Abstract
The objective of the present investigation was to evaluate the physical stability of emulsions (w/o) with a gel as an oil phase. Two oil phases (canola and coconut oil) were used to assess the impact of the different vegetable oils. Monoglycerides were used as the gelling agent and polyglycerol polyricinoleate as the surfactant. Micrographs, differential scanning calorimetry, and rheology tests were performed. The presence of crystalline structures was observed in the continuous phase characteristic of some organogels and a smaller distribution of sizes as a function of time. Also, a change in the crystallization profile of the aqueous and oily phases concerning to time was also found, the crystallization signals coinciding (≈ − 40 °C), indicating a better organization by the phases. No variability was found in modules G′ and G″, so these systems have good mechanical stability. The properties of the organogelated emulsions are explained by the interface-interface interactions present between the particles and the reduced mobility, which slows the phase separation.
Similar content being viewed by others
1 Introduction
In the last decade, interest in the introduction of micronutrients and nutraceuticals as part of a food matrix or products with a contribution to health has grown exponentially. Some of these products or compound matrices require emulsions to have specific characteristics. Also, pharmaceutical and food industries have used emulsions to load some bioactive compounds and include them in a final product [1, 2].
Organogel-emulsions (w/o) has a continuous oil phase structured from a self-assembling gelling agent giving semi-solid characteristics like those of an organogel [3]. The w/o emulsions have water droplets surrounded by an interfacial film where some surfactants can be found, these drops are dispersed in the continuous oil phase. Emulsions can be obtained by different methods, which can be classified as high and low energy. The low energy emulsions drop out the phase in minor proportion to become under certain specific conditions of concentration, temperature, and agitation metastable systems [4].
The development of w/o emulsions is based on dispersion and force methods, the so-called high-energy physical mechanisms, which by disruptive forces mechanically disintegrate the aqueous phase into small droplets that disperse in the continuous oil phase. Therefore, it is possible to obtain emulsions by mechanisms such as mixing and homogenization. However, it is well known that the drop size reduction is limited by the viscosity of the oil phase [5, 6]. By reducing the droplet size in emulsions, the interaction area between droplets is increased, presenting greater stability to the aggregation of particles, and reducing the phenomena of coalescence and flocculation. It is also important to consider the concentration of surfactant since a suitable thickness of the interface surrounding the drops provides a greater viscosity to the dispersed phase that in conjunction with a structured continuous phase can provide gel or paste characteristics for different applications, where semisolid behavior is required [6]. It has also been shown that smaller droplet sizes increase the bioavailability of certain types of lipophilic substances loaded in emulsions [7].
Emulsions based on structuring with monoglycerides (MG) are widely used in food products and cosmetics [8]. The MG are low molecular weight lipids, which have a single fatty acid chain attached to a glycerol main chain, providing amphiphilic characteristics and being important emulsifiers. These molecules self-assemble both in the presence of water and oil in various types of mesophases depending on the type of continuous phase and their concentration. It has been reported that it is possible to obtain structured emulsions, resembling trans-free vegetable fats, which can be used in different food products [9, 10].
In o/w emulsions structured with MG, structural and rheological properties have been studied finding that in the lamellar phase, after the homogenization, the MG and the co-emulsifiers assemble themselves in hydrated lamellar structures and form a gel network like fat and organogels, in which the oil droplets are surrounded by alternating MG bilayers and water [11, 12]. However, some emulsions structured only with MG are prone to phase separation and syneresis of water after four weeks of storage at room temperature (about 20 °C).
The destabilization of structured emulsions is mainly caused by a polymorphic transformation of MG [13]. The polymorphic transition of the MG limits the useful life of emulsions due to the loss of nanostructured water. However, it is possible to increase the stability of structured organogel-emulsions by slowing the transition from the α-gel to the coagel phase. These transitions have already been well characterized in MG-water interactions, where heating the MG-water mixture above its Kraft temperature (Tk), the MG molecules self-assemble into a crystalline liquid phase Lα (α-gel) [14, 15]. By cooling the Lα phase below Tk, the hydrocarbon chains of the MG lose mobility and transform into a Lα′ phase (sub-α-gel phase), in which thick layers of water are retained between the MG bilayers. The α-gel phase is thermally reversible to the sub-α-gel phase [16]. However, both the sub-α-gel phase and the α-gel phase are thermodynamically unstable and will gradually crystallize in the more densely compacted Lβ phase (coagel), accompanied by a release of water at room temperature [17].
Although the coagel phase is the thermodynamically most favorable phase in organogels, this transition prevents the stability of an emulsion [16]. A slow cooling rate, as well as avoiding cooling during storage after homogenization also improves the stability of the α-gel phase [18].Also, the use of surfactants in conjunction with MG can reduce the release of water during polymorphic transitions by reinforcing the interfacial film [19].
The objective of the present investigation was to develop structured organogel-emulsions (w/o) with MG and a lipophilic surfactant. The microstructural, thermodynamic, and rheological behavior in function to time above ambient temperature (25 °C) was also evaluated.
2 Materials and methods
2.1 Materials
Used oils had the following compositions (according to their manufacturer’s labels): canola oil (CAO), 61% monounsaturated, 32% polyunsaturated and 7% saturated; and extra virgin coconut fat oil (CNO), 86% saturated, 13% monounsaturated and 1% polyunsaturated. Both oils were purchased at a local supermarket store (Durango, Mexico). Myverol (18-04 PK Cod. 20070857, Lot: 1056067) is a mixture of monoglycerides (mainly 49% glycerol monostearate, 48% glycerol monopalmitate and 3% calcium silicate) kindly provided by Kerry (SW food technology, SA de CV, Nuevo Leon, Mexico). The surfactant PGPR (E 476), which is a complex mixture of partial esters of polyglycerol with linearly esterified polyricinoleic acid derived from castor oil, was purchased from Palsgaard (San Luis Potosí, Mexico).
2.2 Emulsions preparation
Emulsions (w/o) were prepared following the method of [3] with 25% disperse phase. The oil phase was obtained by mixing each of the oils with myverol (MY)(10% w/w) and PGPR (1% w/w) at 80 °C, 10 min by magnetic stirring (1000 rpm). Once the mixture was homogenized, it was mixed with the aqueous phase using an Ultra-Turrax homogenizer (60 °C, 6500 rpm, 7 min), while it was immersed in a refrigerated bath (5 °C) until it reached 25 °C. They were refrigerated at 5 °C for 12 h and then stored at 25 °C for 28 days.
2.3 Microscopy
Photographs of the different systems were taken every 7 days. The samples were placed on glass slides and observed under a Nikon optical microscope (Eclipse LV100N POL) with a bright field and polarized light filter at a total magnification of 100 × (25 °C), and equipped with a digital camera (Q-Imaging 3.3 RTV) to observe drops and interphases, as well as the crystalline structures characteristic of organogel systems. Measurements of the diameter of observed drops were taken with the help of Nis Elements AR software. A record of the different diameters was made to observe the size distribution of each system.
2.4 Differential scanning calorimetry
The Thermal evaluation was performed in a differential scanning calorimetry (DSC) equipment from TA, Instruments (2920, New Castle, DE, USA), equipped with a refrigerated cooling system and a nitrogen flow of 50 mL/min. Samples of 3-6 mg of each of the organogel-emulsions were placed in hermetically sealed aluminum capsules (25 °C). They were maintained at 25 °C for 10 min, cooled to − 65 °C at a rate of 2 °C/min to obtain the crystallization profile, then kept for 3 min at − 65 °C and warmed up to 80 °C at a speed of 5 °C/min, obtaining the profile of structure loss and the fusion endotherms. Thermograms were obtained from each of the systems, in which enthalpies and temperatures were determined by analyzing the graphs with the TA Instruments Universal Analysis 2000 software.
2.5 Rheology
Rheological tests were carried out in an Anton Paar rheometer with parallel plate geometry PP50/TG (air pressure 5 bar, means position 1 mm). Deformation sweep (ramp log 0.001–100%, 1 Hz, 0 N) and simple shear tests (10 s−1, 25 °C) were performed, followed by a recovery stage (0.004%, 1 Hz, 0 N, 25 °C, 60 min). The flow point, the range of linear viscoelasticity (r = 0.999), yield point, phase angles, and the different corresponding modulus (G′, G″ and G*) were determined at each point. The recovery percentage was determined in the last stage. The tests were performed every 7 days for 28 days.
2.6 Statistical analysis
Comparisons were made in function of the storage time with the STATISTICA software (Ver. 7.0, 2007); using factorial design and ANOVA with a comparison of means (Tukey < 0.05).
3 Results and discussion
3.1 Emulsion microstructure
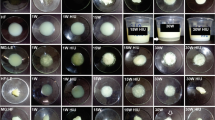
The photographs of organogel-emulsions of canola (OECAO) and coconut (OECNO) oils are shown in Fig. 1. Micrographs of them are shown in Fig. 2 (A for clear field and B for polarized field). At day one, it is possible to observe many solid oil aggregates and the presence of few water droplets in the coconut (OECNO) and canola emulsions (OECAO), the aggregates can be observed as oil stains in the polarized field (Fig. 2b) [8]. At longer times, it was observed that the solid aggregates were separated and evenly distributed in the continuous oil phase in the form of crystalline fibrillar structures by the MG-oil interactions (Fig. 2b). In the bright field, it was observed the appearance of a greater number of drops of smaller size (1–5 μm) at 28 days in OECAO, while in OECNO a greater size distribution (1–30 μm) was observed with less number of drops (Fig. 2a). Similar results on drop size have been reported by other research groups using the same oils, but no surfactant and gelator, reporting free water by coalescence after 20 days [20]. The microstructural changes in OECAO and OECNO indicate a more organized structuring with in function to time, even when at rest at 25 °C.
The constant structural organization in organogel-emulsions is due to polymorphic transitions of the MG from the α-gel phase to sub-α-gel and subsequently to the coagel phase. Cassin et al. [17], found that α-gel is stable when stored at temperatures above 45 °C because the driving force for coagel formation is at lower storage temperatures. The polymorphic behavior of glycerol monostearate (MSG) in water has been well characterized by different research groups, which report that maintaining a higher temperature during the storage of organogel-emulsions delays polymorphic transitions due to aging and the use of surfactants. Temperature can increase the electrostatic repulsion between MG bilayers and slow down the polymorphic change to coagel [11].
In oil-MG systems become structured with glyceryl monostearate (MSG) or glyceryl monopalmitate (MSG), when the α-gel phase is below 55 °C and the sub-α phase, below 36 °C [21]. However, the α-gel is only in the metastable state and is thermodynamically unstable; so that the α-gel will crystallize gradually in the Lβ phase, which is more organized and densely packed, also called β-gel or coagel [14, 17]. Also, it is known that in the transition to the coagel phase, the bilayers reorganize structurally and expel the water between the bilayers. The system cannot return to the α-gel phase without heating above the Kraft temperature and re-forming the lamellar phase [22, 23].
Organogel-emulsions formed with MG-PGPR seem to maintain prolonged metastability regardless of the type of oil used. They are more organized in their structure because the PGPR surfactant in conjunction with the MG reinforces the interface, increasing the electrostatic repulsions and better dispersed water that avoids expelling it during the polymorphic transitions. The storage at 25 °C may be another factor that delays polymorphic transitions while maintaining metastability. Similarly, there are reports that in structured systems with MG, impurities such as free fatty acids and diglycerides help to structure water [24].
3.2 Emulsion crystallization and fusion profiles
The crystallization and melting profiles of the organogel-emulsions were obtained. No significant differences were found in the melting peaks of oils and water in function to time (Fig. 3 Canola oil, coconut oil, water and myverol).
No significant differences were found in the melting of the water due to the influence of the type of oil (Table 1). The fusion of MG can be considered as the loss of structure of the organogel-emulsions (Fig. 3 Myverol). Higher temperature values were found at the start during the loss of structure when using canola oil (CAO); however, there were no differences with respect to time in each sample. No significant differences were found in the enthalpy values either (Table 2). Lower onset values were found in the loss of structure for OECNO, which is related to the sensitivity to temperature changes of coconut oil (CNO) (Table 2).
In the crystallization profile, no significant differences were found in the crystallization peaks of CNO (about 13 °C) with respect to time (Fig. 4 Coconut oil).
However, the crystallization peak of CAO (about − 40 °C) presents an increase in enthalpy as a function of time because the aqueous phase moves to lower crystallization temperatures as a function of time, coinciding at − 40 °C with the crystallization peak of the CAO (Fig. 5).
In OECNO, water is divided into two main crystallization peaks, one at 13 °C, indicating that this fraction of water can be found relatively free within the system and another at − 40 °C, which corresponds to smaller sizes and confined droplets (Tables 3 and 4) [25, 26]. Considering that the freezing is the result of a nucleation phenomenon, all the drops do not freeze at the equilibrium point, but their subzero temperatures can be varied depending on the energy required to access to them [27].
The results obtained in DSC correspond to those observed in the micrographs. The microstructural organization of the organogel-emulsions (w/o) over time forms smaller water droplets, being more confined in the structured oil phase, which is reflected at a lower crystallization temperature [28]. The division of two peaks of water crystallization into OECNO may be related to a greater dispersion of droplet sizes (Fig. 4 water). It is known that in addition to the storage temperature, the dispersion of droplet size also affects the morphology of structured emulsions [27, 28]. Similarly, a smaller droplet size increases the solubility in the continuous phase, making possible the mass transfer between droplets, which is identified with differences in the enthalpy values.
The mechanism of mass transfer can be influenced by the presence of surfactants in the continuous medium that can further increase the solubility [29]. The selection of a suitable surfactant to the type of emulsion can slow the mass transfer [30]. found that in emulsions (w/o) a lower water content resulted in a finer and monodisperse system with a freezing point at about − 40 °C, in which nucleation was more homogeneous than in systems with a higher content of water. It was also concluded that there is an intermediate behavior between the homogeneous nucleation and the release of water, in which part of the water crystallized at − 40 °C and another part at − 27 °C. Changes in the nucleation and dispersion of droplet sizes by polymorphic transitions could explain the changes observed over time in the micrographs (Fig. 2) and the DSC thermograms.
3.3 Emulsions mechanical behavior
It was found that the organogel-emulsions of canola and coconut oils obey the model of the law of potency, with a behavior index between 0 and 1; thus, it is confirmed that these systems are pseudo plastic materials (Fig. 6a). Rheological measurements showed similar behavior for OECAO and OECNO. The glycerol hydrophilic head is smaller and less bulky in comparison to PGPR. A less voluminous conformation allows the approximation of molecules that can interact more closely with their neighbors. These interactions are weak forces of short_range (hydrophobic interactions, dipole moment, and H-bonding) that led to the formation of stronger elastic structures and resistance to deformation [31,32,33].
In deformation sweep experiments, it was found that with saturated medium-chain vegetable oils the mechanical modulus (G′ and G″) are higher than in systems with unsaturated long-chain oils (Fig. 6b). The presence of water droplets between the structured bilayers of MG-oil led to a less crooked spatial arrangement, which increases the interaction energy, favoring the stability of the organogel-emulsions. Also, G′ predominated over G” in both systems during the strain sweep. The complex module (G*) was graphed against the percentage of deformation, making a comparison between oils inside the linear viscoelasticity range (LVR) (Fig. 6c). It was found that LVR is wider in the coconut organogel-emulsion (0.223% strain) compared to the canola one (0.043% strain) (Fig. 6c, d).
No significant differences were found in the LVR during the experimental time (28 days) in both systems. The phase angles in LVR for both emulsions showed values between 0 and 45 confirming that a semi-solid is obtained at relatively weak stresses.
A central value of G* was taken into the LVR and evaluated as a function of time for CAO and CNO systems (Fig. 6d). A linear behavior by G* with respect to time was observed indicating important stability of its mechanical behavior for 28 days regardless of the type of oil (Fig. 6d). Shear and recovery tests were performed for each system during the time evaluated. Recovery percentages were obtained at about 100% with minimal variations in OECAO and no significant differences in OECNO with related to time. The rheological properties and the mechanical stability of structured emulsions (w/o) in the long term depending on the physical aging of the crystalline network by compaction of the structures, the speed at which these changes occur, and their capacity for retaining the dispersed phase during its restructuring. The aging of a crystalline network will depend in turn on the growth of crystals and their aggregation; this in sequence will depend on the composition of the system, as well as the storage conditions [34, 35].
4 Conclusion
Organogel-emulsions are metastable systems, which mean that although there may be constant changes in their morphology, they do not necessarily promote phase separation in all cases. The low concentration of dispersed aqueous phases in conjunction with the presence of PGPR and MG, in relatively high-temperature storage (25 °C) could influence inverse laminar packing. Therefore, water droplets trapped in the center of the MG-oil bilayers may be considered a “metastable association”. The presence of surfactants and the glycerol group of the MG molecules could reinforce the interfacial film surrounding the water droplets, preventing their release during the polymorphic transitions of the MG structured with the oil phase after 28 days, and even being able to slow down their microstructural changes.
References
McClements DJ (2010) Emulsion design to improve the delivery of functional lipophilic components. Ann Rev Food Sci Technol 1:241
Sagalowicz L, Leser ME (2010) Delivery systems for liquid food products. Curr Opin Colloid Interface Sci 15:61
Rocha-Amador OG, Gallegos-Infante JA, Huang Q, Rocha-Guzmán NE, Moreno-Jiménez MR, González-Laredo RF (2014) Influence of commercial saturated monoglyceride, mono-/diglycerides mixtures, vegetable oil, stirring speed, and temperature on the physical properties of organogels. Intl J Food Sci 2014:513641
Rao JJ, McClements DJ (2010) Formation of flavor oil microemulsions, nanoemulsions and emulsions: influence of composition and preparation method. J Agric Food Chem 58:7059
McClements DJ (2011) Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 7:2297
McClements DJ, Rao J (2011) Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr 51:285
Acosta E (2009) Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Inter Sci 14:3
Batte HD, Wright AJ, Rush JW, Idziak SHJ, Marangoni AG (2007) Phase behavior, stability, and mesomorphism of monostearin–oil–water gels. Food Biophys 2:29
Calligaris S, Manzocco L, Valoppi F, Nicoli MC (2013) Effect of palm oil replacement with monoglyceride organogel and hydrogel on sweet bread properties. Food Res Intl 51:596
Blake AI, Marangoni AG (2015) Factors affecting the rheological properties of a structured cellular solid used as a fat mimetic. Food Res Intl 74:284
Wang FC, Marangoni AG (2015) Effect of intrinsic and extrinsic factors on the stability of the α-gel phase of a glyceryl monostearate–water system. R Soc Chem Adv 5:43121
Verstringe S, Moens K, De Clercq N, Dewettinck K (2015) Revealing the influence of tempering on polymorphism and crystal arrangement in semicrystalline oil-in-water emulsions. Cryst Growth Design 15:5693
Larsson K, Fontell K, Krog N (1980) Structural relationships between lamellar, cubic and hexagonal phases in monoglyceride-water systems. Possibility of cubic structures in biological systems. Chem Phys Lipids 27:321
Krog N, Larsson K, Krog N, Larsson K (1968) Phase behaviour and rheological properties of aqueous systems of industrial distilled monoglycerides. Chem Phys Lipids 2:129
Krog N, Borup AP (1973) Swelling behaviour of lamellar phases of saturated monoglycerides in aqueous systems. J Sci Food Agric 24:691
Wang FC, Marangoni AG (2014) Nature and dynamics of monostearin phase transitions in water: stability and the sub-α-gel phase. R Soc Chem Adv 4:50417
Cassin G, de Costa C, van Duynhoven JPM, Agterof WGM (1988) Investigation of the gel to Coagel phase transition in monoglyceride—water systems. Langmuir 14:5757
Da Pieve S, Calligaris S, Co E, Nicoli MC, Marangoni AG (2010) Shear nanostructuring of monoglyceride organogels. Food Biophys 5:211
Marangoni AG, Idziak SHJ, Vega C, Batte H, Ollivon M, Jantzi PS, Rush JWE (2007) Encapsulation-structuring of edible oil attenuates acute elevation of blood lipids and insulin in humans. Soft Matter 3:183
Ghosh S, Rousseau D (2009) Freeze–thaw stability of water-in-oil emulsions. J Colloid Interface Sci 339:91
Chen CH, Van Damme I, Terentjev EM (2009) Phase behavior of C18 monoglyceride in hydrophobic solutions. Soft Matter 5:432
Wang FC, Marangoni AG (2016) Microstructural basis for water release from glycerol monostearate structured emulsions upon transformation from the α-gel to the coagel phase Food. Structure 7:1
Zetzl A, Ollivon M, Marangoni A (2009) A coupled differential scanning calorimetry and X-ray study of the mesomorphic phases of monostearin and stearic acid in water. Crystal Growth Design 9:3928
Goldstein A, Seetharaman K (2011) Effect of a novel monoglyceride stabilized oil in water emulsion shortening on cookie properties. Food Res Intl 44:1476
Chen G, He G (2003) Separation of water and oil from water-in-oil emulsion by freeze/thaw method. Sep Purif Technol 31:83
Chen CH, Terentjev EM (2010) Effects of water on aggregation and stability of monoglycerides in hydrophobic solutions. Langmuir 26:3095
Clausse D, Gomez F, Pezron I, Komunjer L, Dalmazzone C (2005) Morphology characterization of emulsions by differential scanning calorimetry. Adv Colloid Interface Sci 117:59
Clausse D, Gomez F, Dalmazzone C, Noik CJ (2005) A method for the characterization of emulsions, thermogranulometry: application to water-in-crude oil emulsion. J Colloid Interface Sci 287:694
Elwell MW, Roberts RF, Coupland JN (2004) Effect of homogenization and surfactant type on the exchange of oil between emulsion droplets. Food Hydrocolloids 18:413
Gasperlin M, Kristl J, Smid-Korbar J (1994) Kerc, The structure elucidation of semisolid w/o emulsion systems. J Intl J Pharm 107:51
Cerqueira MA, Fasolin LH, Picone CSF, Pastrana LM, Cunha RL, Vicente AA (2017) Structural and mechanical properties of organogels: role of oil and gelator molecular structure. Food Res Intl 96:161
Toro-Vazquez JF, Morales-Rueda J, Torres-Martínez A, Charo-Alonso MA, Mallia VA, Weiss RG (2013) Cooling rate effects on the microstructure, solid content, and rheological properties of organogels of amides derived from stearic and (R)-12-hydroxystearic acid in vegetable oil. Langmuir 29:7642
Toro-Vazquez JF, Mauricio-Pérez R, González-Chávez MM, Sánchez-Becerril M, Ornelas-Paz J, Pérez-Martínez JD (2013) Physical properties of organogels and water in oil emulsions structured by mixtures of candelilla wax and monoglycerides. Food Res Intl 54:1360
Lupi FR, Greco V, Baldino N, de Cindio B, Fischer P, Gabriele D (2016) The effects of intermolecular interactions on the physical properties of organogels in edible oils. J Colloid Interface Sci 483:154
Patel AR, Schatteman D, De Vos WH, Lesaffer A, Dewettinck K (2013) Preparation and rheological characterization of shellac oleogels and oleogel-based emulsions. J Colloid Interface Sci 411:114
Acknowledgements
Author J.I. Contreras-Ramírez is grateful for graduate scholarship from CONACYT (National Council of Science and Technology) Mexico. Funding from the Strengthening and Development of Scientific and Technological Infrastructure program (Grants Nos. 253333 and 224651) and the Basic Science program (Grant No. 241241) also from CONACyT is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Contreras-Ramírez, J.I., Gallegos-Infante, J.A., Pérez-Martínez, J.D. et al. Influence of vegetable oil, monoglycerides and polyglycerol polyricinoleate into the physical stability of organogel-emulsion (w/o) systems. SN Appl. Sci. 2, 1343 (2020). https://doi.org/10.1007/s42452-020-3144-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3144-y