Abstract
This study assesses two parameters that can result in high efficiency in the recovery of the microalgae Monoraphidium contortum. The significant contribution of this paper is to test different coagulants in different conditions of concentration in the coagulation and flocculation processes followed by sedimentation to evidence the best coagulant and the best condition for harvesting of Monoraphidium contortum biomass. So the proposed methodology aimed to perform preliminary tests using a tannin-based cationic coagulant (TANFLOC SG®), FeCl2, and Al2 (SO4)3, where they were performed at concentrations of 0, 20, 40, 60, 80, and 100 mg L−1 at a fast mixing speed of 400 RPM. The tests determined 20 mg L−1 of Tanfloc SG® as the most efficient turbidity reduction in the preliminary test. The obtained results were used to construct a non-factorial central composite planning. Therefore, after a design of experiments, the study outcome shows the best turbidity removal range from the main tests came at 35 mg L−1 and 550 RPM of fast mixing speed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the biomass of microalgae, which is considered a third-generation raw material, may generate biofuels, food supplements, animal food, products for pharmaceutical use, and be used for other applications [1, 2]. Concerning the use of microalgae as a nutritional supplement, it is mainly due to richness in nutritional elements such as omega 3, fatty acids, which could be important for food supplements, cosmetics products, and fish farming [3]. However, one of the difficulties in the production and use of microalgae lies in the efficient harvesting of microalgae, since the size of its cells and the electrostatic repulsion resulting from its negative surface charge contribute to this fact [4]. In addition, its mass, which varies from 0.1 to 1 g L−1 [5], density and radius may hinder the harvest due to the low sedimentation speed.
In order to carry out the microalgae harvesting process, there are several types of methodologies, which include flocculation and sedimentation. Such methodologies have positive points such as the percentage of cell recovery, which is higher than 90%. There are also several types of existing coagulants applicable to microalgae harvesting that are of low cost. However, a negative point of the methodology is linked to the need to remove flocculants, chemical contamination, fragile flakes, and possible long sedimentation times [6].
Smith and Davis [7] state that the sedimentation process of non-flocculated microalgae is very slow due to the values referring to the diameter (5 μm), specific density (1.08 g cm−3), and sedimentation speed (0.4 cm h−1). Flocculation is an alternative to accelerate the sedimentation process, aggregating its primary particles into larger particles to form flakes. Just like a colloid particle, the decrease in turbidity for microalgae is gradual and slow, taking a much longer time to reach the values obtained under the influence of the used coagulants, which have the function of aggregating the microalgae particles, carrying out the process of flocculation and sedimentation.
Flocculation is a highly complex process, as it is influenced by the properties of the cell surface of the particle that interact with the flocculant, in addition to the ambient pH, cell concentration, ionic strength, flocculant type, and dosage [4]. Brennan and Owende [8] and Milledge and Heaven [9] say that the method is influenced by the density and radius of the microalgal cell, as well as the sedimentation speed that varies between 0.1 and 2.6 cm h−1. Sedimentation, on the other hand, is an inactive process that allows the flakes formed to sediment under the influence of gravity [10]. In this sense, the sedimentation speed is a valuable parameter in the design of sedimentation systems, also emphasizing that coagulant/flocculant concentration is the most important criterion for assessing the overall efficiency of the process [11]. Therefore, it is possible to relate the low specific density as a cause of long sedimentation times in crops [12].
Şirin et al. [13] state that in chemical flocculation processes, flocculants are divided into three groups: inorganic flocculants (metal salts of aluminum and iron), inorganic polymers (polyelectrolytes, polyaluminium chloride, and polyacrylamide), and organic polymers (chitosan, glutamic acid, and cationic starch). A specific type of organic polymer is called tannin. Tannins are biodegradable phenolic molecules that form complexes with proteins and other macromolecules and minerals, being extracted from the peel of vegetables, such as Acacia mearnsii de Wild (black wattle) [14]. Tannins are used as a coagulation aid to remove particles when they are anionic, in addition to its use as polyelectrolyte when it is of the cationic type for water treatment [15].
The present study aimed to compare initially the efficiency in turbidity removal as a parameter for biomass recovery for three coagulants (Tanfloc SG®, Al2(SO4)3, and FeCl2) in different concentrations in 120 min of sedimentation. After the preliminary tests, we aimed to find the best concentration and fast mixing speed through the design of experiments from the fixation as a central point at 20 mg L−1 of Tanfloc SG® in 400 RPM of fast mixing speed.
2 Methods
2.1 Cultivation
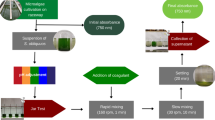
The strain of the microalgae Monoraphidium contortum was kindly provided by Prof. Dr. Pedro Augusto Arroyo, responsible for the Heterogeneous Catalysis and Biodiesel Laboratory (LCHBio) of the Chemical Engineering Department-DEQ, of State University of Maringá (UEM). The experiments were carried out at the Pollution Laboratory, of the Department of Environment, at the State University of Maringá, Umuarama campus (23º 46ʹ 47ʺ S; 53º 19ʹ 31ʺ O).
The cultivation of the microalgae Monoraphidium contortum was carried out in a 250 L capacity tank, maintaining the natural photoperiod, room temperature and agitation produced by the injection of atmospheric air for 24 h, using air pumps (BIG AIR A420, BIG AIR A320), with a capacity of 3.5 L min−1. The microalgae cultivation took place for 27 days, starting on August 15, 2019 and ending on September 12, 2019.
One liter of DM culture medium Watanabe [16] was daily supplied. The culture medium is composed of [1 g L−1 of Ca (NO3) 2.4H2O], [0.25 g L−1 KCl]; [0.55 g L−1 of MgSO4.7H2O]; [0.26 g of KH2PO4 L−1]; [0.02 g L−1 of FeSO4.7H2O] and [1 mL of Solution A5]. While the A5 solution is composed of: [2.9 g L−1 of H3BO3]; [1.81 g L−1 of MnSO4]; [0.11 g L−1 of ZnCl2]; [0.08 g L−1 of CuSO4] and [0.018 g L−1 of 3(NH4) 2O.7MoO3.4H2O]. During the cultivation, pH and turbidity were monitored. The pHwas measured using a Lucadema pH meter model LUCA-210P, previously calibrated. And turbidity was measured using a Del Lab turbidimeter model DLT-WV.
2.2 Treatments
Solutions were prepared for the preliminary treatments with 3 powder coagulants of 5 g (Tanfloc SG®, FeCl2, and Al2(SO4)3). For the preparation of solutions, the coagulants were weighed on an analytical scale (Shimadzu AUY 220), placed in a 1L volumetric flask, in which the concentration was dissolved in 0.5 L of distilled water, and were subsequently diluted to concentrations of 20, 40, 60, 80 and 100 mg L−1. Such preparation also occurred in the main treatments, having concentrations related to the Design of Experiments (DOE), providing the concentrations of 5, 20, 35, and 41.2 mg L−1 for Tanfloc SG® (Tanac SA, Brazil). Experiments were carried out in Jar test, model Floccontrol III of the polycontrol brand with 6 jars of 2 L, to obtain the best concentrations of each coagulant.
The conditions used for preliminary treatments were fast mixing speeds of 400 RPM and slow mixing of 30 RPM, the fast mixing time of 40 s and slow mixing of 20 min. For evaluation of the efficiency of microalgae removal, turbidity was determined by sample means collected at 0, 2, 4, 6, 8, 10, 20, 30, 60, 90, and 120 min, after the end of the slow stirring.
For main treatments, the DOE method was performed to determine the ideal conditions of the experiment. The experimental factors were established as coagulant concentration and fast mixing speed, while the response variable was the removal of turbidity, from the fixation as a central point at 20 mg L−1 of Tanfloc SG® and 400 RPM of fast mixing speed. The results are shown in Table 1.
All the results were evaluated using the Student's t-test, which identified the significant effects of the factors studied. Statistical analyzes of the t-test were verified using a significance level of 5%.
3 Results and discussions
3.1 Preliminary treatments
Figure 1 shows the values obtained in the preliminary tests according to the pre-established concentrations of Tanfloc SG®, Al2(SO4)3, and FeCl2. Results for Tanfloc showed that the lowest concentration used, which was 20 mg L−1, presented the highest final efficiency (98.7%), despite having less efficiency at the beginning of the process (47.6%). After it, the higher efficiencies were followed by the concentration of 40 mg L−1 (98.4%), 60 mg L−1 (97.9%), 80 mg L−1 (96.6%), 100 mg L−1 (96%) and 0 mg L−1 (55.9%). Barrado-Moreno et al. [17] also observed that increase in concentration did not increase the percentage of microalgal cell removal.
The results for the coagulant Al2(SO4)3 showed a behavior of increasing efficiency to the increase in the concentration of coagulant, with concentration of 100 mg L−1 (97.5%) followed by 80 mg L−1 (95.4%), 60 mg L−1 (87.0%), 40 mg L−1 (77.8%), 20 mg L−1 (74.1%) mg L−1 and 0 mg L−1 (55.9%). This efficiency trend with an increase in concentration was also observed by Zhu et al. [10]. The authors used Al2(SO4)3 nH2O with concentrations varying from 0.1 to 10 g L−1.
In relation to FeCl2, the concentration of 100 mg L−1 (89.8%) was the most promising, followed by the concentrations of 80 mg L−1 (89.3%), 60 mg L−1 (89.2%), 40 mg L−1 (87.4%), 20 mg L−1 (81.1%) and 0 mg L−1 (55.9%). These results show an increase in the efficiency directly proportional to the increase in the concentration of coagulant. Also, the results show that this coagulant presented the lowest efficiency in turbidity removal compared to other coagulants. Kandasamy and Shaleh [18] also found efficiency higher than 80% using FeCl2 (0.1 gL−1).
Table 2 shows the results of the Student’s t-test performed for the coagulant Tanfloc SG®, FeCl2, and Al2(SO4)3 in the preliminary treatments. From the results obtained, for Tanfloc SG®, it is possible to observe that the hypothesis of homogeneity of variance is rejected (P-value < 0.05) for the specific concentrations (0–20 mg L−1), (0–40 mg L −1), (0–60 mg L−1), (0–80 mg L−1), (0–100 mg L−1), and (40–100 mg L−1). For FeCl2 concentrations, it is possible to observe that the hypothesis of homogeneity of variance is rejected in the concentrations (0–40 mg L−1), (0–60 mg L−1), (0–80 mg L−1), and (0–100 mg L−1). Finally, for coagulant Al2(SO4) 3, the hypothesis of homogeneity of variance is rejected in the concentration (0–40 mg L−1), (0–60 mg L−1), (0–80 mg L−1), (0–100 mg L−1), (20–80 mg L−1), (20–100 mg L−1), (40–80 mg L−1), (40–100 mg L−1), and (60–100 mg L−1).
3.2 Discussions of preliminary treatments
It is possible to verify that Tanfloc SG® presented higher efficiencies in lower concentrations. Thus, Tanfloc SG® presented the highest biomass removal efficiency at a concentration of 20 mg L−1, reaching 98.7% of efficiency.
The high values achieved in the removal are linked to the coagulation/flocculation process, which destabilizes the negatively charged particles (microalgae) suspended in the water [19], by means of an electrostatic bridge between the suspended algae cells and the flocculant [20]. In addition, the degree of flocculation is directly associated with the extent of polymer coverage, that is, the concentration of polyelectrolytes. Still, the concentrations of the flocculant, above or below the ideal, result in impediment or insufficient access [21], culminating in less than ideal efficiency, meeting the results obtained in the present work that had a reduction in efficiency as the concentration of the natural flocculant increased.
The result was similar to the results found by Mezzari et al. [21], with an increase in efficiency in smaller concentrations. The authors found the best concentration of 11 mg L−1 using a tannin organic polymer to recover over 95% of Chlorella vulgaris biomass. Barrado-Moreno et al. [22] using another tannin-based coagulant (Acquapol C1-derived tannin) found the efficiency of Oocystis microalgae biomass removal up to 80% with low dosage (5 mg L−1).
Gutiérrez et al. [23] using Tanfloc SG® found an increase in biomass recovery with the increase of coagulant concentration. They found 93.3% of biomass recovery in 60 mg L−1, smaller efficiency compared to results seen in this study at 60 mg L−1 (97.9%). In another study, Cassini et al. [24] also found high efficiency (95.6%) using a tannin-based coagulant-flocculant (Tanfloc ® POP) for the harvest of microalgae biomass (Chlorella sp.) at 100 mg L−1. This value is close to the observed for this concentration in this study (96%). Therefore, the results show that Tanfloc has a great capacity to agglomerate microalgae cells in the process of flocculation, for later sedimentation.
Tonhato Junior et al. [25] used the coagulant Tanfloc SL® liquid to optimize brewery wastewater that contained microalgae. The authors found turbidity removal values for two treatments: in pH 8.0 with 43.75% efficiency to 0.120 mL L−1 and efficiency of 59.53% to 0.180 mL L−1. However, the best performance range came at pH 4.5 for the same concentrations respectively, reaching 98.58% and 95.85%. These results indicate the importance of pH on coagulation and flocculation processes. Still, results for Tanfloc SL® liquid presented lower efficiency compared to Tanfloc SG® powder (98.7%; 20 mg L−1). In addition, it is necessary to recognize that the flocculation pH of the liquid medium is relevant and is directly linked to the behavior of the coagulant.
Therefore, the choice of Tanfloc SG® Coagulant to main treatment was due to the fact that it has the best relationship between coagulant concentration and turbidity removal, presenting itself as the most promising coagulant. Presenting a removal greater than 90% in its low concentrations, a fact that made it stand out among the others used causing its choice for continuity of treatments. Barrado-Moreno et al. [17] also found the best cell removal at a low concentration of Tanfloc, and the recovery did not improve at higher Tanfloc concentrations. Still, the authors affirm that this fact is due to charge density.
Based on the turbidity and efficiency graphs over time of the preliminary tests, it was possible to carry out a more targeted treatment, using specific concentrations, thus being able to achieve the best range of turbidity removal efficiency for the coagulant chosen in the main stage of the experiments. The values obtained by the concentration of 0 mg L−1, will be inherent in the samples that did not have any type of coagulant, serving as a basis for the standard behavior of the sedimentation of microalgae, behavior that occurs due to the natural effect performed by gravity.
The t-test hypothesis, on the other hand, is based on determining whether the behavior of the concentration curves are in fact significantly different, when compared individually, with rejection being characterized by P values < 0.05 and non-rejection being characterized by P values > 0.05. Due to the characteristic that the p-value has to vary between 0 and 1, values that approach 0 tend to reject the hypothesis, and those that approach 1 tend to non-rejection. Thus, for α = 5% if the hypothesis is confirmed we do not want to reject it incorrectly more than 5% of the time. Therefore, due to the fact that the type of analysis is statistical and not experimental, if the hypothesis is rejected, these values are statistically “equal” and if not rejected, they are statistically “different”.
In the use of inorganic salts, the harvest had a lower efficiency compared to the use of Tanfloc. Inorganic salts are multivalent cations that form polyhydroxylated complexes at ideal pH, neutralizing microalgae surface charges. Its effectiveness depends on electronegativity and solubility [20], in addition to the efficiency in mixing salt with the medium, since aluminum sulfate reacts with water and forms aluminum hydroxides in a time of 1–7 s after the addition, in which phase the aluminum ions (Al3+), aluminum hydroxide ions and aluminum hydroxides adsorb to the surface of the microalgae (negative charge) with an estimated time between 0.2 and 1 s [26]. According to Li et al. [27] other free cations, such as Fe3+, Mg2+, and their hydrolysates are positively charged and can neutralize the negative charges of algae cells, promoting the collision and aggregation of cells to form flakes.
Aluminum sulfate required an increase in concentrations to provide an increase in removal efficiency, with 20 mg L−1 stabilizing close to 74%, while 80 and 100 mg L−1 surpassed the efficiency of 95.0%. While ferric chloride had an efficiency that stabilized close to 80% (20 mg L−1) and 90% (100 mg L−1) as a maximum. This characteristic is observed for aluminum sulfate by Cassini et al. [24] in which doses greater than 80 mg L−1 were necessary to achieve an efficiency of 60–70% in basic conditions, just as in this study. Still, it is important to highlight that inorganic salts can leave residuals in the biomass and can cause environmental and health problems [20, 28].
3.3 Main Treatments
Figure 2 shows the behavior of the Tanfloc SG® coagulant in the main treatments. At a concentration of 5 mg L−1, it is possible to verify higher efficiency at 550 RPM (98.3%) than at 250 RPM (97.7%). The efficiency of turbidity removal increased at 20 mg L−1, where the highest efficiency for this concentration was seen at 612.9 RPM (98.8%). However, the best results were seen at a concentration of 35 mg L−1 (99%). The efficiency was slightly higher at 550 RPM. Therefore, the central composite design can provide adequate grounds for reaching the test ranges in question.
Turbidity × Time (blue) and Efficiency × Time (red) for 5 mg L−1 (250 RPM), 5 mg L−1 (550 RPM), 20 mg L−1 (187.9 RPM), 20 mg L−1 (400 RPM), 20 mg L−1 (2) (400 RPM), 20 mg L−1 (612.9 RPM), 35 mg L−1 (250 RPM), 35 mg L−1 (550 RPM), 41.2 mg L−1 (400 RPM). The blue dashed line means the zero for y-axis when the initial efficiencies are lower or close to zero
The t-tests for the main treatments using Tanfloc SG® are shown in Table 3. From the results obtained, the main tests that presented the hypothesis of homogeneity of rejected variance are in the conditions of 0 mg L−1 (400 RPM)-5 mg L −1 (250 RPM) up to 0 mg L−1 (400 RPM)-20 mg L−1 (400 RPM) (Center point)-2, in addition to the range that incorporates the 5 mg L−1 range (250 RPM)-20 mg L−1 (400 RPM) (Center point)-1 and 5 mg L−1 (250 RPM)-20 mg L−1 (400 RPM) (Center point)-2.
3.4 Discussions of main treatments
In the main treatments, it was observed that the tannin-based coagulant acted better in an extensive range of fast mixing speed and concentration suggesting that both of these parameters are important parameters to increase the efficiency in microalgal biomass harvesting. In addition, comparing to initial results, the efficiency increased from 98.4% (20 mg L−1; 400 RPM) to 99% (35 mg L−1; 550 RPM). In Fig. 2, it is possible to verify that the most efficient range to coagulate, is in the range that occurs from 5 to 41.2 mg L−1 at fastest mixing speeds with a focus on the best removal efficiency range that came to 35 mg L−1 (550 RPM), reaching 99% removal efficiency.
Zhu et al. [10] achieved more than 90% of Chlorella Vulgaris biomass recovery using a fast mixing speed of 150 RPM in 1 min, however, the authors used a concentration of 1.2 g L−1 of chitosan. Bracharz et al. [29] stirred algae suspension at 300 RPM for 3 min, reaching higher efficiencies using Tanin and Chitosan in concentrations of 40 and 60 mg L−1. Kandasamy and Shaleh [18] also used 300 RPM as a fast mixing speed, where the efficiencies were 75.50% in 3 min of mixing time, 80.30% in 6 min of mixing time, and 78% in 9 min of mixing time using MBPE flocculant.
It was also possible to visualize that, in general, in the experiments carried out using the Tanfloc SG® coagulant, an appropriate electrostatic destabilization of the colloids (microalgae) was decisive. Despite the different speeds and concentrations used, there was the occurrence, for example, in the concentration of 20 mg L−1 (187.9 RPM) that even having a lower rotation range has an efficiency of removal almost equivalent to the central points with a concentration of 20 mg L−1 (400 RPM) and is still capable of being equivalent to the second central point.
The performance of the coagulant was extremely efficient, with small differences between the figures, showing specific characteristics, which are visible for comparison in the concentrations of 5 mg L−1 in the rotations of 250 and 550 RPM, and 35 mg L−1 in the rotations of 250 and 550 RPM. The best efficiencies came with higher speed ranges except for the 41.2 mg L−1 concentration (400 RPM), which had no comparison criteria, as there was no other test at a different speed with the same concentration used.
Still, Fig. 2 shows the main treatment behaviors that have become intrinsic in all concentration ranges and rapid mixing speed. After the initial minutes, there are extremely satisfactory efficiencies, occurring in contrast only in the concentrations of 20 mg L−1 (187.9 RPM), 20 mg L−1 (612.9 RPM), 35 mg L−1 (550 RPM), and 41.2 mg L−1 (400 RPM), when occurrences of negative initial efficiencies were seen at the beginning of the sedimentation process.
The efficiencies in question give a false impression that the process is not being effective, however, at the time of the first readings (1–2 min), flocculation of the microalgae is still occurring for sedimentation, reducing the speed of the water that keeps the flakes suspended, as well as a higher concentration of cells in the aggregates. As an effect, this results in an instantaneous and brief increase in turbidity, due to the rupture of aggregates by the collection. But immediately after such an event, in the next collection, they presented increasing values and efficiency above 97% as a result of the overcoming of the forces that kept the flakes in suspension.
4 Conclusions
Through the analysis of the experiments, it was seen how important the proper adjustment is for the optimization of the fast mixing speed ranges and coagulant concentrations. Thus, being able to determine the reason for the coagulant to be effective or not in the situations in which it was used. The experiments in question demonstrated the highest efficiency at a concentration of 35 mg L−1 and a fast mixing speed of 550 RPM, reaching 99% efficiency of removal of turbidity, being the most expressive result using the DOE method in this study. The method provided adequate concentrations and fast mixing speeds efficient in the harvesting process. Finally, the treatments in conjunction with mathematical tools proved to be of great value in determining fast mixing speeds and concentration for the coagulant, proving that it is possible to optimize the results to the point of acquiring increasingly better values, as more simulations are carried out. Still, the results show that it is possible to carry out the coagulation, flocculation, and sedimentation process even more effectively when compared to the preliminary treatments, in case small adjustments occur in the dependent and independent variables that are part of the process responsible for obtaining satisfactory harvests.
Date Availability
Not applicable.
Abbreviations
- FeCl2 :
-
Iron (II) Chloride
- Al2 (SO4)3 :
-
Aluminum Sulfate
References
Farooq W, Lee Y-C, Han J-I, Darpito CH, Choi M, Yang J-W (2013) Efficient microalgae harvesting by organo-building blocks of nanoclays. Green Chem 3:749–755
Trevisan E, Godoy RFB, Radomski FAD, Crisigiovanni EL, Branco KBZF, Arroyo PA (2020) Chlorella vulgaris growth in different biodigested vinasse concentrations: biomass, pigments and final composition. Water Sci Technol 5:1–9
Wang Z, Hou J, Bowden J, Belovich JM (2013) Evaluation of an inclined gravity settler for microalgae harvesting. J Chem Technol Biotechnol 89(5):714–720
Papazi A, Makridis P, Divanach P (2010) Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol 22:349–355
Castrillo M, Lucas-Salas LM, Rodríguez-Gil C, MARTÍNEZ, D. (2013) High pH-induced flocculation–sedimentation and effect of supernatant reuse. Biores Technol 128:324–329
Brányikova I, Prochazkova G, Potocar T, Zuzana J, Branyik T (2018) Harvesting of microalgae by flocculation. Fermentation 4(93):1–12
Smith BT, Davis RH (2012) Sedimentation of algae flocculated using naturally-available, magnesium-based flocculants. Algal Res 1(1):32–39
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14(2):557–577
Milledge JJ, Heaven S (2013) A review of the harvesting of micro-algae for biofuelproduction. Rev Environ Sci Bio Technol 12:165–178
Zhu L, Li Z, Hiltunen E (2018) Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels 11(183):1–10
Vandamme D, Muylaert K, Fraeye I, Foubert I (2014) Floc characteristics of Chlorella vulgaris: Influence of flocculation mode and presence of organic matter. Bioresour Technol 151:383–387
Epsztein R, Felder A, Mishelevitz A, Gitis V (2012) Efficient separation of Nannochloropsis salina using minerals to optimize algae sedimentation. J Chem Technol Biotechnol 87:1512–1519
Şirin S, Trobajo R, Ibanez C, Salvadó J (2012) Harvesting the microalgae Phaeodactylum tricornutum with polyaluminum chloride, aluminium sulphate, chitosan and alkalinity-induced flocculation. J Appl Phycol 24:1067–1080
Beltrán-Heredia J, Sánchez-Mártin J, Mártin-Sanchez C (2011) Remediation of dye-polluted solutions by a new tannin-based coagulant. Ind Eng Chem Res 50(2):686–693
Graham N, Gang F, Fowler G, Watts M (2008) Characterisation and coagulation performance of a tannin-based cationic polymer: a preliminary assessment. Colloids Surf A 1–3:9–16
Watanabe A (1960) List of algal strains in collection at the institute of applied microbiology, University of Tokyo. J Gen Appl Microbiol 5:284–292
Barrado-Moreno MM, Beltrán-Heredia J, Martín-Gallardo J (2016) Microalgal removal with natural coagulants. Phycologia 55(6):688–695
Kandasamy G, Shaleh SRM (2016) Harvesting of the microalgae Nannochloropsis sp. by bioflocculation with Mung Bean protein extract. Appl Biochem Biotechnol 3:1–12
Kim S, Moon B, Lee H (2001) Effects of pH and dosage on pollutant removal and floc structure during coagulation. Microchem J 68:197–203
Singh G, Patidar SK (2018) Microalgae harvesting techniques: a review. J Environ Manag 217:499–508
Mezzari MP, Silva MLB, Pirolli M, Perazzoli S, Steinmetz RLR, Nunes EO, Soares HM (2014) Assessment of a tannin-based organic polymer to harvest Chlorella vulgaris biomass from phycoremediation process treating swine wastewater digestate. Water Sci Technol 6:1–7
Barrado-Moreno MM, Beltrán-Heredia J, Martín-Gallardo J (2015) Removal of Oocystis algae from freshwater by means of tannin-based coagulant. J Appl Phycol 3:1–7
Gutiérrez R, Passos F, Ferrer I, Uggetti E, García J (2015) Harvesting microalgae from wastewater treatment systems with natural flocculants: effect on biomass settling and biogas production. Algal Res 9:204–211
Cassini ST, Francisco SA, Pereira PA, Oss RN, Keller R (2017) Harvesting microalgal biomass grown in Anaerobic Sewage treatment effluent by the coagulation-flocculation method: effect of pH. Braz Arch Biol Technol 3:1–12
Tonhato Junior A, Hasan S, Sebastien NY (2019) Optimization of coagulation/flocculation treatment of brewery wastewater employing organic flocculant based of vegetable Tannin. Water Air Soil Pollut 230(202):115
Korpijärvi J, Laine E, Ahlstedt H (2000) Using CFD in the study of mixing in coagulation and flocculation. In: Chemical water and wastewater treatment VI. Springer, Berlin, Heidelberg, pp 89–99
Li S, Hu T, Xu Y, Wang J, Chu R, Yin Z, Zhu L (2020) A review on flocculation as an efficient method to harvest energy microalgae: mechanisms, performances, influencing factors and perspectives. Renew Sustain Energy Rev 131:110005
Matter IA, Bui VKH, Jung M, Seo JY, Kim Y, Lee Y, Oh Y (2019) Flocculation harvesting techniques for microalgae: a review. Appl Sci 9:1–27
Bracharz F, Helmdach D, Aschenbrenner I, Funck N, Wibberg D, Winkler A, Bohnen F, Kalinowski J, Mehlmer N, Bruck TB (2018) Harvest of the Oleaginous Microalgae Scenedesmus obtusiusculus by flocculation from culture based on natural water sources. Front Bioeng Biotechnol 6:1–11
Acknowledgements
The authors would like to thank the opportunity to use the Pollution laboratory at University of Maringá campus Umuarama.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruggeri, M.V.R., Godoy, R.F.B., Arroyo, P.A. et al. Evaluation of natural flocculant efficiency in the harvest of microalgae Monoraphidium contortum. SN Appl. Sci. 3, 627 (2021). https://doi.org/10.1007/s42452-021-04614-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04614-4