Abstract
This study presents an optimized method that is applicable in monitoring the occurrence of pharmaceuticals in a wide range of aquatic environments. The optimised Solid Phase Extraction method is based on Bond Elut Plexa cartridges for the identification and quantification of three non-steroidal anti-inflammatory drugs, three antiretroviral drugs and a lipid regulator in the coastal area of Durban city, South Africa covering four seasons. The extracted compounds are qualitatively and quantitatively detected by a high-performance liquid phase chromatographic instrument coupled to a photodiode array detector. The recoveries range from 62 to 110% with a Relative Standard Deviation of 0.56−4.68%, respectively, for the determination of emtricitabine, tenofovir, naproxen, diclofenac, ibuprofen, efavirenz, and gemfibrozil. The analytical method is validated by spiking estuarine water samples with 5 µg L− 1 of a mixture containing the target pharmaceuticals and the matrix detection limit is established to be 0.62–1.78 µg L− 1 for the target compounds. The optimized method is applied to seasonal monitoring of pharmaceuticals at chosen study sites from winter and spring of 2019 and summer and autumn of 2020. The results indicate the concentration of the pharmaceuticals studied varies with the type of aquatic environment and season.
Highlights
-
The Solid Phase Extraction method is optimised for the pre-concentration of pharmaceuticals in coastal waters.
-
Non-steroid anti-inflammatory drugs and Antiretroviral drugs are successfully quantified in coastal waters.
-
The concentration of pharmaceuticals detected in coastal waters varies with each season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, studies in South Africa have shown consistent detection of the presence of pharmaceuticals in the country’s water bodies, therefore, raising concerns about the inappropriate discharge of these pharmaceuticals in water systems [28, 30, 37, 57]. This continues to raise concerns as many of our South African citizens still depend on river water due to lack of access to clean drinking water, especially those people residing in rural areas. South Africa in 2019 had a population of about 58.78 million and Kwa-Zulu Natal (KZN) is the second largest province in the country with the largest population estimated at 11.9 million people, making up about 19.2% of the country’s population [49]. Generally, South Africa has recorded an increase in population in recent years. Subsequently, this has caused an increase in the production of treatment by the pharmaceutical industries. Pharmaceuticals make their way into the water systems through excretion, direct disposal of expired drugs, disposal of empty tablet containers containing pharmaceutical residue, defecation as well as discharge from wastewater treatment plants into rivers and the sea [48, 60, 3].
It has been reported that wastewater treatment plants (WWTPs) are the main contributors to the presence of pharmaceuticals in water systems as a result of inadequate or partial removal during the water treatment process, hence discharging of the effluent water containing pharmaceuticals into the rivers [21, 51, 7]. The source of pharmaceuticals in wastewater treatment sectors includes sewage from households, effluent from pharmaceutical formulating industries and hospitals (healthcare facilities). The use of sludge from the WWTPs for irrigation and manure is another way for pharmaceuticals to reach the environment, which can reach rivers through runoff and drinking water [31]. Another major source is the leaking of raw sewage into river systems due to the aging wastewater treatment plant system. Most of the infrastructure is old and some pipes tend to leak raw sewage, especially in developing countries where many municipalities are struggling financially.
Non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac, ibuprofen, and naproxen are pharmaceuticals that are frequently detected in South African water bodies due to their easy access to patients as over-the-counter drugs and free availability in clinics, hospitals and use in the veterinary application. These NSAIDs are classified as weak organic acids that are used to treat inflammation, arthritis, pain and fever [50, 46, 33, 52]. Their high detection in the water streams is due to their properties which include high water solubility, polarity and human excretion rates as un-metabolized drugs [8, 19, 23]. The presence of NSAIDs in the aquatic systems has proven to be great environmental concern over the years, a high number of these pharmaceutical residues are continuously detected in WWTPs, rivers, sediments and plants [31, 35, 6].
Furthermore, Human Immune Virus (HIV) is a global challenge; the situation is worse in undeveloped countries, particularly in South Africa where about 6.4 million of the country’s population is HIV positive [49, 44]. Their treatment is based on Antiretroviral drugs (ARVs). ARVs are a class of pharmaceutical drugs that are also of concern. There is very little information on the presence of ARVs in the South African water bodies. ARVs are used to maintain and suppress the human immune virus. However, they are not a cure for the HIV but act as an inhibitor to maintain the spread of the virus in the human body [12, 32]. ARV drug inhibitors such as emtricitabine, tenofovir disoproxil, and efavirenz are commonly used in the treatment of HIV in South Africa. A standard dose of typical antiretroviral therapy (ART) medication contains active compounds in the range of 300–600 mg [36]. Daily administration implies approximately 600 mg of these active compound(s) per patient per day. For instance, the ART formulation for Trivenz contains 600 mg Efavirenz, 200 mg Emtricitabine and 300 mg Tenofovir disoproxil fumarate. Similarly to NSAIDs, they find their way into water streams through excretion as the parent compound or metabolite into the sewage plant. Due to the polarity and high solubility in the water, they form part of the water that is discharged into surface water as part of the effluent or directly into the sea. This emphasizes the need for analytical methods capable of simultaneous detection of all the therapeutic classes of ARVs.
The presence of these pharmaceuticals in European coastal areas has been reported, Gonzalez-Rey and Bebianno [15] reported on NSAIDs in aquatic environments such as surface water. Several other studies have reported on the presence of ARVs in various aquatic environments [4, 11, 13, 14, 42, 53]. A few studies have reported on the presence of ARVs in African countries [36, 1, 18, 43].
Environmental monitoring of these pharmaceuticals involves the use of chromatographic techniques after pre-concentration with a suitable solid phase extraction (SPE) technique. The most frequently used cartridges for these studies are Oasis hydrophilic-lipophilic balance sorbent (HLB), mixed-mode anionic Exchange (MAX), and molecularly imprinted polymers (MIPs). HLB is used when target compounds show hydrophilic and lipophilic properties. Studies have reported using the aforementioned techniques for the extraction of NSAIDs such as diclofenac, naproxen, and ibuprofen [37, 31]. Oasis MAX is another cartridge used by Madikizela and Chimuka [23] which is made up of a mixed-mode polymer sorbent containing both reverse-phase and mixed-mode functionalities. Another more selective sorbent that has been reported is MIPs where a single or multi-template synthesis is used. These smart polymers have been used for their excellent removal efficiencies, and pre-concentration of target pharmaceutical compounds [30, 23, 2, 16].
This study optimised an SPE technique for selected pharmaceuticals from wastewater, surface water, estuarine water, and seawater using Bond Elut Plexa cartridges. This sorbent was chosen because it can extract compounds with a wide range of physicochemical properties. Polar bases are extracted with high recovery and allow simple sorbent optimisation with this sorbent. The optimized SPE method was applied for the isolation and pre-concentration of target pharmaceuticals before quantitative analysis by HPLC-PDA. Although several studies have been reported on the extraction, identification, and quantification of pharmaceutical compounds in South African environmental compartments [23, 2, 17], still more data is needed especially in coastal areas as this helps to understand the contribution of mainland pollution to the coastal areas and seawater. It is important to come up with a long-term solution to coastal and seawater pollution as well as the influence of climate change in detecting these compounds. Hence, seasonal variation was investigated in this study. The specific objectives of the study were to:(1) develop an optimised method for detection and quantification of selected classes of pharmaceuticals (pollutants) in different South African water environments (systems); and (2) apply the method in the assessment and comparison of the concentration of the pharmaceuticals in the different water systems during different seasons.
The paper has the following structure: after the introduction, we consider the experimental data of this study in Sect. 2 where chemicals and materials are presented. Section 2.1, instrumentation being used Sect. 2.2, the analysis procedure Sect. 2.3, sample collection and pre-treatment in Sect. 2.4, and Sect. 2.5 the geographical coordinates and climate of the study sites, Sect. 3 presents the results and discussion, Sect. 4 presents the study’s conclusion and, Sects. 5–6 presents acknowledgements and references respectively.
2 Experimental
2.1 Chemicals and materials
High purity (> 98%) standards of target pharmaceutical compounds such as diclofenac (DICLO) salt, ibuprofen (IBU), naproxen (NAP), emtricitabine (FTC), efavirenz (EFV), and tenofovir disoproxil (TENO) were purchased from Sigma-Aldrich (Steinheim, Germany). Gemfibrozil (GEM) was purchased from J&H Chemicals Co. Ltd (Hangzhou, China). The physicochemical properties of the studied compounds are shown in Supplementary Table S1. Hydrochloric acid (HCl) and formic acid (> 98%) were also purchased from Sigma-Aldrich (Steinheim, Germany). The HPLC-grade solvents acetonitrile (> 99%), methanol (99, 5%), and acetic acid (> 98%) were purchased from Merck (Darmstadt, Germany) and they were used for the SPE as well as HPLC mobile phase. Sodium chloride (NaCl) was purchased from Associated Chemical Enterprise (Johannesburg, South Africa). Ultrapure water was obtained using a Purite select HP 40 system (Purite Ltd, Oxfordshire, UK). A 100 mg L− 1 stock solution containing a mixture of the 7 target pharmaceutical compounds was prepared by dissolving the drugs in acetonitrile. A series of working standards in a concentration range of 0.001-10 mg L− 1 were prepared in a mixture of acetonitrile and 0.2% formic acid (60:40).
2.2 Instrumentation
Water samples were filtered through 0.45 μm hydrophilic polypropylene membrane filter papers and Whatman (70 mm × 100 circles), filter papers were purchased from GE Healthcare UK Ltd Supplies (South Africa). The pH of the water samples was measured with a Bante900P portable multi-parameter water quality meter obtained from Bante instruments (Shanghai, China). For the extraction of the target compounds from the water samples, a vacuum SPE manifold sourced from Phenomenex (California, USA) attached to a vacuum pump purchased from Pall Corporations (Fribourg, Switzerland) was used. Various SPE cartridges were utilized in this study, namely, MAX sorbent (OASIS MAX, 6 cc, 200 mg), HLB sorbent (OASIS HLB, 6 cc 200 mg) from Waters Corporation (Milford, MA, USA) and Agilent Bond Elute Plexa, (3ml, 200 mg) purchased from Chemetrix (Johannesburg, South Africa). The HPLC instrument, purchased from Shimadzu Corporation (Kyoto, Japan), contained an online mobile phase degasser unit (DGU-20A3), and a ternary pump (LC-20AB). All samples and standards were injected onto a Rheodyne 7010 injector equipped with a 20 µL sample loop (California, USA). The compounds were separated using a C18 Kromasil column (150 mm × 4.60 mm × 5 μm) obtained from Phenomenex (California, USA) and detected using a PDA detector (SPD-M20A).
2.3 HPLC-PDA analysis procedures
Chromatographic separation was achieved by applying a mobile phase containing 0.2% formic acid in water (solvent A) and Acetonitrile (solvent B). A multi-step gradient elution program was employed by using 15% solvent B for the first 3 min at a flow rate of 1 mL min− 1 and at 3.01 min ramped up to 60% solvent B until 12 min at a flow rate of 2 mL min− 1 and 12.01 min changed to 15% solvent B and held at a flow rate of 2 mL min− 1 to 19.50 min and thereafter changed back 1 mL min− 1 for equilibration of the system. The total run time for the analysis was 20 min. The PDA wavelengths were set according to the absorbance of each compound. Naproxen, ibuprofen, gemfibrozil, tenofovir disoproxil and efavirenz were monitored at 254 nm and emtricitabine and diclofenac were monitored at 280 nm. The chromatogram in Fig. 1 is a representation of a standard solution for the separation of these compounds studied at a known concentration.
2.4 Sample collection and pre-treatment
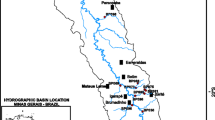
Water samples were collected from 12 different sampling sites located around Durban city of KwaZulu-Natal province, South Africa. Table 1 lists the GPS coordinates of the sampling sites. These sites include wastewater treatment plants (Northern WWTP and Kingsburgh WWTP) where both influent and effluent were collected, surface water (Umgeni River and Kingsburgh river), seawater (Blue Lagoon Beach, Glen Ashley Beach, and Warner Beach (top and bottom at 2 km apart) and estuary (Warner Beach estuary and Umgeni estuary). Samples were collected in pre-cleaned 3.5 L amber glass bottles and kept in a cooler box with ice during transportation to the laboratory. They were filtered immediately for the removal of solid matter and thereafter stored in the refrigerator at 4 °C until analysis.
2.5 Seasons and climate of the study site
Kwa-Zulu Natal (KZN) is the easternmost province of South Africa. It is distinguished by a subtropical coastline, grasslands in the east, and the Drakensberg Mountain range in the west. The Indian Ocean, particularly the warm Agulhas current, has a considerable impact on the climate of KZN, which accounts for the high humidity, mild temperatures, and summer rainfalls. During the austral summer and spring, tropical temperate troughs, east coast low-pressure systems, and southeast coast ridging high-pressure systems predominate [20]. During the winter, the northward movement of the Intertropical Convergence Zone (ITCZ) and the strengthening of the anticyclonic circulation restrict moisture from the tropics, reducing precipitation. Winter is considered a dry season. During the winter, however, disturbances in the westerly circulation, such as anticyclones and ridging high-pressure systems behind cold fronts, predominate [45]. Table 2 shows the sampling dates and season of sampling.
2.6 Solid-phase extraction of water samples
The SPE extraction method reported by Rimayi and colleagues [43] was optimized to get maximum recovery. The parameters that were optimized are the amount of salt in the water sample, sample volume and its flow rates. The rest of the parameters were taken from Rimayi and colleagues [43]. Optimizations were performed by varying one parameter while holding others constant.
At optimum conditions, the Bond Elut Plexa SPE cartridges (3 mL, 200 mg Styrene divinyl benzyl) were conditioned with 5 mL methanol and equilibrated with 5 mL ultrapure water adjusted to a pH 5.8, before loading the 500 mL water samples at a flow rate of 10 mL min− 1. The cartridges were dried under a gentle vacuum for 30 min. Elution of the retained compounds was performed with 3 mL methanol followed by 3 mL acetic acid in methanol (20:80) at a flow rate of 5 mL min− 1. The extracts were evaporated under a gentle stream of nitrogen gas at a temperature of 40 °C and then reconstituted with a 1 mL mixture of acetonitrile and 0.2% formic acid in water (60:40). Finally, the extracts were analyzed by HPLC-PDA.
3 Results and discussion
3.1 Optimization of solid-phase extraction
3.1.1 Salting effect
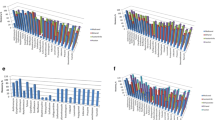
The effect of added salt in the sample on the extraction of the target compounds is shown in Fig. 2. Therefore, 500 mL deionised water aliquots with NaCl content ranging from 0 to 4% w/v were spiked with a 5 µg L− 1 mixture of the target compounds. The range of the salt content was selected because seawater is estimated to contain 3.5% w/v of salt [58]. The results suggest that the presence of salt has minimal effect on the percent recovery (Fig. 2). It is essential to investigate the effects of salt as the samples collected from the estuaries and seawater may contain high levels of salt. In a study done by [54] and [37], the ionic strength of the water samples was also found to have little or no effect on the adsorption of the investigated compounds. The extent to which ionic strength from the salt affects recovery depends on its amount and the physicochemical properties of the target compounds. In this case, we found it does not affect the percent recoveries as they ranged from 70 to 100%.
3.1.2 Effects of sample volume
The effect of sample volume on recoveries was investigated by extracting 500 mL and 1000 mL deionized water samples that were spiked with a 5 µg L− 1 mixture of the target pharmaceuticals at pH 5.8. Figure 3 shows the results obtained. The 500 mL sample volume was chosen as the optimum because at 1000 mL, the recovery decreased for most compounds. Recoveries ranging from 79 to 121% were obtained for the 500 mL sample volume while for 1000 mL ranged from 63 to 94%.
The decrease in recovery at high sample volume suggests that the sample breakthrough volume was exceeded. This observation has been noted by other researchers as well [23, 9, 39]. Also choosing a lower sample volume helped with the efficiency of the experiment.
3.1.3 Effects of elution flow rate
The results of the elution flow rate studies are shown in Fig. 4. In this case, the elution flow rate was investigated from 1 to 10 mL min− 1. A sample elution flow rate reported by Rimayi and colleagues [43] was used in the study. The results show that the lower the elution flow rate, the higher the recovery. This is expected as a slow flow rate gives time to desorb more of the analytes from the sorbent. The flow rate at which the sample is percolated and eluted into the SPE cartridge is thus an important parameter as it influences how the target compounds are retained and eluted from the cartridge [37]. Even though 5 mL min− 1 gave good results and would help shorten analysis time this lowered the recovery of EMI and IBU to recoveries of 40 and 50% respectively, hence 1 mL min− 1 elution flow rate was selected to get maximum recoveries for all compounds ranging from 80 to 110% with an RSD of 0.56–4.68%.
3.2 Quality assurance parameters of the method
The linearity was calculated by plotting the concentration of each analyte against its peak area attained in the HPLC analysis. The instruments detection limit was calculated as the limit of detection (LOD) and limits of quantification (LOQ) using a statistical equation of 3.3 and 10 times the standard error of y-intercept divided by the slope of the graph. To study the influence of sample matrix on recovery deionized water and estuarian water were spiked with 5 µg L− 1 concentration of target compounds and thereafter subjected to SPE before analysis at optimized conditions. Table 3 gives a summary of the results obtained. The results showed that extraction of samples increased the sensitivity and reduced the LOD to those expected in environmental samples. This shows that after the Solid-phase extraction the analytes were pre-concentrated and were more sensitive to detection compared to direct injection of the sample in the instrument before SPE. The recoveries in deionised water and those obtained in estuary river water were similar and slightly higher when compared to those found in river water. This shows that the developed method was not influenced by the sample’s matrix found in river water.
3.3 Environmental monitoring of target compounds based on the seasons
3.3.1 Seasonal comparison of the concentrations found in samples
The sum of concentrations of each compound obtained per given season at each sampling site, for each study site and season is given in Fig. 5. Samples were collected during summer which is a wet season (December to February), Winter a dry season (June to August) and autumn and spring rational seasons (March to May) and (September to November). Summer is accompanied by warm temperatures in the 32–40° range and significant rains, while the winter is humid and cold, with temperatures ranging from 4 to 20° and less to no rain. Autumn and spring interchange between being windy and dry and sometimes, with rainfalls. Looking at the concentration of the total sum of emerging pollutants (Fig. 5) for the Northern WWTP influent, high concentrations were observed in autumn and spring with concentrations of 117.11 and 114.4 µg L− 1, respectively, where there is minimal to no rainfall. Similarly to its effluent site, high concentrations were observed in winter and spring with concentrations of 43.97 and 42.59 µg L− 1, respectively. The Kingsburgh WWTP had a high concentration of 144.88 µg L− 1 in the winter season. Its effluent site had high concentrations during the summer season with a concentration of 63.8 µg L− 1.
The Umgeni and Kingsburgh river had concentrations of 18.3 and 22.66 µg L− 1 during the spring and winter seasons, respectively. Because rainfall is limited throughout these seasons, the high levels during the dry season may be attributable to the lack of dilution. Blue lagoon and Glen Ashely had a high concentration in spring with ƩEP concentrations of 46.75 and 13.3 µg L− 1. Warner beach’s Top and bottom had a ƩEP concentration of 6.94 and 24.96 µg L− 1 in autumn and summer. One might expect no recoveries of these pharmaceuticals as the sea is highly diluted. These water samples were collected on the surface of the ocean where most people are likely to be found swimming. The Umgeni and Warner beach estuaries had high concentrations observed in winter with concentrations of 20.97 and 37.9 µg L− 1, respectively.
3.3.2 Pollutants in the Wastewater treatment plant
The concentrations of pharmaceuticals studied in the influent and effluent sites of the Northern and Kingsburgh WWTPs varied considerably among the two wastewater treatment plants (refer to Supplementary Table S2). High concentration levels of all the target compounds were detected in the Northern WWTP influent. Spring had the most frequently detected pharmaceuticals with concentrations ranging from 1.98 to 44.7 µg L− 1. Diclofenac is the least detected compound in this season. See similar concentrations were detected by Madikizela [26] in the Lady Smith wastewater treatment plant, and concentrations of 1.24 µg L− 1 were detected. The ARV drug emtricitabine was the most frequently detected compound in this site in all seasons with concentrations higher than that detected by Mosekiemang [36] who detected 172 ng/mL emtricitabine. The total ƩEP in this study site was found to be 114.4 µg L− 1 in Spring (Fig. 4). This could be due to exposure to the pre-exposure prophylaxis drug prescribed over the counter and provided free in clinics, which contains a large dose of these compounds [41]. Also, the Northern WWTP receives both industrial and domestic waste as well as runoff. These compounds were found to be more dominant in the Autumn season in this WWTP with the ƩEP of 117.11 µg L− 1 in this season and concentrations ranging from 1.81 to 101.2 µg L− 1, Diclofenac being the least recoverable compound. Various studies have reported poor recovery of diclofenac [37, 23, 22]. Emtricitabine was found to have the highest concentration in this water sample in winter and was found to have a total ƩEP of 83.14 µg L− 1 with a concentration in a range of 5.07–37.5 µg L− 1, again diclofenac and emtricitabine being the least and most detected compounds in all these seasons. Summer had concentrations in the range 0.591–26.4 µg L− 1 compared to 24,000 ± 1400 ng L− 1 determined by [1] in the same site. This suggested that these compounds were not effectively removed after the purification process. In the Northern WWTP effluent site, only diclofenac and efavirenz were detected in summer with concentrations of 2.098, and 6.83 µg L− 1 respectively, and were lower than what each compound was found to be in the inlet. In autumn, concentrations of 4.17, 5.56, and 5.70 µg L− 1 were determined for diclofenac, efavirenz and gemfibrozil, respectively. In winter, concentrations in the range of 5.42–25.03 µg L− 1 were determined, naproxen had the lowest concentration while tenofovir disoproxil was the highest. In spring, concentrations ranged from 0.82 to 36.75 µg L− 1 efavirenz being the lowest detected compound and emtricitabine being the highest detected compound. The trend showed that after treatment some of these compounds are removed but a large amount remains in these water systems.
The Kingsburgh WWTP was found to have lower concentrations of these pharmaceuticals detected than the Northern WWTP (Industrial sewage plant) and concentrations varied with each season. Winter was found to have the highest total ƩEP of 144.88 µg L− 1, concentrations ranged from 2.94 to 48.4 µg L− 1, emtricitabine being the least detected and the highest being Tenofovir disoproxil. This was followed by Spring with a total ƩEP of 95.8 µg L− 1, and concentrations ranged from 1.18 to 36.3 µg L− 1, diclofenac and emtricitabine were the lowest and highest detected compounds respectively. Followed by Summer with a total ƩEP of 95.81 µg L− 1, with concentrations ranging from 7.25 to 39.38 µg L− 1 with gemfibrozil being the lowest detected compound and Tenofovir disoproxil being the highest detected compound. Autumn had the lowest concentrations ranging from 1.55 to 16.52 µg L− 1. Ibuprofen had higher concentrations of 22.8, 35.5, and 6.06 µg L− 1 respectively for each season compared to those determined by Madikizela and Chimuka [24] of 2.1 µg L− 1 in the same study site. These pharmaceuticals were frequently detected in winter, which is the flu and cold season leading to frequent consumption of these pharmaceuticals by patients with arthritis for pain. The effluent of this site showed low concentrations of these pharmaceuticals except for emtricitabine with the highest concentrations of 32.7, 44.7 and 45.8 µg L− 1 in spring, winter and summer respectively.
Diclofenac had the lowest concentration for all seasons. Up to 63.8 µg L− 1 total ƩEP of these pharmaceuticals were detected in summer at this site. The percentage removal efficiencies for individual pharmaceuticals in all seasons are shown in Table 4. In the Northern WWTP, these pharmaceuticals were almost completely removed or slightly removed except for tenofovir, diclofenac, gemfibrozil which accumulated in this site in autumn. Spring was the only season with low removal efficiency for all pharmaceuticals. In the Kingsburgh WWTP emtricitabine and efavirenz were the only two pharmaceuticals that had accumulated in the effluent in all seasons except in autumn, but low removal was observed with only 36.7% efavirenz being removed, these results were comparable to those found by Schoeman and colleagues [47] were these drugs accumulated after the chlorination process. Emtricitabine had 93% accumulation after treatment. The Northern and Kingsburgh employ similar treatment technologies, hence low removal efficiency of the ARV drugs is observed in both sites.
This suggested that wastewater treatment plants are still incapable of effectively removing these pharmaceuticals in the chlorination process, High quantities of bioactive pharmaceuticals, such as ARV drugs, have been reported to impair the efficiency of WWTPs [33, 29].
3.3.3 Pollutants in Surface water
The Umgeni River flows through several distant and rural areas that are slightly populated, therefore, the potential for pollution is low. However, when the Umgeni River reaches Durban, pharmaceuticals were detected along various sampling areas. Umgeni River and Kingsburgh River were the sites of interest. The Umgeni River had low concentrations of these pharmaceuticals (Table S2). Diclofenac, Ibuprofen and efavirenz were the only compounds detected in the summer season with concentrations of 0.73, 1.60, and 2.34 µg L− 1, respectively. Efavirenz had a higher concentration compared to [43] who reported 138 ng L− 1 efavirenz at the same site. Ibuprofen had lower concentrations detected compared to those detected by [30, 37, 17, 5] of 68.14, 0.100 µg L− 1 in summer, 12.94 and 0.36 µg L− 1 respectively in the same sampling site. Autumn had most of these pharmaceuticals detected ranging from 0.40 to 2.71 µg L− 1, efavirenz had the lowest concentration and tenofovir with the highest concentration.
High concentrations of ibuprofen, gemfibrozil, and efavirenz were detected in the Kingsburgh River with concentrations of 1.4, 5.55, and 0.32 µg L− 1 respectively in summer. Similar results were found by Madikizela and Chimuka [24] in the Kingsburgh effluent site. In autumn, most of these pharmaceuticals were detected with concentrations ranging from 0.53 to 12.2 µg L− 1, diclofenac being the lowest detected compound and emtricitabine being the highest detected concentration. Winter had concentrations ranging from 2.14 to 9.89 µg L− 1, ibuprofen being the lowest detected compound and efavirenz with the highest detected concentration in this season. Ibuprofen and gemfibrozil were the only detected pharmaceuticals in spring with a concentration in the general order of 6.73 and 5.19 µg L− 1. Tenofovir was not detected in all seasons while naproxen was found to be below the quantification limit. This could be due to the large sampling area instigating reduction of the target pharmaceutical. These pharmaceuticals were frequently detected in autumn 2020 with the total ƩEP being 22.5 µg L− 1 which showed a 0.16 µg L− 1 difference in the concentration of these pharmaceuticals in these water bodies as the years progressed. This is supported by considering the 2019 winter concentration results when the first sampling was done, which yielded the ƩEP of 22.66 µg L− 1. This slightly remained the same in the following seasons.
3.3.4 Pollutants in the estuarine water
The Umgeni and Warner Beach estuaries were the estuarian areas of interest in this study. In summer only efavirenz was detected with a concentration of 0.55 µg L− 1. In Autumn low concentrations of these pharmaceuticals were found ranging from 0.394 to 6.69 µg L− 1, emtricitabine being the lowest recoverable compound and gemfibrozil having the higher concentration. This was expected considering the accumulation of gemfibrozil in this season in the Northern WWTP. Winter concentrations ranged from 0.95 to 9.6 µg L− 1, diclofenac having the lowest concentration and gemfibrozil with the highest concentration. Concentrations of gemfibrozil found in this study are lower compared to those found by Fang and colleagues [10] in groundwater they reported 19.4 µg L− 1 of gemfibrozil. In Spring concentrations of 0.22, 0.14, and 3.04 µg L− 1 were detected for emtricitabine, diclofenac, and efavirenz, respectively. Diclofenac was detected in all seasons in this study site compared to Ngubane and colleagues who did not detect the compound in the same site [37]. The presences of these pharmaceuticals in the estuarian water are not surprising as the Msunduzi and Umgeni river flow into this stream and they have been reported to have high concentrations of these compounds [30, 17, 5, 34].
Warner Beach estuary was one of the study sites of interest, where naproxen was only detected in autumn with a concentration of 1.82 µg L− 1 and efavirenz with a concentration of 3.92 µg L− 1. Tenofovir disoproxil was not detected in all seasons in this study area. In summer, concentrations ranging from 0.21 to 11.95 µg L− 1 were detected, diclofenac having the lowest concentration and emtricitabine having the highest concentration. The concentration of emtricitabine detected in this site was found to be higher compared to those detected by [35, 13] in their influent site, with concentrations of 980 ng. L and 0.033 µg L− 1, respectively. In Spring only emtricitabine and diclofenac were detected with concentrations of 18.2 µg L− 1 and 0.55 µg L− 1 respectively. In Winter only diclofenac, efavirenz, and gemfibrozil were detected with concentrations of 1.26, 12.3, 24,35 µg L− 1 respectively. Warner Beach estuary was found to contain high concentrations of these pharmaceuticals compared to the Umgeni estuary, the total ƩEP in Umgeni was found to be 20.97 µg L− 1, winter being the season having the highest concentrations detected and the total ƩEP in Warner beach estuary being 37.9 µg L− 1 in the same season. This is due to the size of this estuary, which is smaller than the Umgeni estuary. Hence, lower recoveries were detected at Umgeni due to the large water dilution on the site. According to our knowledge, this study is the first to report the presence of these pharmaceuticals in this study area.
3.3.5 Pollutants in seawater
The presence of these pharmaceuticals in seawater has been reported [37, 38]. However, there is a lack of information on the presence of these pharmaceuticals in South African seawater, therefore, Blue Lagoon, Glen Ashley, and Warner beach at the top and bottom were areas of interest. In Blue Lagoon over the summer season, concentrations of 2.01, 0.68, and 0.81 µg L− 1 were detected for emtricitabine, diclofenac, and efavirenz, respectively. In autumn concentrations of 2.67, 0.21, and 3.54 µg L− 1 were detected for diclofenac, efavirenz, and gemfibrozil. As well as in winter the same compounds were detected with concentrations of 0.43,0.64, and 6.52 µg L− 1, respectively. In spring concentrations of 6.1, 0.31, 38.3, and 2.04 were detected for emtricitabine, diclofenac, ibuprofen, and efavirenz, respectively. Naproxen and ibuprofen were found to have concentrations below the quantification limit. Compared to results reported by Ngubane and Colleagues on the same study site, diclofenac was detected in all seasons. Naproxen and ibuprofen were found to be lower than the concentrations they detected [37]. The presence of these pharmaceuticals was expected as the Northern WWTP discharges into the Umgeni river which flows into the Umgeni estuary and feeds into the Indian ocean within a couple of meters from the Blue Lagoon. One might argue that because of how large the sea is there should be high dilution, these samples were collected on the surface of the sea, where most people are found to be swimming. Most things discarded by the sea are found as sediments on the surface of the sea.
The seawater study site Glen Ashley in summer only detected diclofenac, ibuprofen, and efavirenz with concentrations of 1.51, 1.92, and 1.72 µg L− 1, respectively. Gemfibrozil was detected but could not be quantified in all the seasons. In Autumn concentrations of 1.39, 0.67, and 0.21 µg L− 1 for emtricitabine, diclofenac and efavirenz, respectively were the only ones detected. In winter only emtricitabine was detected with a concentration of 0.22 µg L− 1. In spring concentrations ranging from 0.47 to 5.44 µg L− 1 were detected with efavirenz having the lowest concentration and ibuprofen with the highest concentration detected. The concentrations of ibuprofen in seawater in this area are higher than those reported in Tromso, Norway of 0.007 µg L− 1 and Santos, Brazil 2.09 µg L− 1, respectively [55, 40]. Naproxen was not detected in all the seasons.
Warner beach Top and Bottom were found to have high concentrations of these pharmaceuticals. Warner Beach Bottom, situated 1 km away from the Top, had a higher concentration compared to the Bottom as it is the most frequently used beach for swimming and fishing. In summer concentrations of 0.22, 24.3, and 0.44 µg L− 1 for diclofenac, ibuprofen, and efavirenz were detected respectively. In autumn concentrations of 0.36–3.63 µg L− 1 efavirenz was the lowest concentration detected and the highest concentration was gemfibrozil. Concentrations of gemfibrozil detected were higher than those detected in Singapore ranging from 1 to 9 ng/L [59]. In winter only efavirenz was detected with a concentration of 0.31 µg L− 1. In spring concentrations ranged from 0.78 to 3.38 µg L− 1, efavirenz having the lowest concentration and tenofovir disoproxil with the highest concentration detected.
Warner beach Top in summer only had a concentration of 2.56 µg L− 1 emtricitabine detected on the site, the rest of the compounds were below the detection limit. In autumn concentrations ranged from 0.402 to 2.92 µg L− 1 efavirenz having the lowest concentration and gemfibrozil with the highest concentration detected. Concentrations detected in seawater were low due to the high dilution factor of seawater. Comparing all these seawater sites, summer and winter had the largest ƩEP of 24.96 and 37.9 µg L− 1.
3.4 Removal efficiencies from wastewater treatment plants
Based on the raw influent and effluent concentrations, the removal efficiency of each pharmaceutical in the WWTPs was determined. As shown in Table 4, the percentage removal efficiency for target compounds varied with each season for each WWTP. In the Northern WWTP, summer had a percent removal of 32.3–100% and efavirenz having the lowest percentage removal efficiency. Autumn percentage removal ranged from 15.8 to 100% and efavirenz being the lowest percent removed, however, gemfibrozil and tenofovir disoproxil had an accumulation of 16.3% after treatment compared to what was in the inlet. In winter diclofenac had an 85.6% accumulated after treatment compared to what was determined in the inlet. Poor removal of diclofenac in WWTP has been reported in various studies [22, 27]. Spring percentage removal ranged from 17.7 to 100% and emtricitabine having the lowest removal percentage. Naproxen was 100% effectively removed in all seasons. The Kingsburgh WWTP had an accumulation of several compounds in various seasons after the treatment process. In summer emtricitabine and efavirenz had an accumulation of 16 and 1.62% respectively, and in winter 93 and 45.5% respectively. In spring only efavirenz had an accumulation of 68.14% after the treatment process compared to the influent. Accumulation of the ARV drug emtricitabine and efavirenz has been reported in other studies in Africa [48, 36, 56]. The Kingsburgh WWTP had a poor removal efficiency compared to the Northern WWTP and yet they use similar treatment technologies. Wastewater treatment facilities are the primary units for removing or degrading contaminants from wastewater. The activated sludge system is the standard biological treatment procedure used in WWTPs. The removal efficiency varies depending on the physicochemical properties of the compounds as well as environmental conditions such as biological reactor configuration and operational parameters such as retention time and pH. Further work is required to effectively remove these pharmaceuticals and rectify this issue.
4 Conclusions
The determination of selected NSAIDs, ARVs and a lipid regulator in various aquatic environments was performed using a rapid analytical method that uses an SPE technique employing Bond Elut Plexa cartridges, followed by quantitation using HPLC-PDA. Tenofovir and naproxen were the least detected pharmaceuticals in these study sites across all seasons. This study presents a survey of NSAIDs and ARV drugs in various aquatic sites. Generally, the treatment technique employed in these study sites appeared to be effective in the removal of most of the pharmaceuticals, apart from emtricitabine, efavirenz and tenofovir disoproxil. High concentrations and recoveries of ARV drugs were detected across all studied water bodies. Most of these pharmaceuticals were detected in high concentrations in the summer and autumn of 2020. The discharge points of the Northern and Kingsburgh WWTPs could be the source of the presence of these drugs in the rivers, estuaries, and sea. The degradation kinetic and breakdown of these pharmaceuticals need to be further investigated. The accumulation of efavirenz concentration after treatment is a concern, especially in the FDC therapy that includes this drug in a single tablet. As a result, the prevalence of this resistant drug is anticipated to rise. The presence of these pharmaceuticals suggested incomplete removal of these compounds by the wastewater treatment sectors as well as the state of contamination in the KwaZulu-Natal water bodies. Climate change seems to have an influence on the range in concentration of these compounds, winter, spring, and autumn had high concentrations of these pharmaceuticals as expected as they are known to be dry and rational seasons. The high concentration of these pharmaceuticals serves as motivation for continuous monitoring of these acidic compounds seasonally, and highly effective removal techniques to be studied in the future. The Agilent bond Elut Plexa cartridges are recommended for retaining more of these pharmaceuticals compared to OASIS HLB, and OASIS MAX used by other researchers. It is recommended that more rapid and sensitive techniques such as the LC/MS be employed in future.
Data Availability
The data for this study is available upon reasonable request to the corresponding author Sigonyasisonke@gmail.com, and profstan4christ@yahoo.com.
References
Abafe OA, Späth J, Fick J, Jansson S, Buckley C, Stark A, Pietruschka B, Martincigh BS (2018) LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 200:660–670
Agunbiade FO, Moodley B (2014) Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ Monit Assess. https://doi.org/10.1007/s10661-014-3926-z
Agunbiade FO, Moodley B (2016) Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ Toxicol Chem. https://doi.org/10.1002/etc.3144
Aminot Y, Le Menach K, Pardon P, Etcheber H, Budzinski H (2016) Inputs and seasonal removal of pharmaceuticals in the estuarine Garonne River. Mar Chem 185:3–11
Amos Sibeko P, Naicker D, Mdluli PS, Madikizela LM (2019) Naproxen, ibuprofen, and diclofenac residues in river water, sediments and Eichhornia crassipes of Mbokodweni river in South Africa: An initial screening. Environ Forensics 20:129–138
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446
Biel-Maeso M, Baena-Nogueras RM, Corada-Fernández C, Lara-Martín PA (2018) Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.08.279
Dahane S, Gil García MD, Martínez Bueno MJ, Uclés Moreno A, Martínez Galera M, Derdour A (2013) Determination of drugs in river and wastewaters using solid-phase extraction by packed multi-walled carbon nanotubes and liquid chromatography-quadrupole-linear ion trap-mass spectrometry. J Chromatogr A 1297:17–28
Debska J, Kot-Wasik A, Namiesnik J (2005) Determination of nonsteroidal antiinflammatory drugs in water samples using liquid chromatography coupled with diode-array detector and mass spectrometry. J Sep Sci 28:2419–2426
Fang Y, Karnjanapiboonwong A, Chase DA, Wang J, Morse AN, Anderson TA (2012) Occurrence, fate, and persistence of gemfibrozil in water and soil. Environ Toxicol Chem 31:550–555
Fisher IJ, Phillips PJ, Colella KM, Fisher SC, Tagliaferri T, Foreman WT, Furlong ET (2016) The impact of onsite wastewater disposal systems on groundwater in areas inundated by Hurricane Sandy in New York and New Jersey. Mar Pollut Bull 107:509–517
Fox MP, Sanne IM, Long LC, Evans D, Sauls C, Brennan AT, Rosen S (2018) Can routine inpatient mortality data improve HIV mortality estimates? Inpatient mortality at an urban hospital in South Africa. South Afr Med J 108:870
Funke J, Prasse C, Ternes TA (2016) Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Water Res 98:75–83
Giebułtowicz J, Tyski S, Wolinowska R, Grzybowska W, Zaręba T, Drobniewska A, Wroczyński P, Nałęcz-Jawecki G (2018) Occurrence of antimicrobial agents, drug-resistant bacteria, and genes in the sewage-impacted Vistula River (Poland). Environ Sci Pollut Res 25:5788–5807
Gonzalez-Rey M, Bebianno MJ (2014) Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis. Aquat Toxicol 148:818–831
Gumbi BP, Moodley B, Birungi G, Ndungu PG (2017) Assessment of nonsteroidal anti-inflammatory drugs by ultrasonic-assisted extraction and GC-MS in Mgeni and Msunduzi river sediments, KwaZulu-Natal, South Africa. Environ Sci Pollut Res 24:20015–20028
Gumbi BP, Moodley B, Birungi G, Ndungu PG (2017) Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry. Chemosphere 168:1042–1050
Habyalimana V, Mbinze JK, Yemoa AL, Waffo C, Diallo T, Tshilombo NK, Ntokamunda JLK, Lebrun P, Hubert P, Marini RD (2017) Application of design space optimization strategy to the development of LC methods for simultaneous analysis of 18 antiretroviral medicines and 4 major excipients used in various pharmaceutical formulations. J Pharm Biomed Anal 139:8–21
Kermia AEB, Fouial-Djebbar D, Trari M (2016) Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus Chim 19:963–970
Lakhraj-Govender R, Grab S (2019) Temperature trends for coastal and adjacent higher lying interior regions of KwaZulu-Natal, South Africa. Theor Appl Climatol 137:373–381
Larsson N, Petersson E, Rylander M, Jönsson JA (2009) Continuous flow hollow fiber liquid-phase microextraction and monitoring of NSAID pharmaceuticals in a sewage treatment plant effluent. Anal Methods 1:59–67
Lindqvist N, Tuhkanen T, Kronberg L (2005) Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res 39:2219–2228
Madikizela LM, Chimuka L (2017) Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ Monit Assess 189:1–12
Madikizela LM, Chimuka L (2017) Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewater using solid-phase extraction with high performance liquid chromatography. Water SA 43:264–274
Madikizela LM, Mdluli PS, Chimuka L (2018) An initial assessment of naproxen, ibuprofen and diclofenac in Ladysmith water resources in South Africa using molecularly imprinted solid-phase extraction followed by high performance liquid chromatography-photodiode array detection. South Afr J Chem 70:145–153
Madikizela L, Tavengwa N, Pakade V (2018) Molecularly imprinted polymers for pharmaceutical compounds: synthetic procedures and analytical applications. Recent Res Polym. https://doi.org/10.5772/intechopen.71475
Madikizela LM, Tavengwa NT, Chimuka L (2018) Applications of molecularly imprinted polymers for solid-phase extraction of non-steroidal anti-inflammatory drugs and analgesics from environmental waters and biological samples. J Pharm Biomed Anal 147:624–633
Madikizela LM, Ncube S, Chimuka L (2020) Analysis, occurrence and removal of pharmaceuticals in African water resources: A current status. J Environ Manage. https://doi.org/10.1016/j.jenvman.2019.109741
Márta Z, Bobály B, Fekete J, Magda B, Imre T, Szabó PT (2018) Simultaneous determination of ten nonsteroidal anti-inflammatory drugs from drinking water, surface water and wastewater using micro UHPLC-MS/MS with on-line SPE system. J Pharm Biomed Anal 160:99–108
Matongo S, Birungi G, Moodley B, Ndungu P (2015) Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa. Environ Sci Pollut Res 22:10298–10308
Matongo S, Birungi G, Moodley B, Ndungu P (2015) Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere 134:133–140
Mayosi BM, Lawn JE, Van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM (2012) Health in South Africa: changes and challenges since 2009. Lancet 380:2029–2043
Mbhele ZE, Ncube S, Madikizela LM (2018) Synthesis of a molecularly imprinted polymer and its application in selective extraction of fenoprofen from wastewater. Environ Sci Pollut Res 25:36724–36735
Mlunguza NY, Ncube S, Nokwethemba Mahlambi P, Chimuka L, Madikizela LM (2019) Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J Environ Chem Eng 7:103142
Mlunguza NY, Ncube S, Mahlambi PN, Chimuka L, Madikizela LM (2020) Determination of selected antiretroviral drugs in wastewater, surface water and aquatic plants using hollow fibre liquid phase microextraction and liquid chromatography - tandem mass spectrometry. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121067
Mosekiemang TT, Stander MA, de Villiers A (2019) Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid chromatography - tandem mass spectrometry. Chemosphere 220:983–992
Ngubane NP, Naicker D, Ncube S, Chimuka L, Madikizela LM (2019) Determination of naproxen, diclofenac and ibuprofen in Umgeni estuary and seawater: A case of northern Durban in KwaZulu–Natal Province of South Africa. Reg Stud Mar Sci. https://doi.org/10.1016/j.rsma.2019.100675
Nikolaou A, Meric S, Fatta D (2007) Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal Bioanal Chem 387:1225–1234
Paíga P, Delerue-Matos C (2013) Response surface methodology applied to SPE for the determination of ibuprofen in various types of water samples. J Sep Sci 36:3220–3225
Pereira CDS, Maranho LA, Cortez FS, Pusceddu FH, Santos AR, Ribeiro DA, Cesar A, Guimarães LL (2016) Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci Total Environ 548–549:148–154
Plosker GL (2013) Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis. Drugs 73:279–291
Prasse C, Schlüsener MP, Schulz R, Ternes TA (2010) Antiviral drugs in wastewater and surface waters: a new pharmaceutical class of environmental relevance? Environ Sci Technol 44:1728–1735
Rimayi C, Odusanya D, Weiss JM, de Boer J, Chimuka L (2018) Contaminants of emerging concern in the Hartbeespoort Dam catchment and the uMngeni River estuary 2016 pollution incident, South Africa. Sci Total Environ 627:1008–1017
Ritchwood TD, Selin A, Pettifor A, Lippman SA, Gilmore H, Kimaru L, Hove J, Wagner R, Twine R, Kahn K (2019) HIV self-testing: South African young adults’ recommendations for ease of use, test kit contents, accessibility, and supportive resources. BMC Public Health 19:1–10
Roffe SJ, Fitchett JM, Curtis CJ (2019) Classifying and mapping rainfall seasonality in South Africa: a review. South Afr Geogr J 101:158–174
Saleh A, Larsson E, Yamini Y, Jönsson J (2011) Hollow fiber liquid phase microextraction as a preconcentration and clean-up step after pressurized hot water extraction for the determination of non-steroidal anti-inflammatory drugs in sewage sludge. J Chromatogr A 1218:1331–1339
Schoeman C, Mashiane M, Okonkwo OJ (2015) Quantification of selected antiretroviral drugs in a wastewater treatment works in South Africa using GC-TOFMS. J Chromatogr Sep Tech 06:1–7
Schoeman C, Dlamini M, Okonkwo OJ (2017) The impact of a Wastewater Treatment Works in Southern Gauteng, South Africa on efavirenz and nevirapine discharges into the aquatic environment. Emerg Contam. https://doi.org/10.1016/j.emcon.2017.09.001
Statisa Stats SA (2019) Midyear population estimate 2019. Popul Estim 24. www.statssa.gov.za. accessed: 21.06.2022
Strauch S, Midha KK, Jantratid E, Dressman JB, Junginger HE, Stavchansky S, Barends DM, Shah VP, Kopp S (2011) Biowaiver monographs for immediate release solid oral dosage forms: lamivudine. J Pharm Sci 100:2054–2063
Tijani JO, Fatoba OO, Petrik LF (2013) A review of pharmaceuticals and endocrine-disrupting compounds: sources, effects, removal, and detections. Water Air Soil Pollut. https://doi.org/10.1007/s11270-013-1770-3
Tran NH, Reinhard M, Gin KYH (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133:182–207
Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castaño-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M (2007) Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 168:148–156
Wasilewska A, Madajczyk M, Szymanski A, Urbaniak W (2011) Determination of trace quantities of dutasteride in water and wastewater by solid phase extraction and high performance liquid chromatography. Ars Separatoria Acta 8:39–49
Weigel S, Berger U, Jensen E, Kallenborn R, Thoresen H, Hühnerfuss H (2004) Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere 56:583–592
Wood TP, Basson AE, Duvenage C, Rohwer ER (2016) The chlorination behaviour and environmental fate of the antiretroviral drug nevirapine in South African surface water. Water Res 104:349–360
Wood TP, Du Preez C, Steenkamp A, Duvenage C, Rohwer ER (2017) Database-driven screening of South African surface water and the targeted detection of pharmaceuticals using liquid chromatography - High resolution mass spectrometry. Environ Pollut 230:453–462
Wu J, Boyle EA (1997) Low blank preconcentration technique for the determination of lead, copper, and cadmium in small-volume seawater samples by isotope dilution ICPMS. Anal Chem 69:2464–2470
Wu J, Qian X, Yang Z, Zhang L (2010) Study on the matrix effect in the determination of selected pharmaceutical residues in seawater by solid-phase extraction and ultra-high-performance liquid chromatography-electrospray ionization low-energy collision-induced dissociation tandem mass spectr. J Chromatogr A 1217:1471–1475
Yang Y, Ok YS, Kim KH, Kwon EE, Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.04.102
Acknowledgements
The work was supported by the National Research Foundation of South Africa (NRF) (Grant Number:122766). I thank Mrs Devrani Naicker, and Dr Talent Makhanya for their continued support and assistance during sampling and the continued encouragement throughout this research and mostly I take thank Prof Chimuka for his support during this study.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sigonya, S., Onwubu, S.C., Mdluli, P.S. et al. Method optimisation and application based on solid phase extraction of non steroidal anti-inflammatory drugs, antiretroviral drugs, and a lipid regulator from coastal areas of Durban, South Africa. SN Appl. Sci. 4, 231 (2022). https://doi.org/10.1007/s42452-022-05120-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05120-x