.092759.109
Hüffer T, Metzelder F, Sigmund G, Slawek S, Schmidt TC, Hofmann T (2019) Polyethylene microplastics influence the transport of organic contaminants in soil. Sci Total Environ 20(657):242–247. https://doi.org/10.1016/j.scitotenv.2018.12.047
Udofia UU, Edet UO, Antai SP (2018) Potential benefits of applying “Omics” technology in cleaning up incessant crude oil spillages in the Niger Delta Region of Nigeria. Adv Res. https://doi.org/10.9734/AIR/2018/41489
Corradini F, Meza P, Eguiluz R, Casado F, Huerta-Lwanga E, Geissen V (2019) Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci Total Environ 25(671):411–420. https://doi.org/10.1016/j.scitotenv.2019.03.368
Zhao T, Lozano YM, Rillig MC (2021) Microplastics increase soil pH and decrease microbial activities as a function of microplastic shape, polymer type, and exposure time. Front Environ Sci. https://doi.org/10.3389/fenvs.2021.675803
Asitok AD, Antai SP, Ekpenyong MG, Eyong UE (2019) Growth responses of pseudomonas fluorescens and vibrio fluvialis to trivalent chromium toxicity. Int J Innov Sci Res Technol 4:517–526
Cao Y, Zhao M, Ma X, Song Y, Zuo S, Li H, Deng W (2021) A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci Total Environ 20(788):147620. https://doi.org/10.1016/j.scitotenv.2021.147620
Guo JJ, Huang XP, Xiang L, Wang YZ, Li YW, Li H, Cai QY, Mo CH, Wong MH (2020Apr) Source, migration and toxicology of microplastics in soil. Environ Int 137:105263. https://doi.org/10.1016/j.envint.2019.105263
Pinnell LJ, Turner JW (2019) Shotgun metagenomics reveals the benthic microbial community response to plastic and bioplastic in a coastal marine environment. Front Microbiol 7(10):1252. https://doi.org/10.3389/fmicb.2019.01252
Costa JP, Nunes AR, Santos PSM, Girão AV, Duarte AC, Rocha-Santos T (2018) Degradation of polyethylene microplastics in seawater: insights into the environmental degradation of polymers. J Environ Sci Health A Tox Hazard Subst Environ Eng 53(9):866–875. https://doi.org/10.1080/10934529.2018.1455381
Romera-Castillo C, Pinto M, Langer TM, Álvarez-Salgado XA, Herndl GJ (2018) Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat Commun 9(1):1430. https://doi.org/10.1038/s41467-018-03798-5
Royer SJ, Ferrón S, Wilson ST, Karl DM (2018) Production of methane and ethylene from plastic in the environment. PLoS ONE 13(8):e0200574. https://doi.org/10.1371/journal.pone.0200574
Zhu YG, Zhao Y, Zhu D, Gillings M, Penuelas J, Ok YS, Capon A, Banwart S (2019) Soil biota, antimicrobialresistance and planetary health. Environ Int 131:105059. https://doi.org/10.1016/j.envint.2019.105059
Drudge CN, Warren LA (2012) Prokaryotic horizontal gene transfer in freshwater lakes: implications of dynamic biogeochemical zonation. J Environ Prot (Irvine Calif) 3:1634–1654
Acknowledgements
We appreciate Dr Ekpenyong Okon (Chemical Science Dept., Arthur Jarvis University) for characterization of the sachet water plastic. Our special appreciation also goes to Mr Mathew Odey who analyzed the soil samples for physico-chemical parameters and heavy metals ex-situ.
Funding
There was no specific funding received for this article.
Author information
Authors and Affiliations
Contributions
UOE and APJ took up the conceptualization while UOE, FON, HUO, BOE and DEB handled data curation. APJ and AN did the formal analysis while UOE and AN handled other logistics. Authors UOE, APJ and FON handled investigation while the methods and project administration was handled by UOE. AN and FON took care of resources, and APJ and UOE handled software and supervision while AN took care of validation. Visualization was handled by APJ and UOE while the UOE wrote the original manuscript draft. APJ and UOE did the initial review and editing. HUO arranged figures. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors do declare that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Edet, U.O., Joseph, A.P., Nwaokorie, F.O. et al. Impact of “sachet water” microplastic on agricultural soil physicochemistry, antibiotics resistance, bacteria diversity and function. SN Appl. Sci. 4, 323 (2022). https://doi.org/10.1007/s42452-022-05206-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05206-6