Abstract
Interest in biochar production from organic waste has been growing in recent years due to its broad applicability, availability, and smoother production. Biochar production techniques are being continuously modernized to improve the production rate and quality. Though numerous methods have been reported in the recent past, a systematic classification of the same is yet to be explored. Based on the advancement of the techniques being employed for biochar production and modification of conventional methods, we have categorized all major techniques of biochar production into two primary classes. In the traditional approach, ancient methods and conventional pyrolysis techniques (Slow and Fast pyrolysis) are included, whereas, in modern approaches, several advanced technologies such as Gasification, Torrefaction, Hydrothermal carbonization, Electro-modification, along with modified traditional methods (Flash pyrolysis, Vacuum pyrolysis, and Microwave pyrolysis) are comprised. Further, the systematic review was intended to evaluate various types of feedstocks (agricultural biomass, forest/woody biomass, aquatic biomass, urban waste, and paper waste) with their potential to produce biochar. It was observed that the feedstock containing high cellulose was found to be helpful in improving the overall properties of biochar, including enhanced adsorptive action and retention of nutrients.
Similar content being viewed by others
1 Introduction
Population explosion, rapid industrialization, and urbanization result in the massive generation of organic wastes, including agro-wastes, municipal solid waste (MSW), industrial waste, sea waste, forestry waste, etc. A small fraction of agro and forestry waste is utilized in domestic and in-farm activities such as cattle feeding, cooking, composting, and biogas production [1]. However, a major portion gets disposed of either by burning or dumping in the fields or landfill that results in air, water, and soil pollution. Thus, several researchers recommended the composting of organic wastes [1,2,3,4]. However, its slow degradation rate and laborious operation make it an unattractive choice [5]. Therefore, the utilization of organic wastes for biochar production could offer a solution to the existing issues. Biochar production is a rapid process that is also economically feasible due to the value of the final product obtained in the process. Further, it has other potential benefits such as improving soil fertility, encouraging seed germination, enhancing vegetative growth of plants, increasing disease resistance of soil, adsorbing toxic pollutants, improving water retention capacity of the land, etc. [6, 7]. Besides, biochar can be used as energy fuel and carbon sink. Biochar is a carbon-rich solid by-product produced through high-temperature pyrolysis or degasification of organic material under low or no oxygen environment, which prevents combustion. The relative yield of product formation in pyrolysis varies with temperature. More char is produced at temperatures between 400–500 °C (752–932 °F), while temperature above 700 °C (1292 °F) favors the yield of liquid and gas fuel components [8]. High-temperature pyrolysis (above 700 °C), which is also known as gasification, can produce biochar as well. However, the yield in gasification was found to be relatively low [9, 10].
The production and application of biochar for improving soil fertility is an old tradition commonly used by farmers from India, Europe, China, Japan, and America. It is being produced by smoldering agricultural waste in pits or trenches [11]. As per their views, the amelioration of soil with biochar can improve the retention of nutrients in the soil, which ultimately increases soil fertility [11, 12]. Several reports suggested that the effect of biochar on soil fertility and crop productivity showed a positive impact, especially where the biochar is mixed with fertilizers [13, 14]. Biochar amendment also improved the seasonal NPP (net primary production) accumulation arising from atmospheric CO2 assimilation [15]. Mosses, which generally grow on peatland, need phosphorus for the vegetative growth. Phosphate rock fertilization is generally used for such peatland restoration [16]. However, the use of biochar could significantly assist the ecological restoration that helps the recovery of the degraded, damaged, or destroyed ecosystem. Further, soil amendment with biochar enhances the nutrient uptake, which reduces dependency on chemical fertilizer and is essentially important in developing countries such as India, where most of the farmers cannot afford chemical fertilizers. Nevertheless, there are several reports surfaced in the recent past stating neutral or negative plant growth responses to the soil amended with sole biochar [17, 18]. Therefore, research needs to be focused concerning the effects of biochar on the increment of nutrient availability, seed germination, vegetative growth, and enhancement in protein and chlorophyll content. Feedstock composition, pyrolysis conditions, and biochar production methods are the vital factors controlling the physical and chemical properties of the resulting biochar and eventually deciding its end application. Thus, the present review emphasizes on feedstocks for biochar production, biochar production techniques with their modernization and application of biochar with special focus on soil amendment, soil fertility, crop productivity, and nutrient availability. The systematic review was intended to evaluate the recent advancement in the field of biochar production along with its applications. In order to obtain all relevant data, multiple engines (Web-of-Science and Scopus) were employed in a systematic search. Considering the recent reports published in the last decade, state–of–art examples were primarily considered while approaching a systematic review of the same. Though numerous examples are considered and included from distinct regions of the world, special attention was given to the reports from India to get an insight into the current scenario in the country.
2 Feedstock availability for biochar
Feedstock availability and its composition are some of the most important factors for the efficient and economical production of biochar. Though there is a huge availability of feedstocks, their proper classification and characterizations are essential for its appropriate utilization. Thus, the present section emphasizes on the feedstock resources, compositions, and their availability. A wide variety of feedstocks being used for biochar production comprising agricultural residues, urban waste, paper waste, woody biomass, aquatic biomass, animal and human excreta, industrial waste, food and kitchen waste, dairy and paper mill waste, poultry waste, etc.
2.1 Agricultural biomass
Huge quantities of agro-wastes are being generated through agricultural operations all over the world and developing countries in particular. As per the report of the Imperial College Centre for Energy Policy and Technology, the total land area across the world is about 13 Gha, of which 1.5 Gha accounts for agricultural operation [19]. Total land occupied by India is 0.297 Gha, of which 10.57% (0.0314 Gha) constitutes for agricultural operations, where the total agricultural waste generation in India is about 600 MT [20]. The agro-waste is primarily composed of cellulose, hemicellulose, and lignin [21]. Cellulose is the most abundant organic material on the earth, and approximately 4 × 1010 t of cellulose is produced annually by plants [22]. Cellulose is insoluble in water. It has high tensile strength, and much higher tolerance to degradation compared to glucose and starch. In its natural form, cellulose is a linear polymer containing thousands of glucose units linked together by β-1,4 glycosidic linkage. Cellulose is highly resistant to microbial and chemical degradation. Cellulose binds with lignin and hemicelluloses by ether and hydrogen bond, respectively [23, 24]. Hemicellulose is a complex, branched, and heterogeneous polymeric network and structurally similar to cellulose. Glycosidic linkage (β-1,4) connects pyrosyl units. Hemicellulose is a polymer of a pentose sugar, especially xylose unit; however, other pentoses (arabinose, mannose, galactose, etc.) are also present. The composition of hemicellulose in softwood (grasses, agricultural waste, and coniferous tree) and hardwood (forest waste and woody biomass) are different. Though the hemicelluloses in softwood are composed of galactan (arabino) and glucans (xylo), the major portion is comprised of glucomannan along with arabino-glucuronoxylan, and galacto-glucomannan [25]. Nevertheless, softwood contains glucomannan, a primary form of hemicellulose. The presence of a profoundly branched structured polymer chain connected via acetyl groups brings a lack of crystallinity to the hemicellulose structure. Typically, hemicellulose can facilitate hydrolysis at a lower temperature than cellulose, which allows it to be water-soluble in the presence of acids at elevated temperatures. After cellulose and hemicellulose, lignin is found in lignocellulosic biomass with an abundance. Lignin is an integral part of plant cells and mainly found in grasses, soft/hardwood, algae, etc. It is a highly complex arrangement than cellulose/hemicellulose conferring overall rigidity to the plant structure. Typically, the lignin polymer incorporates aromatic alcohols (i.e., coniferyl, sinnapyl, and p-coumaryl) in its structure. Despite its strengthening role in plants, lignin plays several biological as well as ecological functions. Lignin fills spaces in the cell wall between cellulose, hemicelluloses, and pectin components, especially in tracheid, sclereid, and xylem cells. It is covalently linked to hemicelluloses and, therefore, crosslinks different plant polysaccharides, conferring mechanical strength to the cell wall and by extension, the plant as a whole. It is particularly abundant in compression wood but scarce in tension wood. Lignin plays a crucial part in conducting water in plant stems. Polysaccharide components of plant cell walls are highly hydrophilic and thus, permeable to water, whereas, lignin is more hydrophobic [26]. Compositional analysis of selected agro-wastes is depicted in Table 1. It was estimated that the cellulose (35–50%), hemicelluloses (15–40%), and lignin components (15–25%) makes the composition of agro-waste [1, 24]. Most commanly used agricultural waste for biochar production is cotton stalk [28], rice and wheat straw [26, 27], maize stover [31, 32], corn straw [33], sugarcane bagasse [30], below-ground peanut biomass and switchgrass [34], etc.
Biochar from agricultural wastes, which are mostly rich in cellulose fiber, shows a significant influence on nitrogen and nutrient uptake from the soil and also provides a home to various kinds of soil biota, which increases soil fertility [29, 31]. Coconut shell and Palmyra nutshell are generally used for biochar production by anaerobically burning at 400 °C [35]. Similarly, hazelnut shells, grape seed, and chestnut shells have also been employed for biochar production [36].
2.2 Urban waste
Due to rapid urbanization and uncontrolled population growth, urban waste/municipal solid waste (MSW) has become a big challenge not only for India but also for most of the developing and developed countries. Urban waste is composed of organic and inorganic fractions (Fig. 1). Organic fraction is classified into biodegradable and non-biodegradable fractions. Biodegradable organic fraction is a collection of food remnants, kitchen, fruits and garden wastes, cloths, papers, leather materials, etc. [37]. A non-biodegradable organic fraction consists of plastic bags, bottles, and electronic waste, while inorganic fraction contains glassware, electric waste, metals, sandstone, etc. [38]. Most of the non-degradable organic and inorganic fractions are recyclable, while biodegradable fractions decompose biologically. The rapid industrial growth increases the urban population, which in turn increases urban waste generation. Such a huge generation of waste gives a burden to Municipal Corporations for their proper management. As per the report [39], nearly 90 million tons of MSW has generated annually in India, with 0.337 t per generation capita rate [20, 40]. The organic fraction of urban waste, which is nearly 30–45%, has the potential to produce energy, soil conditioner, nutrient-rich manure or compost, and biochar. The segregated organic fraction is useful for biogas generation in various dumpsites. A lot of municipalities have started composting of organic waste all over the world. Urban wastes are one of the most potent feedstocks for biochar production and can be categorized into MSW, industrial wastewater, sewage sludge, livestock, and poultry wastes. MSW is used for biochar preparation, which is further used as an adsorbent for the removal of dyes, minerals, pollutants, toxicants, etc. Several researchers have used MSW to produce biochar [41,42,43,44,45,46,47]. A group [41] prepared biochar from MSW by using pyrolysis in a custom-designed packed bed reactor at 400–800 °C, which was further used to remove the azo dye. In another report [48], researchers used MSW biochar for the prevention of pollution from landfill leachate. Similarly, the effect of MSW biochar on the removal of aqueous arsenic (V) from wastewater was studied [42].
2.3 Paper waste
The waste paper constitutes a considerable share of municipal and industrial wastes even though recycling efforts have been strengthened in recent years. According to a study [49] and Food and Agricultural Organization report (FAO) [50], the paper production in India is 10.5 × 109 kg y−1. It was further estimated that almost 5.7 × 109 kg y−1out of the total paper and cardboard production in India, is collected and disposed of in the form of mixed MSW. In another study, MSW constitutes 7–12% of paper waste, which contributes 6–9 MT per year in India, which also matched with the estimates [49]. As per FAO data, 1.9 × 109 kg of paper reused per year; however, the remaining portion (nearly 4.8 × 109 kg) dumped into landfill sites. When paper waste is recycled repeatedly, it loses its quality. The waste paper could be used as an excellent source of lignocellulosic biomass for ethanol production as it contains a significant and underutilized source of cellulose. Similarly, it should be a good resource for biochar production concerning high cellulose content.
2.4 Forest/woody biomass
Russian Federation, Brazil, Canada, the United States of America, China, the Democratic Republic of the Congo, Australia, Indonesia, Sudan, and India are forest mega-biodiversity regions of the world, which constitute more than 67% of the total forest [50]. The 2013 forest survey of India documents its forest cover of 69.8 million ha. As per the report issued by FAO [51], India produces 3000 metric tons of paper annually, consuming nearly 10,000 metric tons of wood. Nearly 3000–3500 million cubic tones wood is used as raw material for furniture and craft industry, fuelwood, fodder, and value-added products. Wood is also an important feedstock for biochar production concerning the quality. Biochar from wood source shows more calorific value due to the presence of lignin, resin, pectin, and volatile materials. Several researchers have worked on the effective production of biochar from a wood source, e.g., Lai et al. [52] used hardwood, whereas Dong et al. [53], and Hu et al. [54] used Douglas fir wood chips and woody shavings for biochar production, respectively. Though wood residues are a good feedstock for biochar production, it must be used with care as it may provoke deforestation.
2.5 Aquatic biomass
Aquatic biomass includes algae, giant kelp, other seaweeds, and marine microflora with phytoplankton and zooplankton. The dry floor of rivers, lakes, and ponds is the richest source of such aquatic waste. Similarly, a significant amount of waste generates after fishing and other activities. Among the aquatic wastes, algae are a prominent and diverse group of primarily aquatic organisms, often fast-growing and able to grow in freshwater, seawater, or damped oils. They may be unicellular or microscopic and multicellular or macroscopic. According to Bird et al. [55], algal biochar is more nutritionally rich than lignocellulosic biochar. The concentration of macronutrients (N, P, K, Ca, and Mg) and micronutrients (Mo) is higher in algal biochar than lignocellulosic biochar. However, the applicability of algal biochar is limited due to the high cultivation and collection cost of algae. Also, cultivated algae have established markets as food, alginate, agar, and carrageenan, which renders more commercial value. Hence, algal biochar for bioremediation does not compete with these existing markets [56]. However, there are several algal species that have been studied for biochar production [55,56,57,58] (Table 2).
3 Methods of biochar production
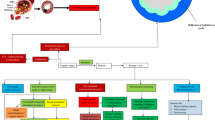
Charcoal forms either naturally as a result of vegetation fire or intentionally induced fire by humans employing burns pits and hand-made structures. A biochar production occurs under anaerobic (limited supply of oxygen) condition. Though a lot of biochar production methods are available in the literature, the proper classification is not available. Thus, in the present section, different approaches for biochar production are highlighted. The biochar production methods are mainly classified into traditional and modern approaches on the basis of their advancements and modernization (Table 3).
3.1 Traditional approaches
Archaeological evidence suggests the production and utilization of biochar by humans started over 2500 years ago. The first evidence was found in the Amazon Basin of South America [72], which was referred as Terra Preta conferring three times higher soil organic matter content and nutrient levels [73]. The ancient people used to pile the wood covered in the soil pits and to burn it slowly with limited or absence of air [74, 75]. In another mode, people used to burn the biomass in open space and immediately cover the half-burned biomass with soil [76, 77]. In ancient times, the soil amendment was not the only application of the production of biochar. The liquid product was also produced from the burning of wood and was used for various purposes such as preservation of dead bodies and meat, house painting, caulking for sealing wood barrels, shipbuilding, and to attach arrowheads to spear shaft [76, 77]. With the continuous evolution of humans and the advancement of science, several traditional biochar production approaches were replaced by modern approaches (Table 3). Handmade reactors such as firebrick pits, clay burners, brick kilns, and iron retorts were employed in the production of biochar. In every method, the common thing was a pit that is surrounded by clay (Clay burner), bricks (Firebrick pits and Brick kilns), and metal (Iron retorts). Such modified methods of biochar preparation are useful in recovering and utilization of volatile compounds produced from pyrolysis [77]. These methods were frequently used until the end of the 19th century and up to the development of labor and time-saving steel ovens [76, 77]. The application of steel oven in biochar production does increase the production rate while improving the standard of biochar quality. Further, it is also useful in the recovery of volatile compounds and bio-oil.
3.1.1 Pyrolysis
Pyrolysis is a thermal degradation process where biomass is heated under anaerobic conditions or a limited supply of oxygen to produce various gaseous and aqueous products as well as char residues (biochar) [78]. Agricultural biomass is composed of lignin, cellulose, hemicelluloses, and silica. Generally, the pyrolysis point of cellulose is 350 °C, whereas lignin melts above 350 °C [79]. Thus, the effective temperature range for pyrolysis was found to be 300–700 °C [80]. Though pyrolysis is an anaerobic heating process, it needs other means of heating, such as hot gases, hot solids, liquid heat transfer media, oxidation, and partial oxidation reactions [81, 82]. Pyrolysis processes have been evolving for decades. Depending upon the process parameters such as temperature, heating rate, and residential time, it is further divided into various modes such as slow and fast pyrolysis. Slow and fast pyrolysis regards to traditional techniques. However, flash pyrolysis, vacuum pyrolysis, and microwave pyrolysis are modern techniques that were modified using modern technologies. Therefore, slow pyrolysis and fast pyrolysis have been classified and added in the section comprising “traditional approaches”. In contrast, flash pyrolysis, vacuum pyrolysis, and microwave pyrolysis have been added in the section containing “modern approaches” (Table 3).
3.1.1.1 Slow pyrolysis
As the name indicates, slow pyrolysis takes several hours to complete the process and produces biochar as a major product. Slow pyrolysis, also known as conventional pyrolysis, where biomass is heated at the temperature in the range of 300–600 °C with a heating rate of 5–7 °C min−1 [52]. Slow pyrolysis yields biochar as a major product (35–45%) along with other products as bio-oil (25–35%), and syngas (20–30%) [52, 67, 83]. A continuous auger/screw pyrolyzer reactor is generally used in the slow pyrolysis [83]. Lai et al. [52] used wood chips to produce biochar by slow pyrolysis, keeping temperature range between 290 to 700 °C with a heating rate of 3 °C min−1 for 2 h. Similarly, Mendez et al. [67] used deinking sludge for the production of biochar by slow pyrolysis process in covered steel cup by employing an electric furnace at a heating rate of 10 °C min−1 for 2 h. Different types of feedstocks have been used to produce biochar by slow pyrolysis such as Conocarpus wood wastes [66], cotton stalks [59], coconut shell, palmyra nutshell, and rice husks [35]. Another report [84] conducted slow pyrolysis with sawdust, bull manure, pinewood, oak wood, dairy manure with rice hulls, hazelnut shells, corn, food waste, and white paper mill sludge in a Daisy Reactor at Best Energies Inc. USA and concluded that the type of pyrolysis and composition of feedstock play an important role in the chemical composition of biochar. A similar experiment was also carried out by Roberts et al. [6] with four different feedstocks using slow pyrolysis in a sealed stainless-steel retort inside a muffle furnace with the inert condition for 60 min and reported that the pyrolysis temperature and biomass rinsing pre-treatment affect the yield of biochar. The yield of biochar decreased with increasing temperature, and the most effective biochar was produced from un-rinsed Ulva processed as flake at a pyrolysis temperature of 300 °C. As per a report [61], a slow heating rate was found to be more effective than a fast heating rate for biochar production.
3.1.1.2 Fast pyrolysis
Fast pyrolysis is nothing but a high-efficiency thermochemical process to produce biomass-derived biofuels [85]. The advantages of fast pyrolysis include short retention time and high product recovery. However, the major products are bio-oil and syngas rather than biochar when subjected to the upgrading process for the production of liquid transportation fuels or fuel additives [86]. The operation of fast pyrolysis is carried out at a temperature above 500 °C with a heating rate of more than 300 °C min−1 in the absence of oxygen. Fast pyrolysis is a rapid process of biochar production and takes seconds to complete. The product yield of fast pyrolysis is reported as 60% bio-oil, 20% biochar, and 20% syngas [85, 86]. It is mostly applied for large scale biochar production. Liu et al. [87] prepared biochar by fast pyrolysis of biomass in a fixed-bed quartz reactor equipped with a temperature controller and a furnace. The furnace was heated to the desired temperature (200–330 °C) with a heating rate of 15 °C min−1 and kept at the desired temperature for less than 20 min. Due to the low temperature [87], more biochar yield was obtained when compared to bio-oil and syngas. A group [88] used the biochar for removal of 4-nitroaniline, salicylic acid, benzoic acid, and phthalic acid from water, and concluded that the biochar from fast pyrolysis has more advantageous for adsorptive actions. According to another report [89], adsorptive removal of salicylic acid and ibuprofen from aqueous solution by employing pinewood pyrolysis biochar was studied, which was prepared by fast pyrolysis at a temperature of 425 °C and residence time of 20–30 min. Moreover, a group [53] prepared a low-cost catalyst from pyrolysis-derived biochar using fast pyrolysis in an Auger pyrolysis reactor at 600 OC for 1 min, which was used for pre-esterification in biodiesel production. Another group [90] prepared biochar by fast pyrolysis from hardwood, which showed a positive impact on soil quality and yield of crop biomass.
3.2 Modern approaches
At the end of the twentieth century, several modifications and changes in biochar production methods were surfaced. Eventually, various approaches have been developed for biochar production, such as modern pyrolysis (flash-, vacuum-, and microwave-pyrolysis), gasification, torrefactions, hydrothermal carbonization, electro-modified techniques, etc. (Table 3).
3.2.1 Gasification
Gasification is a common technique for producing syngas from different solid fuel resources. In comparison with other conventional methods, i.e., pyrolysis, combustion, and fermentation, gasification provides larger syngas volume and lower Levelized emissions. Hydrogen is a major product of gasification. However, a considerable amount of biochar can also get generated during the gasification process. Moreover, biochar generated during gasification is considered as a waste and has several important applications such as dye removal from wastewater, adsorption of chemicals, carbon sequestration, and as a soil amendment agent [91, 92]. Gasification is an effective thermochemical conversion process for biomass into energy fuel while producing biochar as a byproduct [9]. Typically, carbonaceous materials derived from organic fossil fuels can be converted into hydrogen, carbon monoxide, and carbon dioxide employing gasification. In this process, at high temperatures (> 700 °C), the reaction of materials is carried out under a controlled supply of steam and oxygen [10]. As per the requirement of the desired gas composition, varying gasifying agents can be employed in the process [93]. A gas holding a higher content of hydrogen can be obtained via supplying steam that helps to elevate the heating value of syngas [94]. During the gasification process, heat transfer within a particle that increases the localized temperature of biomass leads to the removal of water and follows by the progressive release of pyrolytic volatiles. The precursors of primary volatiles are cellulose, hemicellulose, lignin, and extractives comprising permanent gas species (e.g., CO2, CO, and CH4). Though biomass component decomposes at different temperatures, the overall decomposition ends nearly in the range of 400–500 °C, where biochar is a prime product. Biochar is further converted into fuel at a higher temperature, where secondary decomposition occurs with a variety of secondary reactions to form syngas composed of hydrogen and methane [95]. Another group [96] used sewage sludge for the production of hydrogen and biochar by gasification with the introduction of steam at 800 °C and reported 35–45 vol % hydrogen with a significant amount of char. Coal and petroleum coke are used as the primary feedstock for many large gasification plants worldwide. Municipal solid waste, agricultural and industrial waste, sewage sludge, etc. are also being used as a feedstock while performing gasification operation. The same group [96] also has studied the production of biochar employing gasification where wood pellets, sewage sludge, rapeseed, and miscanthus were utilized in a quartz tubular reactor using steam. Similarly, some other group [97] used steam gasification for pine sawdust and reported an increase in hydrogen volume fraction with increasing temperature as a result of further cracking at 850 °C.
3.2.2 Torrefaction
Burning of the biomass at relatively low temperatures (230 and 300 °C) improves the properties of biomass and widely termed as torrefaction [98, 99]. Alternatively, torrefaction, which can be regarded as a pretreatment step to improve the physical, chemical, and biochemical characteristics of raw biomass, makes the biomass perform better for combustion, gasification, and co-firing purposes [100, 101]. In this process, the hemicellulose fraction of the wood decomposes so that torrefied wood and volatiles are formed, which are more stable and carbon-rich solid products [102]. Torrefaction is the thermochemical treatment of biomass, which is carried out in the absence of oxygen under atmospheric pressure. A fixed bed reactor is generally used for the torrefaction process. Several researchers demonstrated that torrefaction and densification technology was an effective method to form torrefied wood pellets of superior quality, compared to the raw controlled pellets. There are several studies focused on torrefaction of agricultural and forest residues [102,103,104] investigated torrefaction of three agricultural wastes, i.e., coffee residue, sawdust, and rice husk followed by examining the influence of torrefaction on properties and structure of biomass through proximate, elemental, fiber, calorific, thermogravimetric, SEM, and FTIR analysis [105]. It was concluded that the hemicellulose decomposition was more in coffee residue, while all biomass became more hygroscopic compared to raw biomass. The effect of temperature during the torrefaction process on biomass was also investigated by a group [105] using four different varieties of biomass, including bamboo, willow, coconut shell, and wood while focusing on compositional changes. The group concluded that hemicellulose decomposition was observed prominently at temperature 240 °C while cellulose decomposition occurred at severe torrefaction (above 280 °C).
3.2.3 Flash pyrolysis
Flash pyrolysis is an improved and modified form of fast pyrolysis. In flash pyrolysis, biomass decomposes at high temperatures, i.e., more than 1000 °C within a short period, mostly less than a minute. The heating rate sometimes is more than 1000 °C sec−1. Flash pyrolysis is operated at temperatures ranging from 900 to 1200 °C, which can be attained within a second [106, 107]. Such a rapid heating rate with high temperature and low vapor residence time lead to a high bio-oil yield. However, it reduces the biochar yield in the process [108]. Though flash pyrolysis is carried out in a fluidized bed reactor and twin-screw mixing reactor, its industrial applicability is very limited due to the construction of the reactor to operate at high temperature with extremely high heating rate.
3.2.4 Vacuum pyrolysis
Vacuum pyrolysis is a thermal degradation of biomass under vacuum or low pressure in the absence of oxygen. Pressure and temperature range during the vacuum pyrolysis are controlled between 0.05 and 0.20 MPa and 450–600 °C, respectively [19, 109, 110]. Similar to pyrolysis, the heating rate in vacuum pyrolysis is low. Though the heating condition and heating rate are similar to slow pyrolysis, end products are significantly different from each other. This is due to the effective removal of vapor during vacuum pyrolysis. In the vacuum pyrolysis, only vacuum or low pressure is used to remove the vapor generated during pyrolysis that shows a significantly good impact on product quality and yield due to the prevention of devolatilized inorganic [110, 111]. Another group [111] demonstrated that different chemical reactions, i.e., cracking and volatiles-char interactions, are evident to produce bio-oil containing high water content with biochar during vacuum pyrolysis. Vacuum pyrolysis is highly used to produce high-quality biochar, which shows high porosity and, thus, highly useful in adsorption of mineral and nitrogen while applying as soil amendment [112].
3.2.5 Hydrothermal carbonization (HTC)
The high moisture containing feedstocks such as sewage sludge, animal waste, and compost are converted into biochar with the help of hydrothermal carbonization (HTC) method [113]. In the HTC method, there is no need to dry the biomass before processing, where the wet biomass mixture is heated up to temperatures ranging 220–240 °C under high pressure (2–10 MPa) reactor for several hours. HTC process is operated in rotary drums, kilns, and stoves. Most organics HTC processes remain either in dissolved form or transformed into brown coal [114]. HTC process is useful to generate various carbonaceous materials with different sizes, shapes, surfaces, and functional groups. However, most of the initial carbon remains in the soluble form [115]. Important characteristics of HTC produced biochar is the retention of nutrients such as N and P, which is useful in soil fertility. The advantages of HTC process over torrefaction or pyrolysis process for biochar production include a significant reduction in O/C ratio, increased calorific value, better grind ability, and improved hydrophobicity [116]. Various studies on the HTC process for biochar production and their application as soil amendment was conducted by the various researcher [116, 117]. A group [117] prepared loblolly pine hydrochar at the temperature ranging from 200 to 260 °C and observed a 30% increase in calorific value. A similar observation was noted by Yang et al. [118] with biochar produced from nut husk as a feedstock. Reza et al. [119, 120] used pelletized hydrothermal carbonized loblolly pine char to study the effects of biochar on hydrophobicity, abrasion resistance, and density. The group observed a significant increase in these properties of biochar. According to another group [121], hydrothermal carbonized pellets showed considerably superior physicochemical properties when compared to the raw and torrefied pellets. Thus, they concluded HTC is a more promising technology for biochar production compared to torrefaction and hydrothermal.
3.2.6 Microwave pyrolysis
Biochar production through microwave heating is a prominent advanced technique. Microwave heating is advantageous over conventional heating as microwave generates thermal energy through dielectric heating, and the energy is introduced into the reactor remotely without making any contact between the energy source and the reaction mixture [122,123,124]. It is a more rapid and material-selective heating technique than the conventional one [125, 126]. Microwave technology has drawn attention in academic and industrial fields for outstanding thermal characteristics due to rapid, selective, and uniform heating while offering decreased sintering temperature that enhances steam gasification. Biochar produced through microwave heating has more advantages over conventional pyrolysis technique as it reduces temperature requirement by 200 °C while achieving similar results [86]. Several researchers have focused their attention on microwave processing for biochar production. A group [127] investigated experimental conditions for maximum biochar and hydrogen production and reported 450 °C temperature, 400 W microwave power, and 4–6 min resident time as the best conditions for microwave pyrolysis for biochar production. However, maximum hydrogen production was obtained at 700 °C temperature, 400 W microwave power, and 4–6 min retention time. They further studied biochar quality and reported biochar produced through microwave pyrolysis shows more calorific value than conventional biochar. A similar observation was reported by Menedez et al. [128], where they prepared biochar from four types of wet sewage collected from different wastewater treatment plants. A group [128] pyrolyzed the material at 2450 MHz microwave frequency, and 800 °C for 4 min residence time. In another experiment, Huang et al. produced biochar from rice straw through microwave pyrolysis at a frequency of 2450 MHz by using a single-mode microwave device. Moreover, the biochar produced by microwave pyrolysis could potentially be used for CO2 adsorption [129].
3.2.7 Electro-modified biochar
The adsorption capacity of biochar is useful to remove pollutants from soil, water, and air. Also, it is useful in the adsorption of nutrients. The medication of biochar should be done in such a way that it adsorbs a particular group of compounds while easily removing undesired moiety from a particular environment. Such modification of biochar carried out by chemical treatment, and the resultant biochar is termed as modified biochar. The chemical treatment includes mixing of biochar in Fe, Mg, or Al for 2–12 h in the presence of electric current that might alter functional groups on the surface of pores and ultimately improves specific adsorption [130,131,132,133,134,135]. Modern, simple, and time-saving approaches for preparing modified biochar involve the application of an electric field. By this method, i.e., electro-modification, enhancement of the biochar surface area along with impregnation of chemicals on the biochar surface occurs. For such an electrochemical process, strong oxidant, e.g., hypochlorous acid/hypochlorite ions (HOCl/OCl), aluminum ion (Al+3) can be produced by chemical reaction or aluminum electrodes at acidic pH [130]. Jung et al. [130] produced electro-modified biochar by an aluminum electrode-based electrochemical process where a dried brown marine microalga, Laminaria japonica, was used as a feedstock. Macro-alga was dipped in 200 mL of deionized distilled water, stirred for 150 rpm, and supplied current with a density of 0–100 V and 0–12 A for 5 min. After treatment, the macro-algae were separated using filtration followed by pyrolysis at 450 °C at a rate of 5 °C min−1 under an inert atmosphere. The resultant biochar showed improved surface area and nano-sized crystalline beohemite on the biochar surface, which enabled higher adsorption capability for phosphate from aqueous solution. In another parallel experiment, Jung et al. [130] used MgCl2 to improve texture properties of biochar where Mg–Al assembled nano-composites (MgO, spinel MgAl2O4, AlOOH, and Al2O3) were successfully dispersed on the biochar surface with a highly crystalline structure to enhance the phosphate adsorption capability up to several folds.
3.2.8 Magnetic biochar
The enhanced adsorption capacity with complete recovery of adsorbent material has attracted many researchers worldwide, which can intensify research towards novel adsorbents. The magnetic biochar shows tremendous adsorption capacity with complete separation and recovery from water or pollutant site. Magnetic biochar, which is derived from various types of biomass, exhibits a good magnetic property with high surface area and significant morphology through various production methods. Magnetic biochar has been prepared from the addition of Fe ions on the surface of biochar with the help of a binding agent from the chestnut shell at temperature 450 °C under microwave heating. A group [136] prepared magnetic biochar by the addition of gelatine and iron. Another group [74] prepared magnetic biochar using the biomass in a programmable muffle furnace model Wise Therm, at 1000 °C, under vacuum condition with the addition of iron (III) oxide (Fe2O3), iron (II) sulfate heptahydrate (FeSO4.7H2O) and iron (III) chloride hexahydrate (FeCl3.6H2O). Magnetic biochar is useful in adsorption of various pollutants such as arsenic from wastewater [136], Pb+2, and Cu+2 from industrial wastewater [137], and Zn+2 from another source of wastewater [138]. A group [136] demonstrated magnetic biochar as an environment-friendly and low-cost arsenic removal candidate. The adsorption capacity with the application of magnetic biochar increases up to 3–4 folds when compared to conventional biochar. Similar observations were reported by several researchers [74, 133, 137, 138]. Magnetic biochar/c-Fe2O3 was prepared by immersing biomass into the prepared FeCl3 solution for 2 h. The mixture was then dried at 80 °C for 2 h under atmospheric air. The pretreated biomass was pyrolyzed in a furnace at a temperature of 600 °C in N2 supplied environment for 1 h. Biochar/c-Fe2O3 composite produced from the pyrolysis was gently crushed, sieved, and further analyzed.
4 Applications of biochar
4.1 Effects of biochar amendment on adsorption of inorganic nitrogen and phosphate
Nitrogen present in the soil is the most vital element for plant growth, which is present in two forms, i.e., organic and inorganic nitrogen. Most of the organic nitrogen gets converted into inorganic ammonium and nitrate form, which is absorbed by plants [139]. Soluble inorganic nitrogen gets adsorbed on the soil surface, which is utilized by plants. However, various microbial activities cause degradation and removal of nitrogen. Thus, biochar acts as a soil additive and reduces nitrogen loss and ultimately improves soil fertility [140]. Though there are contradictory reports regarding nitrogen adsorption when biochar is applied, most of the report supports a positive impact on the adsorption of soluble nitrogen on soil [141,142,143,144,145]. Several reports suggested that the chemical groups present on the surface of biochar are responsible for the adsorption of nitrogen [146, 147]. Acid functional groups include carboxylic, hydroxyl, lactone, and lactol, which are negatively charged groups, are effectively bind to NH4+ by electrostatic attraction [63, 148]. Similarly, the existence of base functional groups, including chromenes, ketones, and pyrones on biochar, can facilitate NO3− adsorption on biochar [148, 149]. The efficacy of nitrogen adsorption on biochar is also dependent on the time of process and temperature. Old biochar adsorbs more NH4+ than the newer form as hydrophilicity increases during aging [150]. Biochar prepared at high temperature (˃ 600 °C) shows decreased cation exchange capacity (CEC) as acidic functional groups (mainly carboxyl) are converted to neutral or basic fused aromatic groups [151,152,153]. Thus, biochar prepared at moderate temperature was found to be best for the sorption of soluble nitrogen. Moreover, the biomass type also influences the nitrogen adsorption capacity of biochar. Biochar from grassy biomass shows more adsorption than woody biomass due to the presence of more carboxylic groups [152, 154].
Phosphorus is the second most important plant nutrient and essential element in DNA metabolism. It is present in phosphate form in soil or rock. To make it available for plants, a solubilized form of phosphate is crucial. Phosphate solubilizing microorganisms solubilize phosphate that gets adsorbed on biochar and makes it available to plants for a longer duration. One of the most important properties of biochar is the ability to absorb various chemicals, nutrients, and heavy metals on their surface and make them available for plants for a longer period due to its large surface area, porous structure, large cation exchange capacity, and abundant functional group [155,156,157]. Several studies state that biochar could prevent leaching of nitrogen, phosphate, and other nutrients from the compost and make them available for plants [158, 159]. These findings also supported by several other groups [160, 161]. They have reported that the amendment of hardwood biochar to soil would decrease the leaching of nitrogen and phosphate. Moreover, a group [162] demonstrated nutrient conservation of soil by using spent mushroom substrate derived biochar, and both nitrogen and phosphate could get adsorbed on the surface while preventing it from leaching through composting. Zhang et al. reported the concentration of alkaline nitrogen (29%), available phosphorus (77%), and available potassium (100%) significantly increased in the biochar amendment [134]. The group further compared the sorption capacity of biochar against activated carbon and found that the sorption capacity of the biochar for NH4 + was much higher than that on the activated carbon.
4.2 Effects of biochar on soil structure
Incorporation of biochar may improve the physical structure of the soil, especially increased porosity, surface area, water adsorption and holding capacity, oxygen uptake, etc. [163,164,165]. Increased surface area and pore structure are essential to colonize the soil bacteria and fungi, which are useful in the absorption of nutrients from soil [165]. Increased porosity of soil could help it to maintain moisture and aeration, which is essential to microbial life and thus stimulates nitrifier activities [133, 166]. Several other groups demonstrated the stimulation of nitrification due to enhanced porosity of soil by absorbing nitrifier inhibitor (e.g., phenolics) [166,167,168]. However, it is a very slow process and takes several months for biochar to provide habitat for nitrifiers to colonize [143]. Employing the biochar in the soil changes its property that helps nitrogen-fixing bacteria to make habitat inside the biochar pore. Both symbiotic and free-living bacteria show a positive impact of biochar application. Free-living Azotobactor sp. and Azospirillum colonized and multiplied in biochar treated soil due to surplus habitat and required oxygen supply. Similarly, symbiont (e.g., Rhizobia) in biochar treatment also gets activated [169], which results in increased nodulation and nitrogenase activity [142, 170].
4.3 Biochar as a source of nutrients
Biochar can also be a source of micronutrients, for example, boron, molybdenum, K, P, Ca, etc. [171], which are necessary elements for nodulation of Rhizobia. Biochar, in a combination of compost, significantly increases the availability of nutrients and enhances crop productivity as a result [172]. Another group [13] reported 25% enhancement in crop productivity compared to chemical fertilizers and noted the increased soil organic carbon (SOC) from 0.93% (by fertilizer) to 1.25% (Biochar amended), soil water content (SWC) from 18% (by fertilizer) to over 23% (biochar amended) and CEC from 8.9 cmol (+)kg−1 (by fertilizer) to over 10.3 cmol(+)kg−1 (biochar amended) with significant increase in leaf chlorophyll content, nodulation number (NN), leaf nutrient concentration, etc. A chemical fertilizer, when applied to the soil, gets rapidly depleted either by leaching or degrading to another form. Similarly, manure or compost can also get depleted from soil resulting in an increased financial burden to the farmers. Leaching of major plant nutrients such as P, K, and nitrate-nitrogen (NO3− N), potentially lead to environmental pollution [75]. In such conditions, the application of fertilizers or compost in combination with biochar could be more beneficial. A similar observation was reported by a group [172] where they stated increased peanut yield along with an increase in soil pH, available nitrogen/phosphorus, and CEC. Moreover, several studies support the positive influence of biochar on soil fertility and the productivity of a wide range of crops [13, 172,173,174].
4.4 Effects of biochar on microbial diversity and soil enzyme
Effects of biochar on microbial growth, diversity, and soil enzyme are studied by the various researcher and observed stimulatory effects. Several researchers [29, 175] observed an increase in bacterial and fungal growth in biochar added to soil at relatively low concentration (1%) followed by an increasing concentration of more than 5% of biochar resulted in a decrease in microbial biomass. Another group [29] reported an increase in 16S rRNA gene copies by biochar addition in the bacterial dominated microbial community, which was further supported by studies carried out by some others [176]. However, both the studies reported a decrease in bacterial and fungal gene copies by 74 and 25%, respectively, when 5% of biochar was added compared to the control. The possible explanation provided by a group [177] states that the pH value could have played a major role. Slightly alkaline or neutral soil favored bacterial and fungal growth compared to acidic soil [178]. When biochar was applied in the range of 1–2% in soil, pH was in the range of 7–7.5; however, at 5% biochar addition, pH increased up to 8.5 which are again found to be unfavorable to microbial growth and ultimately inhibited bacterial and fungal coding gene copies. A positive effect of biochar on soil fungi (Arbuscular mycorrhizal(AM) and Ectomycorrhizal) was documented by other groups [179, 180]. For such an abundance of microbial and fungal biomass by the addition of biochar, a group [181] hypothesized that biochar might have provided a habitat where bacteria and fungi could sustain themselves from predators and mitigated their diverse requirements of carbon, energy, and mineral nutrients. Not all the bacteria show stimulatory effects with biochar amendments [182]. Denitrifying bacteria could get reduced with biochar amendments [183, 184]. Denitrifying bacteria could also increase N2O emission and reduce soil nitrogen availability. Thus, the biochar amendment again helps in the enrichment of soil N2 by decreasing the population of denitrifying bacteria. Biochar amendment has a positive effect on iron-reducing bacteria (e.g., Clostridia) which increase CH4 emission [185, 186]. Fe reducing bacteria might play an important role in N and C cycling, especially in paddy soil with biochar amendments as biochar provides a stable platform for biofilm formation and supports electron shuttling between microbes and insoluble electron acceptors such as Fe oxides [187, 188]. A similar influence of biochar on iron-reducing bacteria was studied by several groups [150, 170, 189, 190], where they documented that the biochar could potentially serve as a habitat for microorganisms, and thus, improve the soil properties such as water-holding capacity, nutrient availability, and pH buffering capacity altogether.
Soil microbes are responsible for the breakdown of large organic molecules into simple monomers, which can be utilized by plants. For such metabolism, microbes secrete specific extracellular enzymes such as cellulase, urease, invertase, phosphatase, laccase, glucosidase, galactosidase, etc. that have a crucial role in the recycling of C, N, and P [191, 192]. The addition of biochar, manure/compost increases nutrients availability, which in turn increases microbial biomass [193] and ultimately enhances the production and activity of extracellular enzymes [194]. A group [29] studied the effects of biochar on extracellular enzymes such as invertase, urease, alkaline phosphatase, and reported increased enzyme activity by adding biochar up to 1–2%. A further increase in biochar concentration reduced the enzyme activities. A few researchers [68, 195] observed an increase in alkaline phosphatase and alkaline phosphomonoesterase activities with a low concentration (2.5%) of biochar and demonstrated the activity of enzymes related to P cycling. A group [196] reported a positive effect of biochar amendment on other extracellular enzymes, for example, α-1,4- glucosidase, β-D-cellobiohydrolase, and β-1,4-N acetylglucosaminidase and negative effects on β-1,4-glucosidase and phosphatase activities. Few others [179, 197] also supported a decrease in invertase and alkaline phosphatase activities on the addition of biochar. According to these studies, a possible reason for such decrease could be as follows: (i) strong adsorption property of biochar adversely binds the enzyme which limits its catalytic activity [197]; (ii) biochar addition might have detrimental effects on microbial growth and enzyme production [198]; and (iii) high pH might have influenced the metabolic activities due to biochar addition [199].
4.5 Biochar amendment suppresses plant diseases
Biochar in addition to carbon sequestration, nutrient enrichment, improvement in soil quality, and stimulatory effects on microbial diversity and extracellular enzymes, can also measurably reduce disease severity of different pathogen types and even induce system-wide defense responses in host plants [29, 182, 195, 200,201,202]. Another group [203] studied the effect of biochar on lettuce and strawberry plants and reported 3% biochar amendment significantly reduced the susceptibility for the fungal pathogen Botrytis cinerea on both leaves and fruits of strawberry; however, the effect was limited on lettuce plant. In another similar work carried out by a group [201] where strawberry plants were grown in a biochar-amended soil, showed an upregulated salicylic acid-induced (SAR) and jasmonic acid/ethylene-induced (ISR) gene expressions and were primed for gene expression upon infection by Botrytis cinerea and by Podosphaeraaphanis. A group [200] reported pepper and tomato plants were more resistant to Botrytis cinerea and Oidiopsissicula when they were cultivated on biochar-amended soil. Similar observations were also noted by a research group [204] where biochar helped to prevent root rot in asparagus caused by Fusarium oxysporum f. sp. asparagi. A few others [205, 206] documented suppression abilities of biochar for cucumber damping-off caused by Rhizoctonia solani and carrot root-lesion nematode Pratylenchuspenetrans. The disease suppression mechanism for biochar amendment soil can be similar to other organic soil amendments such as composts [207]. However, various researchers [169, 208] have discussed main defense-enhancing mechanisms of biochar amendment soil could be due to following reasons such as (i) better availability of nutrients for host plant; (ii) stimulation of microbial biomass; (iii) removal or neutralization of toxins produced by a pathogen or other infection-relevant substances; and (iv) induced system-wide defense responses in the host plant. Moreover, several others suggested that biochar can also affect the plant-wide systemic response, which can further induce disease-related genes linked to induced systemic resistance (ISR) [201, 205, 209].
5 Future prospects
Low soil fertility is a common problem in many regions around the world, which can be overcome by the use of biochar in the future. Biochar can efficiently improve the water holding capacity of the soil, which is extremely helpful in order to develop healthy plantation in the arid area. Also, biochar can be more beneficial in combination with compost where biochar can adsorb nutrients from the compost and keep deposited inside the holes while slowly releasing and making them available for plants and thus can eliminate the dependency on chemical fertilizers. In the future, many agro-industries may formulate such commercial products and make them available to farmers. Further, the adsorptive capacity of biochar could be proven as a milestone for water purifiers. Biochar not only adsorbs microorganisms but also removes suspended and dissolved solids. The use of biochar filters instead of a carbon filter could be advantageous with respect to cost and work efficiency. Also, due to selective adsorptive properties, it could also be highly useful in dye industries. Further, biochar shows more potential in mitigating climate change, especially in terms of carbon capture or its storage or carbon sequestration. Moreover, it can also be employed in pharmaceutical industries where it can efficiently remove toxicants. In the future, it could immerge as a highly potential commodity for several industries such as food, fertilizer, agriculture, and pharmaceuticals.
6 Conclusion
The recent trends in biochar production methods and their applications have been systematically reviewed. Biochar is being used in an increasing number of fields and has been widely employed in a variety of applications, such as an adsorbent, a source of nutrients, and soil amendment agent where the biochar amendment could further suppress plant diseases as well. Properties of biochar and its applications are highly influenced by the mode of preparation and type of feedstock used. Moreover, the quality and efficiency of biochar to affect soil quality and plant growth vary greatly depending upon the experimental conditions such as pyrolysis temperature, feedstock material, age of produced biochar, etc. Evaluation of field efficiency and economic feasibility of biochar applications should be considered while providing a measure of certainty to the many possible benefits, which is a key challenge to be addressed by further research.
References
Gabhane J, Tripathi A, Athar S, William SPMP, Vaidya AN, Wate SR (2016) Assessment of bioenergy potential of agricultural wastes: a case study cum template. J Biofuels Bioenergy 2:122. https://doi.org/10.5958/2454-8618.2016.00011.0
Patle AV, Prince M, Williams SP, Gabhane J, Dhar H, Nagarnaik P (2014) Mircobial assisted rapid composting of agriculture residues. Int J Sci Eng Res 5:1097–1099
Villaseñor J, Rodríguez L, Fernández FJ (2011) Composting domestic sewage sludge with natural zeolites in a rotary drum reactor. Bioresour Technol 102:1447–1454. https://doi.org/10.1016/j.biortech.2010.09.085
Raut M, Princewilliam S, Bhattacharyya J, Chakrabarti T, Devotta S (2008) Microbial dynamics and enzyme activities during rapid composting of municipal solid waste—a compost maturity analysis perspective. Bioresour Technol 99:6512–6519. https://doi.org/10.1016/j.biortech.2007.11.030
Gabhane J, William SP, Bidyadhar R, Bhilawe P, Anand D, Vaidya AN, Wate SR (2012) Additives aided composting of green waste: effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour Technol 114:382–388. https://doi.org/10.1016/j.biortech.2012.02.040
Roberts DA, Cole AJ, Paul NA, de Nys R (2015) Algal biochar enhances the re-vegetation of stockpiled mine soils with native grass. J Environ Manage 161:173–180. https://doi.org/10.1016/j.jenvman.2015.07.002
Kelly CN, Peltz CD, Stanton M, Rutherford DW, Rostad CE (2014) Biochar application to hardrock mine tailings: soil quality, microbial activity, and toxic element sorption. Appl Geochem 43:35–48. https://doi.org/10.1016/J.APGEOCHEM.2014.02.003
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. https://doi.org/10.1007/s11104-009-0050-x
Guan G, Kaewpanha M, Hao X, Abudula A (2016) Catalytic steam reforming of biomass tar: prospects and challenges. Renew Sustain Energy Rev 58:450–461. https://doi.org/10.1016/J.RSER.2015.12.316
Turner J, Sverdrup G, Mann MK, Maness P-C, Kroposki B, Ghirardi M, Evans RJ, Blake D (2008) Renewable hydrogen production. Int J Energy Res 32:379–407. https://doi.org/10.1002/er.1372
Solomon D, Lehmann J, Thies J, Schäfer T, Liang B, Kinyangi J, Neves E, Petersen J, Luizão F, Skjemstad J (2007) Molecular signature and sources of biochemical recalcitrance of organic C in Amazonian Dark Earths. Geochim Cosmochim Acta 71:2285–2298. https://doi.org/10.1016/j.gca.2007.02.014
Ogawa M, Okimori Y (2010) Pioneering works in biochar research, Japan. Aust J Soil Res 10:1–12. https://doi.org/10.1071/SR10006
Agegnehu G, Bass AM, Nelson PN, Muirhead B, Wright G, Bird MI (2015) Biochar and biochar-compost as soil amendments: effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric Ecosyst Environ 213:72–85. https://doi.org/10.1016/J.AGEE.2015.07.027
Matuštik J, Hnatkova T, Koči V (2020) Life cycle assessment of biochar-to-soil systems: a review. J Clean Prod 259:120998. https://doi.org/10.1016/j.jclepro.2020.120998
Kimetu JM, Lehmann J, Ngoze SO, Mugendi DN, Kinyangi JM, Riha S, Verchot L, Recha JW, Pell AN (2008) Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 11:726–739. https://doi.org/10.1007/s10021-008-9154-z
Graf ER, Valakh V, Wright CM, Wu C, Liu Z, Zhang YQ, DiAntonio A (2012) RIM promotes calcium channel accumulation at active zones of the drosophila neuromuscular junction. J Neurosci 32:16586–16596. https://doi.org/10.1523/JNEUROSCI.0965-12.2012
Meschewski E, Holm N, Sharma BK, Spokas K, Minalt N, Kelly JJ (2019) Pyrolysis biochar has negligible effects on soil greenhouse gas production, microbial communities, plant germination, and initial seedling growth. Chemosphere 228:565–576. https://doi.org/10.1016/j.chemosphere.2019.04.031
Bartoli M, Giorcelli M, Jagdale P, Rovere M, Tagliaferro A (2020) A review of non-soil biochar applications. Materials (Basel) 13:261. https://doi.org/10.3390/ma13020261
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sustain Energy Rev 55:467–481. https://doi.org/10.1016/J.RSER.2015.10.122
Pappu A, Saxena M, Asolekar SR (2007) Solid wastes generation in India and their recycling potential in building materials. Build Environ 42:2311–2320. https://doi.org/10.1016/J.BUILDENV.2006.04.015
Mtui GYS (2009) Recent advances in pretreatment of lignocellulosic wastes and production of value added products. African J Biotechnol 8:1398–1415
Coughlan MP (1985) The properties of fungal and bacterial cellulases with comment on their production and application. Biotechnol Genet Eng Rev 3:39–110. https://doi.org/10.1080/02648725.1985.10647809
Harmsen P, Huijgen W, Lopez L, Bakker R (2010) Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Food Biobased Res 1–49
Patil PD, Yadav GD (2019) Exploring the untapped potential of solar pretreatment for deconstruction of recalcitrant Kraft lignin in fungal biotransformation. Clean Technol Environ Policy 21:579–590. https://doi.org/10.1007/s10098-018-1656-6
Balan V, Sousa LdaC, Chundawat SPS, Marshall D, Sharma LN, Chambliss CK, Dale BE (2009) Enzymatic digestibility and pretreatment degradation products of AFEX-treated hardwoods (Populus nigra). Biotechnol Prog 25:365–375. https://doi.org/10.1002/btpr.160
Erdtman H (1972) Lignins: occurrence, formation, structure and reactions, Sarkanen KV, Ludwig CH (eds.), John Wiley & Sons, Inc., New York, 1971. 916 pp. $35.00. J Polym Sci Part B Polym Lett 10:228–230. https://doi.org/10.1002/pol.1972.110100315
Gabhane J, Vaidya AN (2019) Efficiency of nutrient based compost activator on composting of green biomass: effect on physico-chemical, biological parameter and maturity of compost. Int J Curr Eng Sci Res 6:321–331
Liao N, Li Q, Zhang W, Zhou G, Ma L, Min W, Ye J, Hou Z (2016) Effects of biochar on soil microbial community composition and activity in drip-irrigated desert soil. Eur J Soil Biol 72:27–34. https://doi.org/10.1016/J.EJSOBI.2015.12.008
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wang R, Wan J (2017) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 174:545–553. https://doi.org/10.1016/j.chemosphere.2017.01.130
Jeong CY, Dodla SK, Wang JJ (2016) Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 142:4–13. https://doi.org/10.1016/J.CHEMOSPHERE.2015.05.084
Yang X, Meng J, Lan Y, Chen W, Yang T, Yuan J, Liu S, Han J (2017) Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric Ecosyst Environ 240:24–31. https://doi.org/10.1016/J.AGEE.2017.02.001
Intani K, Latif S, Kabir AKMR, Müller J (2016) Effect of self-purging pyrolysis on yield of biochar from maize cobs, husks and leaves. Bioresour Technol 218:541–551. https://doi.org/10.1016/J.BIORTECH.2016.06.114
Ouyang W, Zhao X, Tysklind M, Hao F, Wang F (2015) Optimisation of corn straw biochar treatment with catalytic pyrolysis in intensive agricultural area. Ecol Eng 84:278–286. https://doi.org/10.1016/J.ECOLENG.2015.09.003
Srinivasan P, Sarmah AK, Smernik R, Das O, Farid M, Gao W (2015) A feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposite feedstock: production, characterization and potential applications. Sci Total Environ 512–513:495–505. https://doi.org/10.1016/J.SCITOTENV.2015.01.068
Jegajeevagan K, Mabilde L, Gebremikael MT, Ameloot N, De Neve S, Leinweber P, Sleutel S (2016) Artisanal and controlled pyrolysis-based biochars differ in biochemical composition, thermal recalcitrance, and biodegradability in soil. Biomass Bioenerg 84:1–11. https://doi.org/10.1016/J.BIOMBIOE.2015.10.025
Özçimen D, Ersoy-Meriçboyu A (2010) Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew. Energy. 35:1319–1324. https://doi.org/10.1016/J.RENENE.2009.11.042
Bhange VP, William SP, Sharma A, Gabhane J, Vaidya AN, Wate SR (2015) Pretreatment of garden biomass using Fenton’s reagent: influence of Fe(2 +) and H2O2 concentrations on lignocellulose degradation. J Environ Heal Sci Eng 13:12. https://doi.org/10.1186/s40201-015-0167-1
Jha MK, Sondhi AK, Pansare M (2003) Solid waste management—a case study. Indian J Environ Prot 23:1153–1160
Sharholy M, Ahmad K, Mahmood G, Trivedi RC (2008) Municipal solid waste management in Indian cities—a review. Waste Manag 28:459–467. https://doi.org/10.1016/J.WASMAN.2007.02.008
Devasahayam S, Dowling K, Mahapatra MK (2016) Sustainability in the mineral and energy sectors. CRC Press, Cambridge
Agarwal M, Tardio J, Mohan SV (2015) Pyrolysis biochar from cellulosic municipal solid waste as adsorbent for azo dye removal: equilibrium isotherms and kinetics analysis. Int J Environ Sci Dev 6:67–72. https://doi.org/10.7763/IJESD.2015.V6.563
Jin H, Capareda S, Chang Z, Gao J, Xu Y, Zhang J (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: adsorption property and its improvement with KOH activation. Bioresour Technol 169:622–629. https://doi.org/10.1016/J.BIORTECH.2014.06.103
Ateş F, Miskolczi N, Borsodi N (2013) Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: product yields, gas and pyrolysis oil properties. Bioresour Technol 133:443–454. https://doi.org/10.1016/J.BIORTECH.2013.01.112
Bernardo M, Lapa N, Gonçalves M, Mendes B, Pinto F, Fonseca I, Lopes H (2012) Physico-chemical properties of chars obtained in the co-pyrolysis of waste mixtures. J Hazard Mater 219–220:196–202. https://doi.org/10.1016/J.JHAZMAT.2012.03.077
Cao X, Ma L, Gao B, Harris W (2009) Dairy-Manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291. https://doi.org/10.1021/es803092k
Meng J, Wang L, Liu X, Wu J, Brookes PC, Xu J (2013) Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour Technol 142:641–646. https://doi.org/10.1016/J.BIORTECH.2013.05.086
Uchimiya M, Lima IM, Klasson KT, Wartelle LH (2010) Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere 80:935–940. https://doi.org/10.1016/J.CHEMOSPHERE.2010.05.020
Jayawardhana Y, Kumarathilaka P, Herath I, Vithanage M (2016) Municipal solid waste biochar for prevention of pollution from landfill leachate. Environ Mater Waste. https://doi.org/10.1016/B978-0-12-803837-6.00006-8
Nandy B, Sharma G, Garg S, Kumari S, George T, Sunanda Y, Sinha B (2015) Recovery of consumer waste in India—a mass flow analysis for paper, plastic and glass and the contribution of households and the informal sector. Resour Conserv Recycl 101:167–181. https://doi.org/10.1016/J.RESCONREC.2015.05.012
Food and Agriculture Organization of the United Nations (2010) Global forest resources assessment 2010 : main report. Food and Agriculture Organization of the United Nations
Asia-Pacific forestry sector outlook study II india forestry outlook study by the ministry of environment and forests government of india food and agriculture organization of the united nations regional office for asia and the pacific. (2009)
Lai WY, Lai C-M, Ke GR, Chung R-S, Chen CT, Cheng C-H, Pai CW, Chen SY, Chen CC (2013) The effects of woodchip biochar application on crop yield, carbon sequestration and greenhouse gas emissions from soils planted with rice or leaf beet. J Taiwan Inst Chem Eng 44:1039–1044. https://doi.org/10.1016/J.JTICE.2013.06.028
Dong T, Gao D, Miao C, Yu X, Degan C, Garcia-Pérez M, Rasco B, Sablani SS, Chen S (2015) Two-step microalgal biodiesel production using acidic catalyst generated from pyrolysis-derived bio-char. Energy Convers Manag 105:1389–1396. https://doi.org/10.1016/J.ENCONMAN.2015.06.072
Hu Q, Yang H, Yao D, Zhu D, Wang X, Shao J, Chen H (2016) The densification of bio-char: effect of pyrolysis temperature on the qualities of pellets. Bioresour Technol 200:521–527. https://doi.org/10.1016/J.BIORTECH.2015.10.077
Bird MI, Wurster CM, de Paula Silva PH, Bass AM, de Nys R (2011) Algal biochar—production and properties. Bioresour Technol 102:1886–1891. https://doi.org/10.1016/J.BIORTECH.2010.07.106
Roberts DA, de Nys R (2016) The effects of feedstock pre-treatment and pyrolysis temperature on the production of biochar from the green seaweed Ulva. J Environ Manage 169:253–260. https://doi.org/10.1016/j.jenvman.2015.12.023
Nautiyal P, Subramanian KA, Dastidar MG (2016) Adsorptive removal of dye using biochar derived from residual algae after in situ transesterification: alternate use of waste of biodiesel industry. J Environ Manage 182:187–197. https://doi.org/10.1016/j.jenvman.2016.07.063
Chaiwong K, Kiatsiriroat T, Vorayos N, Thararax C (2013) Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenerg 56:600–606. https://doi.org/10.1016/J.BIOMBIOE.2013.05.035
Shen B, Chen J, Yue S, Li G (2015) A comparative study of modified cotton biochar and activated carbon based catalysts in low temperature SCR. Fuel 156:47–53. https://doi.org/10.1016/J.FUEL.2015.04.027
Wang P, Yu H, Zhan S, Wang S (2011) Catalytic hydrolysis of lignocellulosic biomass into 5-hydroxymethylfurfural in ionic liquid. Bioresour Technol 102:4179–4183. https://doi.org/10.1016/J.BIORTECH.2010.12.073
Moralı U, Şensöz S (2015) Pyrolysis of hornbeam shell (Carpinus betulus L.) in a fixed bed reactor: characterization of bio-oil and bio-char. Fuel 150:672–678. https://doi.org/10.1016/J.FUEL.2015.02.095
Chen B, Chen Z (2009) Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 76:127–133. https://doi.org/10.1016/J.CHEMOSPHERE.2009.02.004
Zheng W, Sharma BK, Rajagopalan N (2010) Using biochar as a soil amendment for sustainable agriculture 1–36
Moussavi G, Khosravi R (2012) Preparation and characterization of a biochar from pistachio hull biomass and its catalytic potential for ozonation of water recalcitrant contaminants. Bioresour Technol 119:66–71. https://doi.org/10.1016/J.BIORTECH.2012.05.101
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res 48:516. https://doi.org/10.1071/SR10058
Yuan H, Lu T, Wang Y, Chen Y, Lei T (2016) Sewage sludge biochar: nutrient composition and its effect on the leaching of soil nutrients. Geoderma 267:17–23. https://doi.org/10.1016/J.GEODERMA.2015.12.020
Méndez A, Paz-Ferreiro J, Gil E, Gascó G (2015) The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci Hortic (Amsterdam) 193:225–230. https://doi.org/10.1016/J.SCIENTA.2015.07.032
Jin Y, Liang X, He M, Liu Y, Tian G, Shi J (2016) Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: a microcosm incubation study. Chemosphere 142:128–135. https://doi.org/10.1016/J.CHEMOSPHERE.2015.07.015
Liu Z, Han G (2015) Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 158:159–165. https://doi.org/10.1016/J.FUEL.2015.05.032
Wrobel-Tobiszewska A, Boersma M, Sargison J, Adams P, Jarick S (2015) An economic analysis of biochar production using residues from Eucalypt plantations. Biomass Bioenerg 81:177–182. https://doi.org/10.1016/J.BIOMBIOE.2015.06.015
Kumar S, Masto RE, Ram LC, Sarkar P, George J, Selvi VA (2013) Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol Eng 55:67–72. https://doi.org/10.1016/J.ECOLENG.2013.02.011
Glaser B (2007) Prehistorically modified soils of central Amazonia: a model for sustainable agriculture in the twenty-first century. Philos Trans R Soc Lond B Biol Sci. 362(1478):187–196. https://doi.org/10.1098/rstb.2006.1978
Glaser B, Haumaier L, Guggenberger G, Zech W (2001) The “Terra Preta” phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88:37–41. https://doi.org/10.1007/s001140000193
Thines KR, Abdullah EC, Mubarak NM, Ruthiraan M (2017) Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: a review. Renew Sustain Energy Rev 67:257–276. https://doi.org/10.1016/J.RSER.2016.09.057
Barrow CJ (2012) Biochar: potential for countering land degradation and for improving agriculture. Appl Geogr 34:21–28. https://doi.org/10.1016/J.APGEOG.2011.09.008
Emrich W (1985) Commission of the European Communities, L. (Luxembourg). D.-G.I.M. and I. eng: Handbook of charcoal making: the traditional and industrial methods, http://agris.fao.org/agris-search/search.do?recordID=XF2015046878
Reilly J (1925) The technology of wood distillation: with special reference to the methods of obtaining the intermediate and finished products from the primary distillate. Nature 116:779–780. https://doi.org/10.1038/116779a0
Basu P (2010) Biomass gasification and pyrolysis: practical design and theory. Academic Press, Burlington
Czernik S, Bridgwater AV (2004) Overview of applications of biomass fast pyrolysis oil. Energ Fuel. https://doi.org/10.1021/EF034067U
Suárez-Abelenda M, Kaal J, McBeath AV (2017) Translating analytical pyrolysis fingerprints to Thermal Stability Indices (TSI) to improve biochar characterization by pyrolysis-GC-MS. Biomass Bioenerg 98:306–320. https://doi.org/10.1016/J.BIOMBIOE.2017.01.021
Li J, Dai J, Liu G, Zhang H, Gao Z, Fu J, He Y, Huang Y (2016) Biochar from microwave pyrolysis of biomass: a review. Biomass Bioenerg 94:228–244. https://doi.org/10.1016/j.biombioe.2016.09.010
Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45:939–969. https://doi.org/10.1080/10643389.2014.924180
Verma M, Godbout S, Brar SK, Solomatnikova O, Lemay SP, Larouche JP (2012) Biofuels production from biomass by thermochemical conversion technologies. Int J Chem Eng 2012:1–18. https://doi.org/10.1155/2012/542426
McBeath AV, Smernik RJ, Krull ES, Lehmann J (2014) The influence of feedstock and production temperature on biochar carbon chemistry: a solid-state 13C NMR study. Biomass Bioenerg 60:121–129. https://doi.org/10.1016/J.BIOMBIOE.2013.11.002
Dai L, Fan L, Liu Y, Ruan R, Wang Y, Zhou Y, Zhao Y, Yu Z (2017) Production of bio-oil and biochar from soapstock via microwave-assisted co-catalytic fast pyrolysis. Bioresour Technol 225:1–8. https://doi.org/10.1016/J.BIORTECH.2016.11.017
Huang YF, Chiueh P-T, Kuan WH, Lo SL (2016) Microwave pyrolysis of lignocellulosic biomass: heating performance and reaction kinetics. Energy. 100:137–144. https://doi.org/10.1016/J.ENERGY.2016.01.088
Liu S, Xie Q, Zhang B, Cheng Y, Liu Y, Chen P, Ruan R (2016) Fast microwave-assisted catalytic co-pyrolysis of corn stover and scum for bio-oil production with CaO and HZSM-5 as the catalyst. Bioresour Technol 204:164–170. https://doi.org/10.1016/J.BIORTECH.2015.12.085
Karunanayake AG, Todd OA, Crowley ML, Ricchetti LB, Pittman CU, Anderson R, Mlsna TE (2017) Rapid removal of salicylic acid, 4-nitroaniline, benzoic acid and phthalic acid from wastewater using magnetized fast pyrolysis biochar from waste Douglas fir. Chem Eng J 319:75–88. https://doi.org/10.1016/J.CEJ.2017.02.116
Essandoh M, Kunwar B, Pittman CU, Mohan D, Mlsna T (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 265:219–227. https://doi.org/10.1016/j.cej.2014.12.006
Laird DA, Novak JM, Collins HP, Ippolito JA, Karlen DL, Lentz RD, Sistani KR, Spokas K, Van Pelt RS (2017) Multi-year and multi-location soil quality and crop biomass yield responses to hardwood fast pyrolysis biochar. Geoderma 289:46–53. https://doi.org/10.1016/J.GEODERMA.2016.11.025
Al-Rahbi AS, Williams PT (2017) Hydrogen-rich syngas production and tar removal from biomass gasification using sacrificial tyre pyrolysis char. Appl Energy 190:501–509. https://doi.org/10.1016/J.APENERGY.2016.12.099
González JF, Román S, Bragado D, Calderón M (2008) Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process Technol 89:764–772. https://doi.org/10.1016/J.FUPROC.2008.01.011
Shen Y, Zhao P, Shao Q, Takahashi F, Yoshikawa K (2015) In situ catalytic conversion of tar using rice husk char/ash supported nickel–iron catalysts for biomass pyrolytic gasification combined with the mixing-simulation in fluidized-bed gasifier. Appl Energy 160:808–819. https://doi.org/10.1016/j.apenergy.2014.10.074
Luo S, Xiao B, Hu Z, Liu S, Guo X, He M (2009) Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: influence of temperature and steam on gasification performance. Int J Hydrogen Energy 34:2191–2194. https://doi.org/10.1016/J.IJHYDENE.2008.12.075
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20:848–889. https://doi.org/10.1021/EF0502397
Sattar A, Leeke GA, Hornung A, Wood J (2014) Steam gasification of rapeseed, wood, sewage sludge and miscanthus biochars for the production of a hydrogen-rich syngas. Biomass Bioenerg 69:276–286. https://doi.org/10.1016/J.BIOMBIOE.2014.07.025
Yan F, Luo S, Hu Z, Xiao B, Cheng G (2010) Hydrogen-rich gas production by steam gasification of char from biomass fast pyrolysis in a fixed-bed reactor: influence of temperature and steam on hydrogen yield and syngas composition. Bioresour Technol 101:5633–5637. https://doi.org/10.1016/J.BIORTECH.2010.02.025
Bourgeois JP, Doat J (1984) Torrefied wood from temperate and tropical species. Advantages and prospects. Bioenergy 84. In: Proceeding of conference 15–21 June 1984, Goteborg, Sweden. Vol. III. Biomass Convers, pp 153–159
Pentananunt R, Rahman ANMM, Bhattacharya SC (1990) Upgrading of biomass by means of torrefaction. Energy. 15:1175–1179. https://doi.org/10.1016/0360-5442(90)90109-F
Tumuluru JS, Wright CT, Hess JR, Kenney KL (2011) A review of biomass densification systems to develop uniform feedstock commodities for bioenergy application. Biofuels Bioprod Biorefining 5:683–707. https://doi.org/10.1002/bbb.324
Almeida G, Brito JO, Perré P (2010) Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: the potential of mass loss as a synthetic indicator. Bioresour Technol 101:9778–9784. https://doi.org/10.1016/J.BIORTECH.2010.07.026
Prins MJ, Ptasinski KJ, Janssen FJJG (2006) Torrefaction of wood. J Anal Appl Pyrolysis 77:35–40. https://doi.org/10.1016/j.jaap.2006.01.001
Huang Y-F, Cheng P-H, Chiueh P-T, Lo S-L (2017) Leucaena biochar produced by microwave torrefaction: fuel properties and energy efficiency. Appl Energy 204:1018–1025. https://doi.org/10.1016/J.APENERGY.2017.03.007
Chen S, Liu C, Peng C, Liu H, Hu M, Zhong G (2012) Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain cladosporium cladosporioides Hu-01. PLoS ONE 7:1–12. https://doi.org/10.1371/journal.pone.0047205
Chen WH, Kuo PC (2010) A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy. 35:2580–2586. https://doi.org/10.1016/J.ENERGY.2010.02.054
Li L, Rowbotham JS, Christopher Greenwell H, Dyer PW (2013) An introduction to pyrolysis and catalytic pyrolysis: versatile techniques for biomass conversion. In: New and future developments in catalysis, pp 173–208. Elsevier
Demirbas A, Arin G (2002) An overview of biomass pyrolysis. Energy Sources 24:471–482. https://doi.org/10.1080/00908310252889979
Tripathi M, Sahu JN, Ganesan P, Monash P, Dey TK (2015) Effect of microwave frequency on dielectric properties of oil palm shell (OPS) and OPS char synthesized by microwave pyrolysis of OPS. J Anal Appl Pyrolysis 112:306–312. https://doi.org/10.1016/J.JAAP.2015.01.007
Carrier M, Hugo T, Gorgens J, Knoetze H (2011) Comparison of slow and vacuum pyrolysis of sugar cane bagasse. J Anal Appl Pyrolysis 90:18–26. https://doi.org/10.1016/j.jaap.2010.10.001
Roy C, Chaala A (2001) Vacuum pyrolysis of automobile shredder residues. Resour Conserv Recycl 32:1–27. https://doi.org/10.1016/S0921-3449(00)00088-4
Uras-Postma Ü, Carrier M, Knoetze J (2014) (Hansie): vacuum pyrolysis of agricultural wastes and adsorptive criteria description of biochars governed by the presence of oxides. J Anal Appl Pyrolysis 107:123–132. https://doi.org/10.1016/J.JAAP.2014.02.012
Uras Ü, Carrier M, Hardie AG, Knoetze JH (2012) Physico-chemical characterization of biochars from vacuum pyrolysis of South African agricultural wastes for application as soil amendments. J Anal Appl Pyrolysis 98:207–213. https://doi.org/10.1016/J.JAAP.2012.08.007
Titirici M-M, Thomas A, Antonietti M (2007) Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J Chem 31:787. https://doi.org/10.1039/b616045j
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici M-M, Fühner C, Bens O, Kern J, Emmerich K-H (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels. 2:71–106. https://doi.org/10.4155/bfs.10.81
Hu B, Wang K, Wu L, Yu S-H, Antonietti M, Titirici M-M (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22:813–828. https://doi.org/10.1002/adma.200902812
Gao Y, Wang X, Wang J, Li X, Cheng J, Yang H, Chen H (2013) Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy. 58:376–383. https://doi.org/10.1016/J.ENERGY.2013.06.023
Lynam JG, Coronella CJ, Yan W, Reza MT, Vasquez VR (2011) Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour Technol 102:6192–6199. https://doi.org/10.1016/j.biortech.2011.02.035
Yang W, Shimanouchi T, Kimura Y (2015) Characterization of the residue and liquid products produced from husks of nuts from Carya cathayensis sarg by hydrothermal carbonization. ACS Sustain Chem Eng 3:591–598. https://doi.org/10.1021/acssuschemeng.5b00103
Reza MT, Lynam JG, Vasquez VR, Coronella CJ (2012) Pelletization of biochar from hydrothermally carbonized wood. Environ Prog. Sustain Energy 31:225–234. https://doi.org/10.1002/ep.11615
Reza MT, Uddin MH, Lynam JG, Hoekman SK, Coronella CJ (2014) Hydrothermal carbonization of loblolly pine: reaction chemistry and water balance. Biomass Convers Biorefinery 4:311–321. https://doi.org/10.1007/s13399-014-0115-9
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sustain Energy Rev 45:359–378. https://doi.org/10.1016/J.RSER.2015.01.050
Gabhane J, Prince William SPM, Vaidya AN, Mahapatra K, Chakrabarti T (2011) Influence of heating source on the efficacy of lignocellulosic pretreatment—a cellulosic ethanol perspective. Biomass Bioenergy 35:96–102. https://doi.org/10.1016/J.BIOMBIOE.2010.08.026
Intanakul P, Krairiksh M, Kitchaiya P (2003) Enhancement of enzymatic hydrolysis of lignocellulosic wastes by microwave pretreatment under atmospheric pressure. J Wood Chem Technol 23:217–225. https://doi.org/10.1081/WCT-120021926
Patil PD, Yadav GD (2018) Application of microwave assisted three phase partitioning method for purification of laccase from Trametes hirsuta. Process Biochem 65:220–227. https://doi.org/10.1016/j.procbio.2017.10.006
Nihon Hakkō Kōgakkai J, Nihon Seibutsu Kōgakkai F, Koshijima T, (Kyoto Univ., U. (Japan). WRI (1989) Enhancement of enzymatic susceptibility of lignocellulosic wastes [sugar cane bagasse, rice straw and rice hulls] by microwave irradiation [1984]. Society of Fermentation Technology, Japan
Warren Communications News (Firm), E. (Kyoto U. (Japan). C. of A., Okamura, K.: Influence of a steam explosion and microwave irradiation on the enzymatic hydrolysis of a coniferous wood [1989]. Warren Communications News (1989)
Arafat Hossain M, Ganesan P, Jewaratnam J, Chinna K (2017) Optimization of process parameters for microwave pyrolysis of oil palm fiber (OPF) for hydrogen and biochar production. Energy Convers Manag 133:349–362. https://doi.org/10.1016/J.ENCONMAN.2016.10.046
Menéndez JA, Domı́nguez A, Inguanzo M, Pis JJ (2004) Microwave pyrolysis of sewage sludge: analysis of the gas fraction. J Anal Appl Pyrolysis 7:657–667. https://doi.org/10.1016/J.JAAP.2003.09.003
Huang YF, Chiueh P, Te, Lo SL (2019) CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. In: Energy Procedia, pp 4435–4440. Elsevier Ltd
Jung K-W, Hwang M-J, Jeong T-U, Ahn K-H (2015) A novel approach for preparation of modified-biochar derived from marine macroalgae: dual purpose electro-modification for improvement of surface area and metal impregnation. Bioresour Technol 191:342–345. https://doi.org/10.1016/j.biortech.2015.05.052
Fang C, Zhang T, Li P, Jiang R, Wang Y (2014) Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int J Environ Res Public Health 11:9217–9237. https://doi.org/10.3390/ijerph110909217
Hu X, Ding Z, Zimmerman AR, Wang S, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216. https://doi.org/10.1016/J.WATRES.2014.10.009
Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130:457–462. https://doi.org/10.1016/J.BIORTECH.2012.11.132
Zhang M, Gao B (2013) Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem Eng J 226:286–292. https://doi.org/10.1016/J.CEJ.2013.04.077
Wan Z, Sun Y, Tsang DC, Hou D, Cao X, Zhang S, Gao B, Ok YS (2020) Sustainable remediation with electroactive biochar system: mechanisms and perspectives. Green Chem 22:2688–2711. https://doi.org/10.1039/d0gc00717j
Zhou Z, Liu Y, Liu S, Liu H, Zeng G, Tan X, Yang C, Ding Y, Yan Z, Cai X (2017) Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem Eng J 314:223–231. https://doi.org/10.1016/J.CEJ.2016.12.113
Peng X, Luan Z, Di Z, Zhang Z, Zhu C (2005) Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb(II) and Cu(II) from water. Carbon N. Y. 43:880–883. https://doi.org/10.1016/j.carbon.2004.11.009
Mubarak NM, Alicia RF, Abdullah EC, Sahu JN, Haslija ABA, Tan J (2013) Statistical optimization and kinetic studies on removal of Zn2 + using functionalized carbon nanotubes and magnetic biochar. J Environ Chem Eng 1:486–495. https://doi.org/10.1016/J.JECE.2013.06.011
Walworth J (2013) Nitrogen in Soil and the environment
Joseph S, Graber E, Chia C, Munroe P, Donne S, Thomas T, Nielsen S, Marjo C, Rutlidge H, Pan G, Li L, Taylor P, Rawal A, Hook J (2013) Shifting paradigms: development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag. 4:323–343. https://doi.org/10.4155/cmt.13.23
Blackwell P, Krull E, Butler G, Herbert A, Solaiman Z (2010) Effect of banded biochar on dryland wheat production and fertiliser use in south-western Australia: an agronomic and economic perspective. Soil Res. 48:531. https://doi.org/10.1071/SR10014
Clough T, Condron L, Kammann C, Müller C, Clough TJ, Condron LM, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy. 3:275–293. https://doi.org/10.3390/agronomy3020275
Bai SH, Reverchon F, Xu C-Y, Xu Z, Blumfield TJ, Zhao H, Van Zwieten L, Wallace HM (2015) Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol Biochem 90:232–240. https://doi.org/10.1016/J.SOILBIO.2015.08.007
Hosseini Bai S, Xu C-Y, Xu Z, Blumfield TJ, Zhao H, Wallace H, Reverchon F, Van Zwieten L (2015) Soil and foliar nutrient and nitrogen isotope composition (δ15 N) at 5 years after poultry litter and green waste biochar amendment in a macadamia orchard. Environ Sci Pollut Res 22:3803–3809. https://doi.org/10.1007/s11356-014-3649-2
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 74:1–8. https://doi.org/10.1016/J.EJSOBI.2016.02.004
Brennan JK, Bandosz TJ, Thomson KT, Gubbins KE (2001) Water in porous carbons
Amonette JE, Joseph S, Joseph S (2012) Characteristics of biochar: microchemical properties, pp 65–84. https://doi.org/10.4324/9781849770552-10
Montes-Morán MA, Suárez D, Menéndez JA, Fuente E (2004) On the nature of basic sites on carbon surfaces: an overview. Carbon N. Y. 42:1219–1225. https://doi.org/10.1016/J.CARBON.2004.01.023
Biochar for Environmental Management: An Introduction
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. Earthscan
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51: 2061–2069. https://doi.org/10.13031/2013.25409
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011) Biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–143. https://doi.org/10.1016/B978-0-12-385538-1.00003-2
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 9:e113888. https://doi.org/10.1371/journal.pone.0113888
Harvey OR, Herbert BE, Kuo L-J, Louchouarn P (2012) Generalized two-dimensional perturbation correlation infrared spectroscopy reveals mechanisms for the development of surface charge and recalcitrance in plant-derived biochars. Environ Sci Technol 46:10641–10650. https://doi.org/10.1021/es302971d
Das J, Patra BS, Baliarsingh N, Parida KM (2006) Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl Clay Sci 32:252–260. https://doi.org/10.1016/J.CLAY.2006.02.005
Mohan D, Sarswat A, Ok YS, Pittman CU (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresour Technol 160:191–202. https://doi.org/10.1016/J.BIORTECH.2014.01.120
Mukherjee A, Zimmerman AR, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163:247–255. https://doi.org/10.1016/J.GEODERMA.2011.04.021
Zheng H, Wang Z, Deng X, Herbert S, Xing B (2013) Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 206:32–39. https://doi.org/10.1016/J.GEODERMA.2013.04.018
Iqbal H, Garcia-Perez M, Flury M (2015) Effect of biochar on leaching of organic carbon, nitrogen, and phosphorus from compost in bioretention systems. Sci Total Environ 521–522:37–45. https://doi.org/10.1016/J.SCITOTENV.2015.03.060
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158:436–442. https://doi.org/10.1016/J.GEODERMA.2010.05.012
Angst TE, Sohi SP (2013) Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy. 5:221–226. https://doi.org/10.1111/gcbb.12023
Lou Z, Sun Y, Bian S, Ali Baig S, Hu B, Xu X (2017) Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 169:23–31. https://doi.org/10.1016/j.chemosphere.2016.11.044
Janus A, Pelfrêne A, Heymans S, Deboffe C, Douay F, Waterlot C (2015) Elaboration, characteristics and advantages of biochars for the management of contaminated soils with a specific overview on Miscanthus biochars. J Environ Manage 162:275–289. https://doi.org/10.1016/J.JENVMAN.2015.07.056
Liu X (2014) Sustainable biochar effects for low carbon crop production: a 5-crop season field experiment on a low fertility soil from Central China. Am. Geophys. Union, Fall Meet. 2014, Abstr. id. B41A-0002
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 45:629. https://doi.org/10.1071/SR07109
Mukherjee A, Lal R, Mukherjee A, Lal R (2013) Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy. 3:313–339. https://doi.org/10.3390/agronomy3020313
Berglund LM, DeLuca TH, Zackrisson O (2004) Activated carbon amendments to soil alters nitrification rates in Scots pine forests. Soil Biol Biochem 36:2067–2073. https://doi.org/10.1016/J.SOILBIO.2004.06.005
Gundale MJ, DeLuca TH (2006) Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For Ecol Manage 231:86–93. https://doi.org/10.1016/J.FORECO.2006.05.004
Thies JE, Rillig MC, Graber ER, Rillig MC, Graber ER (2015) Biochar effects on the abundance, activity and diversity of the soil biota, pp 359–422. https://doi.org/10.4324/9780203762264-20
Quilliam RS, DeLuca TH, Jones DL (2013) Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 366:83–92. https://doi.org/10.1007/s11104-012-1411-4
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708. https://doi.org/10.1007/s00374-006-0152-z
Fischer D, Glaser B (2012) Synergisms between compost and biochar for sustainable soil amelioration. In: Management of Organic Waste. InTech
Lehmann J, Pereira da Silva Jr J, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357. https://doi.org/10.1023/A:1022833116184
Liang P-W, Liao C-Y, Chueh C-C, Zuo F, Williams ST, Xin X-K, Lin J, Jen AK-Y (2014) Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv Mater 26:3748–3754. https://doi.org/10.1002/adma.201400231
Ippolito JA, Stromberger ME, Lentz RD, Dungan RS (2016) Hardwood biochar and manure co-application to a calcareous soil. Chemosphere 142:84–91. https://doi.org/10.1016/j.chemosphere.2015.05.039
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44. https://doi.org/10.1016/J.APSOIL.2013.05.003
Deng L, Zeng G, Fan C, Lu L, Chen X, Chen M, Wu H, He X, He Y (2015) Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Appl Microbiol Biotechnol 99:8259–8269. https://doi.org/10.1007/s00253-015-6662-6
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596. https://doi.org/10.1128/AEM.02775-08
Elzobair KA, Stromberger ME, Ippolito JA, Lentz RD (2016) Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 142:145–152. https://doi.org/10.1016/J.CHEMOSPHERE.2015.06.044
Warnock DD, Mummey DL, McBride B, Major J, Lehmann J, Rillig MC (2010) Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments. Appl Soil Ecol 46:450–456. https://doi.org/10.1016/J.APSOIL.2010.09.002
Thies JE, Rillig MC, Rillig MC (2012) Characteristics of biochar: Biol Propert, pp 117–138. https://doi.org/10.4324/9781849770552-13
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523. https://doi.org/10.1111/gcbb.12266
Case SDC, McNamara NP, Reay DS, Stott AW, Grant HK, Whitaker J (2015) Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol Biochem 81:178–185. https://doi.org/10.1016/J.SOILBIO.2014.11.012
Nelissen V, Rütting T, Huygens D, Ruysschaert G, Boeckx P (2015) Temporal evolution of biochar’s impact on soil nitrogen processes—a 15 N tracing study. GCB Bioenergy. 7:635–645. https://doi.org/10.1111/gcbb.12156
Wang N, Chang Z-Z, Xue X-M, Yu J-G, Shi X-X, Ma LQ, Li H-B (2017) Biochar decreases nitrogen oxide and enhances methane emissions via altering microbial community composition of anaerobic paddy soil. Sci Total Environ 581–582:689–696. https://doi.org/10.1016/j.scitotenv.2016.12.181
Feng Y, Xu Y, Yu Y, Xie Z, Lin X (2012) Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol Biochem 46:80–88. https://doi.org/10.1016/J.SOILBIO.2011.11.016
Kappler A, Wuestner ML, Ruecker A, Harter J, Halama M, Behrens S (2014) Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ Sci Technol Lett 1:339–344. https://doi.org/10.1021/ez5002209
Gabhane J, Kumar S, Sarma AK (2020) Effect of glycerol thermal and hydrothermal pretreatments on lignin degradation and enzymatic hydrolysis in paddy straw. Renew Energy 154:1304–1313. https://doi.org/10.1016/j.renene.2020.03.035
Ameloot N, Graber ER, Verheijen FGA, De Neve S (2013) Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci 64:379–390. https://doi.org/10.1111/ejss.12064
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387. https://doi.org/10.1890/1540-9295(2007)5%5b381:BITB%5d2.0.CO;2
Bell C, Carrillo Y, Boot CM, Rocca JD, Pendall E, Wallenstein MD (2014) Rhizosphere stoichiometry: are C: n: P ratios of plants, soils, and enzymes conserved at the plant species-level? New Phytol 201:505–517. https://doi.org/10.1111/nph.12531
Patil PD, Yadav GD (2018) Comparative studies of white-rot fungal strains (Trametes hirsuta MTCC-1171 and Phanerochaete chrysosporium NCIM-1106) for effective degradation and bioconversion of ferulic acid. ACS Omega. 3:14858–14868. https://doi.org/10.1021/acsomega.8b01614
Witter E, Mårtensson AM, Garcia FV (1993) Size of the soil microbial biomass in a long-term field experiment as affected by different n-fertilizers and organic manures. Soil Biol Biochem 25:659–669. https://doi.org/10.1016/0038-0717(93)90105-K
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234. https://doi.org/10.1016/J.SOILBIO.2012.11.009
Bhaduri D, Saha A, Desai D, Meena HN (2016) Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 148:86–98. https://doi.org/10.1016/J.CHEMOSPHERE.2015.12.130
Foster EJ, Hansen N, Wallenstein M, Cotrufo MF (2016) Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric Ecosyst Environ 233:404–414. https://doi.org/10.1016/J.AGEE.2016.09.029
Bailey VL, Fansler SJ, Smith JL, Bolton H (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43:296–301. https://doi.org/10.1016/J.SOILBIO.2010.10.014
Killham K (1985) A physiological determination of the impact of environmental stress on the activity of microbial biomass. Environ Pollut Ser A Ecol Biol 38:283–294. https://doi.org/10.1016/0143-1471(85)90133-3
Chintala R, Schumacher TE, Kumar S, Malo DD, Rice JA, Bleakley B, Chilom G, Clay DE, Julson JL, Papiernik SK, Gu ZR (2014) Molecular characterization of biochars and their influence on microbiological properties of soil. J Hazard Mater 279:244–256. https://doi.org/10.1016/J.JHAZMAT.2014.06.074
Elad Y, David DR, Harel YM, Borenshtein M, Kalifa H, Ben H, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 100:913–921. https://doi.org/10.1094/PHYTO-100-9-0913
Meller Harel Y, Elad Y, Rav-David D, Borenstein M, Shulchani R, Lew B, Graber ER (2012) Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 357:245–257. https://doi.org/10.1007/s11104-012-1129-3
Spokas KA, Cantrell KB, Novak JM, Archer DW, Ippolito JA, Collins HP, Boateng AA, Lima IM, Lamb MC, McAloon AJ, Lentz RD, Nichols KA (2012) Biochar: a synthesis of its agronomic impact beyond carbon sequestration. J Environ Qual 41:973–989. https://doi.org/10.2134/jeq2011.0069
De Tender CA, Debode J, Vandecasteele B, D’Hose T, Cremelie P, Haegeman A, Ruttink T, Dawyndt P, Maes M (2016) Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl Soil Ecol 107:1–12. https://doi.org/10.1016/J.APSOIL.2016.05.001
Elmer WH, Pignatello JJ (2011) Effect of biochar amendments on mycorrhizal associations and fusarium crown and root rot of asparagus in replant soils. Plant Dis 95:960–966. https://doi.org/10.1094/PDIS-10-10-0741
Jaiswal AK, Frenkel O, Elad Y, Lew B, Graber ER (2015) Non-monotonic influence of biochar dose on bean seedling growth and susceptibility to Rhizoctonia solani: the “Shifted Rmax-Effect”. Plant Soil 395:125–140. https://doi.org/10.1007/s11104-014-2331-2
George C, Kohler J, Rillig MC (2016) Biochars reduce infection rates of the root-lesion nematode Pratylenchus penetrans and associated biomass loss in carrot. Soil Biol Biochem 95:11–18. https://doi.org/10.1016/J.SOILBIO.2015.12.003
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/J.SOILBIO.2011.04.022
Graber ER, Frenkel O, Jaiswal AK, Elad Y (2014) How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag. 5:169–183. https://doi.org/10.1080/17583004.2014.913360
Mehari ZH, Elad Y, Rav-David D, Graber ER, Meller Harel Y (2015) Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 395:31–44. https://doi.org/10.1007/s11104-015-2445-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gabhane, J.W., Bhange, V.P., Patil, P.D. et al. Recent trends in biochar production methods and its application as a soil health conditioner: a review. SN Appl. Sci. 2, 1307 (2020). https://doi.org/10.1007/s42452-020-3121-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3121-5