Abstract
Activated carbon and silica gel adsorbents are commonly used as sampling agents in small glass tube products for work environment measurements of organic solvent vapor in air. In the measurements, extraction efficiency of organic solvent components from sampling agents is very important for accuracy of the determination. We have investigated the effect of two representative efficiency determination methods as established by the Industrial Safety and Health Act in Japan: the direct addition and phase equilibrium methods, using both of these adsorbent materials found in typical recent sampling tube products in Japan and four types of alcohol. The results indicate that the phase equilibrium method has a tendency to show slightly higher values compared with the direct addition method for each adsorbent. In addition, compared with silica gel, petroleum-based activated carbon can be a preferable sampling agent of alcohols in the extremely low concentration region, below approximately 10 ppm.
Similar content being viewed by others
1 Introduction
When evaluating and controlling work environments typified by various types of industrial plants, the measurement techniques of harmful organic solvent vapors in air are very important [1,2,3,4,5,6,7,8,9]. In work environment measurements, organic solvent vapors are collected by a small glass sampling tube loaded with a sampling agent using a suction pump at a constant time and flow rate. The organic solvent components are then extracted from the agent by an extraction solvent, e.g., carbon disulfide or methanol, and the test liquid is analyzed by gas chromatography. Next, the organic vapor concentration in air is calculated from the result and the collected volume of air. This method for measuring the organic vapor concentration in air using solvent extraction is called “the solid collection method.” Conventional activated carbon and silica gel \(\left( {{\text{SiO}}_{2} \cdot n{\text{H}}_{2} {\text{O}}} \right)\) adsorbents are typical sampling agents used in sampling tube products [10,11,12,13,14,15,16,17,18,19,20,21,22] for this method. In the current market, other materials for preconcentration are expensive and not effective in work environment measurements compared with these two types of adsorbents. The extraction efficiency of the organic solvent components is a very important factor for the accuracy of the determination [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. It is desirable to figure out the efficiency before work environment measurements by the solid collection method. Particularly, the efficiency depends on the types and concentration of the organic solvents, and this tendency influences the accuracy of the results. Therefore, understanding the relation between the determination methods and the organic solvent extraction efficiency is a significant issue for precise work environment measurements. In this regard, however, an international standard for determination of processing methods has yet to be defined. At the present, two existing representative methods: the direct addition and phase equilibrium methods (described later), are used to determine efficiency [9]; however, choice between the two methods is left to the judgment of the measurer, and their detailed properties have been unresolved until now. Most recently, clarification of the differences and relative merits of each method has drawn attention for the improvement of work environment measurements. In this study, we have investigated the effect of the two determination methods on efficiency, using activated carbon and silica gel adsorbents from typical recent sampling tube products in Japan and four types of alcohol.
2 Experimental method
When measuring the extraction efficiencies of organic solvent components from adsorbent specimens, it is difficult to achieve sufficient accuracy and repeatability in the preparation and handling of rarefied organic vapors at a few ppm levels as standard samples. Therefore, as established by the Industrial Safety and Health Act in Japan, in many cases of work environment measurements, extraction efficiencies are measured by the use of only a liquid sample and solid-state adsorbents through two representative methods; direct addition and phase equilibrium methods [9, 17,18,19,20]. The schematics of both measurement methods are presented in Supplementary Fig. 1. In the phase equilibrium method, test liquid solutions are prepared with a given amount of the sampling agent, organic solvent of interest comparable to the collection amount and extraction solvent through one-time admixture in a vial container. After settling time (≥ 1 h) with occasional shaking, the concentration of the organic solvent of interest in the test liquid is measured by gas chromatography. The phase equilibrium method has particular benefits in repeatability and simplicity compared with measurements using rarefied organic vapors. In contrast, in the direct addition method, the organic solvent used as the measuring object and the extraction solvent are added to the sampling agent separately. First, an amount of the organic solvent (comparable to the collection amount) is added to a given amount of the sampling agent using a microsyringe or micropipette in a vial container. Then, the vial container is stored in a refrigerator for a certain period of time to precipitate the adsorption of the organic solvent into the sampling agent and its stabilization without volatilization of the organic solvent component. After cooling down, a certain amount of the extraction solvent is added into the vial container, and the subsequent experimental procedure is similar to that of the phase equilibrium method. In each measuring method, test liquid solutions are prepared with a given amount of sampling agent, organic solvent of interest comparable to amount of collection and extraction solvent. After the settling time ( ≥ 1 h) with occasional shaking, the concentration of the organic solvent of interest in the test liquid is measured by gas chromatography. The concentration is calculated from the peak area of data by the gas chromatograph. Finally, the extraction efficiency is calculated from the results and the initial amount of the organic solvent components.
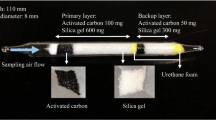
The direct addition method is preferable because it resembles the actual sampling process of organic solvent vapors by the solid collection method in the collection by adsorbent materials and successive extraction from the materials. However, its experimental procedure is a little more complicated than the phase equilibrium method, and the effect of volatilization of the organic solvent components and extraction solvents on accuracy and repeatability are causes for concern. In this study, we aim to clarify the difference and relative merits of each method. Previously [17,18,19], we studied the relation between the material properties and organic solvent extraction efficiency characteristics when determined by the phase equilibrium method. We collected activated carbon and silica gel adsorbents used in typical commercially available sampling tube products in Japan as samples. Then, we investigated the physical and chemical properties of the activated carbon and silica gel specimens according to the methods described in previous reports [17,18,19]. Based on the results, this study was performed using the activated carbon and silica gel specimens, which showed the most preferable results in the previous studies (activated carbon samples F and G [17], and silica gel sample D [19]). The activated carbon specimens were a coconut shell activated carbon and a petroleum-based activated carbon. The principal experimental conditions for the measurement of the extraction efficiency and the properties of the sampling agents are described in Tables 1 and 2.
In many cases of work environment measurements, silica gel adsorbents are used for alcohols and other polar organic substances below approximately a few hundred ppm. We used 1-butanol, 2-butanol, cyclohexanol and isopentyl alcohol (3-methyl-1-butanol) for the measurements of extraction efficiencies in this study. These are often used in the industrial field, as illustrated in the Industrial Safety and Health Act in Japan. The extraction efficiency is affected by the type of extraction solvents, which in this study were chosen as methanol for the silica gel adsorbent and carbon disulfide for the activated carbon adsorbents. Methanol and carbon disulfide are currently in common use in work environment measurements, and for this reason, applied in this study. We used products from FUJIFILM Wako Pure Chemical Corp. for each solvent. A SHIMADZU GC–14B gas chromatograph, which was equipped with a hydrogen flame ionization detector (FID), and a C–R8A Chromatopac data processor was used for the measurements. A capillary column SHIMADZU CBP1–S25–050 or CBP20–S25–050 was also employed. Each measurement was performed at a constant temperature (313–333 K). Helium gas (≥ 99.995%) was used as the carrier gas for each measurement by the gas chromatograph.
3 Results and discussion
The extraction efficiency changes depending on the relative amounts of sampling agent and extraction solvent. In the present study, we standardized the amounts of activated carbon or silica gel and the extraction solvent used for each measurement to facilitate a suitable comparison of the measurement results. In each measurement, a certain amount of the silica gel specimen (approximately 70 mg) and activated carbon specimens (approximately 40 mg) were used. Each amount was decided from suitability of measurements using a vial container of 1.5 mL and the effect of relative amounts of sampling agent and extraction solvent. In this regard, previous reports indicated that when both the mass of the activated carbon sampling agent (~ 100 mg) and the amount of carbon disulfide used as extraction solvent (1–3 mL) show relatively small change, the extraction efficiency does not change significantly [12, 13, 17]. In the phase equilibrium method, we prepared the test liquid solutions in reference to the control concentration of work environment standards established by the Industrial Safety and Health Act in Japan [9] (1-butanol: 25 ppm, 2-butanol: 100 ppm, cyclohexanol: 25 ppm, isopentyl alcohol: 100 ppm, all at 298 K). Test solutions corresponding to 0.01 E, 0.025 E, 0.05 E, 0.075 E, 0.1 E, 0.5 E and 1.0 E (where E = control concentration) of the organic solvents were prepared. The added amount of each organic solvent per mL of methanol and carbon disulfide at 1.0 E was comparable to the content in 5 L of air at 298 K. Approximately, 1 mL of the test solution was added to each sampling agent specimen in a vial container at room temperature (approximately 290–299 K). After 1 h, the solution sample was measured by gas chromatography using a microsyringe. In contrast, in the direct addition method, the organic solvent of interest comparable to the collection amount (0.01 E, 0.025 E, 0.05 E, 0.075 E, 0.1 E, 0.5 E and 1.0 E) was added to a given amount of the sampling agent in the vial container at the beginning. In some cases, the organic solvent component is added as a liquid solution in the extraction solvent because of its low volume. After cooling down in a refrigerator, approximately 1 mL of the extraction solvent was added to the vial container, and the subsequent experimental procedure was similar to that of the phase equilibrium method.
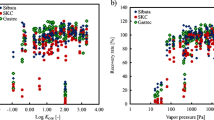
Figure 1 shows the measured extraction efficiencies of the organic solvent components from the silica gel specimen determined by the direct addition and the phase equilibrium methods. The extraction efficiencies depend on the types of organic solvents used. In addition, the efficiencies are markedly unstable for objective organic solvent concentration ≤ 10 ppm. This instability was noticeable in the case of cyclohexanol and isopentyl alcohol. On the other hand, the efficiencies of 2-butanol show a gradual decrease with reduced concentration, and the efficiencies of 1-butanol show relative stability in the data. The deference in tendencies of the extraction efficiencies for each alcohol seems to be connected with their molecular size and shape. In either case, the efficiencies in the figures show a specific tendency toward degradation in the low concentration region for all four types of alcohol.
As a whole, the results of the silica gel specimen indicate that the phase equilibrium method has a tendency to show slightly higher values for measured extraction efficiencies than the direct addition method for the four types of alcohol. However, the overall variation of the efficiencies with concentration of the objective organic solvents in both measuring methods is similar, and the slightly higher values indicated in the phase equilibrium method are attributed to its experimental procedure, particularly the one-time admixture of the sampling agent, organic solvent of interest comparable to the collection amount, and extraction solvent. That is, the slightly higher values recorded by the phase equilibrium method are pseudo-observed data, and the direct addition method is preferable for determining an accurate extraction efficiency, though it has a cumbersome experimental procedure. In the results, no concerning effects from volatilization of the organic solvent components and extraction solvents on the accuracy and repeatability were found in the direct addition method.
Figure 2 shows the extraction efficiencies of the organic solvent components from the coconut shell activated carbon specimen as determined by both the measuring methods. In the results, a similar instability of the extraction efficiencies was also confirmed in experiments using the silica gel specimen in the approximate region of concentration ≤ 10 ppm; however, taken as a whole, the difference between the values recorded by the two measuring methods diminishes compared with the silica gel specimen case. In addition, the variation of efficiencies with concentration of the four types of alcohol in both measuring methods is almost identical. Coconut shell activated carbon is well known as a hydrophobic agent, and silica gel has been recommended as a sampling agent of alcohols in work environment measurements due to its hydrophilic property and because most low-molecular-weight alcohols are soluble in water. Of course, activated carbon is able to collect or adsorb rarefied alcohol vapors at less than a dozen ppm around room temperature [25, 26]. The extraction efficiencies in Fig. 2 are liable to be fluctuating with concentration of the four types of alcohol, and the noted effectiveness of the coconut shell activated carbon specimen was not seen, in contrast with the silica gel specimen. In the figures of this study, several data show extraction efficiencies > 1.00. These results seem to be attributable to effects from the measurement precision of gas chromatography system used in this study. A complete resolution of the experimental error is difficult, and the data > 1.00 was deemed to be almost 1.00.
Figure 3 shows the extraction efficiencies of the organic solvent components from the petroleum-based activated carbon specimen determined by both measuring methods. The distributions of the extraction efficiencies by both measuring methods with the concentration of the four types of alcohol seem to be similar to the case of the coconut shell activated carbon specimen; however, the results are improved and comparatively more stable over a wide range of organic solvent concentrations than for the coconut shell activated carbon specimen. The results in this study correspond to features reported in previous reports [15,16,17,18, 22]. The difference in the values recorded by the two measuring methods also diminishes compared with the silica gel specimen. The activated carbon specimens in this study were microporous materials [17]. In contrast, the silica gel specimen is a mesoporous material [19], and the difference in pore development and pore size distributions are expected to have an effect on the extraction efficiencies recorded by the two measuring methods. That is, the pore development in the mesoporous region of the silica gel specimen seems to give rise to a noticeable difference in the adsorption equilibrium of alcohols between the two measuring methods. On the other hand, the activated carbon specimens in this study do not have a significant pore development in the mesoporous region.
Compared with the silica gel specimen, the petroleum-based activated carbon specimen shows better extraction efficiency of alcohols in the extremely low concentration region (below approximately 10 ppm) (Fig. 4). The results indicate that the high degree of affinity of the silica gel specimen for alcohols poses an impediment in the extraction. As a result, the petroleum-based activated carbon can be a more suitable sampling agent for measurement of alcohols in this region than silica gel. In addition to the type of objective organic solvent, its concentration is also a critical factor in choosing a suitable sampling agent for accurate work environment measurement. This is a subject for future investigation into work environment evaluation improvement.
4 Conclusions
In this study, we have investigated the effect of two representative determination methods of the extraction efficiency: the direct addition and phase equilibrium methods, using activated carbon and silica gel adsorbent materials used in typical recent sampling tube products in Japan and four types of alcohol: 1-butanol, 2-butanol, cyclohexanol and isopentyl alcohol (3-methyl-1-butanol). The results in this study indicate that the phase equilibrium method has a tendency to show slightly higher values than the direct addition method for both types of adsorbent materials. In other words, the direct addition method is more preferable for the accurate determination of extraction efficiency of sampling agents, though it has a cumbersome experimental procedure. In addition, petroleum-based activated carbon can be a more efficient sampling agent of alcohols than silica gel adsorbents, particularly in the extremely low concentration region, below approximately 10 ppm.
References
Whitman NE, Johnston AE (1964) Sampling and analysis of aromatic hydrocarbon vapors in air: a gas–liquid chromatographic method. Am Ind Hyg Assoc J 25:464–469
Reid FH, Halpin WR (1968) Determination of halogenated and aromatic hydrocarbons in air by charcoal tube and gas chromatography. Am Ind Hyg Assoc J 29:390–396
Thain W (1980) Monitoring toxic gases in the atmosphere for hygiene and pollution control. Pergamon, Oxford
American Society for Testing and Materials (1987) Sampling and calibration for atmospheric measurements. In: Taylor JK (ed.), ASTM Special Technical Publication 957, ASTM International, Philadelphia, U.S.A
Berezkin VG, Drugov YS (1991) Gas chromatography in air pollution analysis. In: Journal of chromatography library, vol 49. Elsevier Science Publishers, Amsterdam
Leung MKH, Liu C-H, Chan AHS (2005) Occupational exposure to volatile organic compounds and mitigation by push–pull local exhaust ventilation in printing plants. J Occup Health 47:540–547
Gallego E, Roca FJ, Perales JF, Guardino X (2010) Comparative study of the adsorption performance of a multi-sorbent bed (Carbotrap, Carbopack X, Carboxen 569) and a Tenax TA adsorbent tube for the analysis of volatile organic compounds (VOCs). Talanta 81:916–924
Król S, Zabiegała B, Namieśnik J (2010) Monitoring VOCs in atmospheric air II. Sample collection and preparation. TrAC Trends Anal Chem 29:1101–1112
The Japan Association for Working Environment Measurement (2012) Working environment measurement guidebook Vol. 5 Organic solvents (4th edition). The Japan Association for Working Environment Measurement, Tokyo, Japan (in Japanese)
Tada O, Cai SX (1980) Determination of organic solvents in air using charcoal tube and silica gel tube. J Sci Labour 56:453–467 (in Japanese)
Ashida T, Koike S, Omori K (1981) Research on measurement of organic solvent vapors using activated carbon tube I. J Work Environ 2:53–59 (in Japanese)
Fukabori S, Tada O, Sugai T (1981) Determination of organic solvent vapours in air. J Sci Labour 57:11–23 (in Japanese)
Ashida T, Koike S, Omori K (1983) Research on measurement of organic solvent vapors using activated carbon tube II. J Work Environ 4:52–57 (in Japanese)
Ashida T, Koike S (1984) Research on measurement of organic solvent vapors using activated carbon tube III. J Work Environ 5:51–58 (in Japanese)
Kaifuku Y, Matsunobu K, Wakayama M (2008) Technical information; Spherical activated carbon sampling tube Cat. No. 258 for solvent desorption. J Work Environ 29:35–39 (in Japanese)
Yoshikawa M, Kusumoto J, Nakamura A, Kaifuku Y (2009) Adsorption and desorption characteristics of an active carbon derived from petroleum pitch for twenty organic compounds. J Work Environ 30:53–59 (in Japanese)
Abiko H (2015) The organic solvent extraction efficiency of activated carbon used in sampling tube products. TANSO 2015:201–208
Abiko H (2016) Effect of porosity of activated carbon adsorbents for work environment measurement on extraction efficiency of organic solvents. Sangyo Eiseigaku Zasshi 58:100–105 (in Japanese)
Abiko H (2017) Silica gel adsorbents for sampling tube in work environment measurements and their extraction efficiency of alcohols. J Ceram Soc Jpn 125:175–179
Abiko H (2019) Determination of extraction efficiency of alcohols from silica gel sampling agents in low concentration region. In: The 13th Pacific rim conference of ceramic societies, Okinawa, Japan. Program book 29–P–S06–21
Abiko H (2020) Dependence of the extraction efficiency of activated carbon adsorbents for work environment measurement on the concentration of organic solvents. Sangyo Eiseigaku Zasshi 62:192–197 (in Japanese)
Miyake Y, Tokumura M, Wang Q, Wang Z, Amagai T (2017) Comparison of the volatile organic compound recovery rates of commercial active samplers for evaluation of indoor air quality in work environments. Air Qual Atmos Health 10:737–746
Dommer RA, Melcher RG (1978) Phase equilibrium method for determination of desorption efficiencies. Am Ind Hyg Assoc J 39:240–246
Kuroda D (1987) Round robin tests for determination of organic solvent vapours in air with charcoal tube samplers. Jpn Anal 36:T21–T24 (in Japanese)
Abiko H (2014) Estimation equation of affinity coefficients of organic vapors using molar polarization for several types of activated carbon. Mater Sci Technol Jpn 51:68–74 (in Japanese)
Tamon H, Murakami N, Okazaki M (1997) Correlation of affinity coefficient for gas adsorption by structure–activity parameter. J Chem Eng Jpn 30:735–741
Funding
This study was funded by National Institute of Occupational Safety and Health, Japan Organization of Occupational Health and Safety (Fundamental Research N–F31–02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author has no conflict of interest or competing interest in this study.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Conceptual diagram of the phase equilibrium method and the direct addition method for determining the extraction efficiency of organic solvent components from the adsorbents (DOCX 33 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abiko, H. Extraction efficiency of alcohols from activated carbon and silica gel sampling agents in the low concentration region. SN Appl. Sci. 3, 206 (2021). https://doi.org/10.1007/s42452-020-03997-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03997-0