Abstract
The Industrial Safety and Health Law in Japan established administrative levels for volatile organic compounds (VOCs) in indoor air. In the present study, these 49 VOCs were extracted from the absorbents of commercial active samplers from Sibata Scientific Technology (carbon-bead active sampler), SKC Inc. (Anasorb CSC sorbent tube), and Gastec (bead-shaped activated carbon tube) using carbon disulfide, and the recovery rates were compared. The VOCs were added to the adsorbents at three concentration levels relative to the administrative levels (×0.5, ×1, and ×2). The following mean recovery rates of the 49 VOCs were obtained at the ×0.5, ×1, and ×2 levels: 86, 93, and 92% for the Sibata sampler; 78, 82, and 84% for the SKC sampler; and 94, 93, and 90% for the Gastec sampler. With the Sibata sampler, the recovery rates of 78% (×0.5), 84% (×1), and 90% (×2) of the VOCs measured in this study were adequate (80–120%); the corresponding percentages for the SKC sampler were 67% (×0.5), 69% (×1), and 69% (×2), and those for the Gastec sampler were 92% (×0.5), 86% (×1), and 86% (×2). The effects of the octanol–water partition coefficients and vapor pressures of the VOCs on the recovery rates were investigated. The recovery rates increased with increases in the octanol–water partition coefficient and the vapor pressure and then leveled off. The recovery rates for the o-, m-, and p-cresol isomers were much lower than those obtained for other VOCs at all three concentration levels and with all samplers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To evaluate indoor air quality, active sampling of indoor air pollutants with an adsorbent tube is used for environmental and occupational applications (Ohura et al. 2009; Gallego et al. 2010; Ramírez et al. 2010; Chin et al. 2013; Jumpponen et al. 2013; Tunsaringkarn et al. 2015; Song et al. 2016). Shinohara et al. (2013) used an active sampler to collect samples to measure 11 volatile organic compounds (VOCs), including toluene, p-dichlorobenzene, α-pinene, and aldehydes (formaldehyde and acetaldehyde) in 19 temporary houses in Minami-soma City, Japan, following the Great East Japan earthquake. In another study, nitrogen dioxide concentrations were also measured using an active sampler (Shinohara et al. 2014). In an occupational application, Chen et al. (2014) used an active sampler to collect samples to measure the concentrations of 8 VOCs (e.g., benzene, toluene, and xylenes) in the passenger cabins of 38 taxis in Changsha, China. Their results indicated that VOC concentrations in taxis could be a health risk to passengers and drivers.
Generally, exposure to some VOCs is likely to be higher in occupational settings than in residential indoor and outdoor settings (Jo and Song 2001; Jia et al. 2008; Majumdar et al. 2008; Freberg et al. 2014; Tokumura et al. 2016). Exposure to VOCs from solvent use tends to be high (Leung et al. 2005; Uang et al. 2006; Vitali et al. 2006). Attarchi et al. (2013) reported that workers in a car-manufacturing plant, who were occupationally exposed to VOCs originating from mixed organic solvents (e.g., benzene, toluene, and xylenes), had a high risk of hypertension. In Japan, to protect workers, the Industrial Safety and Health Law established administrative levels for the concentrations of 49 VOCs in indoor work environments, where VOCs are used as solvents (The Japan Association for Working Environment Measurement 2012). The use of active samplers is certified by the Industrial Safety and Health Law in Japan for collection of air samples for VOC analysis.
Nowadays, there are many types of active samplers commercially available (Król et al. 2010; Gallego et al. 2011). Samplers differ in type of adsorbent (e.g., activated carbon, silica gel, and polyurethane foam) and construction (e.g., single layer and double layer), and samplers can be targeted to the physicochemical properties of the VOCs of interest to optimize extraction. Activated carbon is frequently used as an adsorbent for VOCs because it is inexpensive, has a large adsorption capacity, and is adaptable to many types of chemicals. Activated carbons in commercial active samplers can be produced from different precursor materials and differ in their specific surface areas and particle sizes. These characteristics influence adsorption of VOCs and eventually affect the accuracy of the measurement. However, to date, few studies have investigated the effects of these characteristics on accuracy. Borrás et al. (2012) investigated optimization of an active sampler/extraction solvent combination using ORBO-32 activated coconut charcoal (Sigma-Aldrich, St. Louis, MO) and Anasorb CSC coconut charcoal (SKC Ltd., Eighty Four, PA) as the active samplers and hexane and toluene as the extraction solvents. Carbon disulfide in the gas phase was measured to determine the recovery rate, repeatability, reproducibility, and detection limit. According to their results, the Anasorb CSC coconut charcoal sampler in combination with hexane provided adequate sensitivity, good linearity, and a fast and easy protocol for monitoring trace carbon disulfide in air. Abiko (2015) compared the recovery rates of six VOCs (toluene, 1-butanol, acetone, cyclohexanone, ethylene glycol monoethyl ether, and butyl acetate) using eight commercial active samplers to investigate what parameters could directly influence the accuracy of determination. The investigator used activated carbons prepared from coconut shell and petroleum and found that the average particle diameter and the precursor material used to prepare the adsorbent affected the recovery rate. However, not enough samples were analyzed to be able to observe trends in the data, and the recovery rates fluctuated with the type of sampler and VOC. Moreover, the number of VOCs analyzed was limited. Therefore, a more comprehensive study with many kinds of VOCs is required to obtain consistent results.
The aim of this study was to compare the recovery rates obtained with different commercial active samplers for 49 VOCs (Table 1), including isomers, that are included in the Industrial Safety and Health Law in Japan. The commercial active samplers selected were from Sibata, SKC, and Gastec, and the VOCs were added to the absorbents at three concentration levels (0.5, 1, and 2 times the administrative levels) and were extracted using carbon disulfide. The effects of physicochemical properties (octanol–water partition coefficient [log K OW] and vapor pressure) of the VOCs on the recovery rates were also investigated.
Methods
Chemicals and materials
Standards of 1,1,1-trichloroethane and methyl n-butyl ketone were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Isopropyl alcohol and 1,2-dichloroethane were obtained from Kanto Chemical Co. (Tokyo, Japan) and Dojindo Molecular Technologies, Inc. (Rockville, MD), respectively. All other chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Deuterated toluene (toluene-d 8) was obtained from Cambridge Isotope Laboratories (Tewksbury, MA). Carbon disulfide (Wako Pure Chemical Industries, Ltd.) was used as an extraction solvent. Helium gas (99.999%) was supplied by Taiyo Nippon Sanso Corporation (Tokyo, Japan).
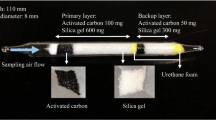
The active samplers purchased for this study were a carbon-bead active sampler (080150–090, Sibata Scientific Technology, Ltd., Saitama, Japan), an Anasorb CSC sorbent tube (SKC 226–01, SKC Inc., Eighty Four, PA, USA), and a bead-shaped activated carbon tube (No. 258, Gastec Co., Ayase, Kanagawa, Japan). The parameters for these commercial active samplers are given in Table 2, and the pictures of them are shown in Fig. S1.
Determination of recovery rates for VOCs from the adsorbents in the commercial active samplers
Taking into account the air sampling volume (1 L) determined by the analytical method established by the Industrial Safety and Health Act in Japan (The Japan Association for Working Environment Measurement 2012), the amounts of addition of VOCs to the sampler adsorbents for each concentration level were determined. To simplify the addition of VOCs in solvent (carbon disulfide) to the sampler adsorbents, a separate VOC mixed standard solution was prepared for each concentration level so that the volume of this mixed standard solution added to the adsorbent was 4 μL. This resulted in two mixed standards for the ×0.5 level, four mixed standards for the ×1 level, and eight mixed standards for the ×2 level. The VOCs in each of the mixed standards are detailed in Tables S1 to S3. For each mixed standard solution, a 4-μL aliquot was added to the adsorbent in a commercial active sampler. Then, the sampler was stored in a refrigerator overnight. The adsorbent was transferred to a 4-mL vial, and 0.5 mL of toluene-d 8 was added as a syringe spike to correct ionization efficiencies of VOCs during their analysis using gas chromatography–mass spectrometry. The concentration of toluene-d 8 in all samples was 100 μg/mL, except for in the Gastec ×0.5 and ×1 samples, which all had a toluene-d 8 concentration of 56 μg/mL. The adsorbed VOCs were extracted by shaking with 1 mL of carbon disulfide for 60 min. The VOCs in the extract were analyzed by gas chromatography–mass spectrometry using the analytical parameters summarized in Table 3. The recovery rate was calculated by dividing the peak area of the VOC in the extract by the average peak area for a blank solution of the same VOC without an adsorbent, and the resulting value was corrected using the toluene-d 8 peak. The number of each type of sampler used was either five or six.
Results and discussion
Recovery rates for the VOCs from adsorbents in the commercial active samplers
The recovery rates of 49 VOCs added to the adsorbents of the 3 commercial active samplers were evaluated after extraction with carbon disulfide, and the means, standard deviations, and relative standard deviations (RSDs) were calculated (Table 4). The raw data (peak areas of the VOCs in the extracts) are given in Tables S1 to S3. The results are also presented in Fig. S2.
For the Sibata sampler, the recovery rates ranged from 5.4% for p-cresol to 113% for cis-1,2-dichloroethylene at the ×0.5 level, 22% for p-cresol to 114% for ethylene glycol monoethyl ether at the ×1 level, and 20% for p-cresol to 113% for 1,2-dichloroethane at the ×2 level. The mean recovery rates for the ×0.5, ×1, and ×2 levels were 86, 93, and 92%, respectively. Satisfaction ratios were calculated as the proportion of VOCs with adequate recovery rates (80–120%). The satisfaction ratios were 78, 84, and 90% for the ×0.5, ×1, and ×2 levels, respectively. Inadequate recovery rates were obtained at some of the concentration levels for 1-butanol (×0.5); o-, m-, and p-cresol (all levels); dichloromethane (×0.5); N,N-dimethylformamide (all levels); ethylene glycol mono-n-butyl ether (×0.5 and ×1); ethylene glycol monoethyl ether (×0.5 and ×2); ethylene glycol monoethyl ether acetate (×1); isobutyl alcohol (×0.5 and ×1); isopropyl alcohol (×1); methyl acetate (×0.5); and methyl ethyl ketone (×0.5). The recovery rates for the cresol isomers were much lower than the recovery rates for any of the other VOCs.
The recovery rates for the SKC sampler ranged from 1.0% for o-cresol to 109% for cis-1,2-dichloroethylene at the ×0.5 level, 3.1% for p-cresol to 104% for trichloroethylene at the ×1 level, and 4.8% for p-cresol to 116% for cis-1,2-dichloroethylene at the ×2 level. The mean recovery rates for the three levels were 78, 82, and 84%, and the satisfaction ratios were 67, 69, and 69%. Inadequate recovery rates were obtained at some of the concentration levels for acetone (×2); 1-butanol (all levels); o-, m-, and p-cresol (all levels); cyclohexanol (all levels); cyclohexanone (all levels); N,N-dimethylformamide (all levels); ethyl ether (×2); ethylene glycol mono-n-butyl ether (all levels); ethylene glycol monoethyl ether (all levels); ethylene glycol monoethyl ether acetate (×0.5 and ×1); isopentyl alcohol (all levels); isopropyl alcohol (×1); methyl acetate (×0.5); methyl ethyl ketone (×0.5); 4-methylcyclohexanol (×0.5 and ×1); and styrene (×0.5 and ×1). The recovery rates for the cresol isomers, N,N-dimethylformamide, ethylene glycol mono-n-butyl ether, and ethylene glycol monoethyl ether were much lower than the recovery rates for the other VOCs at all the concentration levels.
The recovery rates for the Gastec sampler ranged from 11% for p-cresol to 109% for benzene at the ×0.5 level, 13% for p-cresol to 116% for n-hexane at the ×1 level, and 13% for p-cresol to 113% for 1,2-dichloroethane at the ×2 level. The mean recovery rates for the three levels were 94, 93, and 90%, and the satisfaction ratios were 92, 86, and 86%. Inadequate recovery rates were obtained at some of the concentration levels for acetone (×2); o-, m-, and p-cresol (all levels); N,N-dimethylformamide (all levels); ethylene glycol monoethyl ether (×1 and ×2); and p-xylene (×1). The cresols and N,N-dimethylformamide had much lower recovery rates than the other VOCs at all the concentration levels.
A comparison of the recovery rates among the commercial active samplers showed that the Sibata and Gastec samplers showed good recovery rates. The adsorbents in these samplers are petroleum based. According to an earlier study (Abiko 2015), petroleum-based activated carbons tend to show better recovery rates than coconut shell-based activated carbons. This tendency is in good agreement with our results. Among the VOCs, the cresol isomers (o-, m-, and p-cresol) showed the lowest recovery rates at all concentration levels and with all samplers. The recovery rate of N,N-dimethylformamide was also much lower than the recovery rates of other VOCs with all samplers except that from Sibata.
The satisfaction ratios for the RSDs (10 or 15%) were 80% (RSD < 10%) and 94% (RSD < 15%) for the Sibata sampler (the petroleum-based adsorbents), 50% (RSD < 10%) and 76% (RSD < 15%) for the SKC sampler (the coconut shell-based adsorbent), and 81% (RSD < 10%) and 92% (RSD < 15%) for the Gastec sampler (the petroleum-based adsorbents). The cresol isomers, dichloromethane, isopropyl alcohol, and methyl acetate likely had higher RSDs at most concentration levels and with most of the samplers. As was the case for the recovery rates, better RSDs were obtained with the petroleum-based adsorbents (Sibata and Gastec) than with the coconut shell-based adsorbent (SKC).
In summary, the satisfaction ratio of adequate recovery rate with adequately low RSD (10 or 15%) were 69% (RSD < 10%) and 78% (RSD < 15%) for the Sibata sampler, 44% (RSD < 10%) and 63% (RSD < 15%) for the SKC sampler, and 76% (RSD < 10%) and 84% (RSD < 15%) for the Gastec sampler.
Effects of the physicochemical properties of the VOCs on recovery rates
Generally, the recovery rate of a VOC can be affected by its physicochemical properties, and the optimum adsorbent or sampler for a target VOC can be selected on the basis of these properties. In this study, the effects of two physicochemical properties, log K OW and vapor pressure, on the recovery rates of the 49 VOCs added at 3 concentration levels to the adsorbents in the 3 commercial active samplers were evaluated after extraction with carbon disulfide.
For log K OW (Fig. 1a), the general trend observed was that the recovery rates increased with increases in log K OW and then leveled off at around log K OW = 0. The solvent used in this study was carbon disulfide, which is non-polar. Therefore, eluting polar VOCs (which generally have relatively low log K OW values) from the adsorbents with this solvent was difficult. However, there were some outliers, which were the cresol isomers. Although the cresol isomers all have a log K OW of 2.06, their recovery rates ranged from 1 to 31%. With the SKC sampler, ethylene glycol mono-n-butyl ether, ethylene glycol monoethyl ether, and ethylene glycol monoethyl ether acetate did not fit the general trend, which suggested that this sampler was incompatible with these specific VOCs.
For the vapor pressure (Fig. 1b), increases in vapor pressure up to 500 Pa led to higher recovery rates. After this point, the recovery rates leveled off. This trend was similar to that observed for log K OW. Generally, VOCs with lower vapor pressures are more likely to adsorb onto an adsorbent, which could make these VOCs more difficult to desorb than VOCs with higher vapor pressures. Outliers were also found in the vapor pressure data. N,N-Dimethylformamide, ethylene glycol monoethyl ether, and ethylene glycol monoethyl ether acetate did not follow the general trend. The log K OW values of N,N-dimethylformamide, ethylene glycol monoethyl ether, and ethylene glycol monoethyl ether acetate are −0.93, −0.42, and 0.59, respectively, which are the lowest values among the VOCs measured in this study, except for acetone (−0.24). These results indicate that the recovery rates of these VOCs must be affected more by their log K OW values than by their vapor pressures.
On the other hand, polarity of solvent could be a property which could affect the recovery rates of these VOCs. For example, VOCs, which showed low recovery rates (e.g., cresol isomers and N,N-dimethylformamide), could be expected to be more successfully extracted using polar solvent (e.g., acetone). However, polar solvent would not be adequate for extraction of non-polar VOCs.
Conclusions
Forty-nine VOCs, for which administrative levels for work environments were established by the Industrial Safety and Health Law in Japan, were added to the adsorbents in three commercial active samplers (Sibata, SKC, and Gastec) at three concentration levels compared to the administrative levels (×0.5, ×1, and ×2) and were extracted using carbon disulfide. The Sibata and Gastec samplers, which are petroleum based, showed good recovery rates and RSDs for the 49 VOCs. Among the VOCs, cresol isomers (o-, m-, and p-cresol) showed the lowest recovery rates at all the concentration levels and with all samplers. With all samplers except for the Sibata sampler, the recovery rate of N,N-dimethylformamide was much lower than the recovery rates for other VOCs.
An investigation of the effects of two physicochemical properties, log K OW and vapor pressure, of the VOCs on the recovery rates showed that the recovery rates increased with increases in log K OW and vapor pressure up to a certain point. VOCs with log K OW greater than 0 and vapor pressure greater than 500 Pa tended to show good recovery rates.
The comprehensive data of VOC recovery rates could help to select the optimum sampler for evaluation of indoor air quality in work environments.
References
Abiko H (2015) The organic solvent extraction efficiency of activated carbon used in sampling tube products. TANSO 2015:201–208
Attarchi M, Golabadi M, Labbafinejad Y, Mohammadi S (2013) Combined effects of exposure to occupational noise and mixed organic solvents on blood pressure in car manufacturing company workers. Am J Ind Med 56:243–251
Borrás E, Ródenas M, Dieguez JJ, Pérez-García ML, Lomba R, Lavín J, Tortajada-Genaro LA (2012) Development of a gas chromatography–mass spectrometry method for the determination of carbon disulfide in the atmosphere. Microchem J 101:37–42
Chen X, Feng L, Luo H, Cheng H (2014) Analyses on influencing factors of airborne VOCS pollution in taxi cabins. Environ Sci Pollut Res 21:12,868–12,882
Chin JY, Godwin C, Jia C, Robins T, Lewis T, Parker E, Max P, Batterman S (2013) Concentrations and risks of p-dichlorobenzene in indoor and outdoor air. Indoor Air 23:40–49
Freberg BI, Olsen R, Daae HL, Hersson M, Thorud S, Ellingsen DG, Molander P (2014) Occupational exposure assessment of airborne chemical contaminants among professional ski waxers. Ann Occup Hyg 58:601–611
Gallego E, Roca FJ, Perales JF, Guardino X (2010) Comparative study of the adsorption performance of a multi-sorbent bed (Carbotrap, Carbopack X, carboxen 569) and a Tenax TA adsorbent tube for the analysis of volatile organic compounds (VOCs). Talanta 81:916–924
Gallego E, Roca FJ, Perales JF, Guardino X (2011) Comparative study of the adsorption performance of an active multi-sorbent bed tube (Carbotrap, Carbopack X, carboxen 569) and a Radiello® diffusive sampler for the analysis of VOCs. Talanta 85:662–672
Jia C, D'Souza J, Batterman S (2008) Distributions of personal VOC exposures: a population-based analysis. Environ Int 34:922–931
Jo W-K, Song K-B (2001) Exposure to volatile organic compounds for individuals with occupations associated with potential exposure to motor vehicle exhaust and/or gasoline vapor emissions. Sci Total Environ 269:25–37
Jumpponen M, Rönkkömäki H, Pasanen P, Laitinen J (2013) Occupational exposure to gases, polycyclic aromatic hydrocarbons and volatile organic compounds in biomass-fired power plants. Chemosphere 90:1289–1293
Król S, Zabiegała B, Namieśnik J (2010) Monitoring VOCs in atmospheric air II. Sample collection and preparation. TrAC Trends Anal Chem 29:1101–1112
Leung MKH, Liu C-H, Chan AHS (2005) Occupational exposure to volatile organic compounds and mitigation by push-pull local exhaust ventilation in printing plants. J Occup Health 47:540–547
Majumdar D, Dutta C, Mukherjee AK, Sen S (2008) Source apportionment of VOCs at the petrol pumps in Kolkata, India; exposure of workers and assessment of associated health risk. Transp Res Part D: Transp Environ 13:524–530
Ohura T, Amagai T, Shen X, Li S, Zhang P, Zhu L (2009) Comparative study on indoor air quality in Japan and China: characteristics of residential indoor and outdoor VOCs. Atmos Environ 43:6352–6359
Ramírez N, Cuadras A, Rovira E, Borrull F, Marcé RM (2010) Comparative study of solvent extraction and thermal desorption methods for determining a wide range of volatile organic compounds in ambient air. Talanta 82:719–727
Shinohara N, Tokumura M, Kazama M, Yonemoto Y, Yoshioka M, Kagi N, Hasegawa K, Yoshino H, Yanagi U (2014) Indoor air quality and thermal comfort in temporary houses occupied after the Great East Japan earthquake. Indoor Air 24:425–437
Shinohara N, Tokumura M, Kazama M, Yoshino H, Ochiai S, Mizukoshi A (2013) Indoor air quality, air exchange rates, and radioactivity in new built temporary houses following the Great East Japan earthquake in Minamisoma, Fukushima. Indoor Air 23:332–341
Song G, Yu A, Sakai K, Khalequzzaman M, Nakajima T, Kitamura F, Guo P, Yokoyama K, Piao F (2016) Levels of volatile organic compounds in homes in Dalian, China. Air Quality, Atmosphere & Health . doi:10.1007/s11869-016-0422-3In press
The Japan Association for Working Environment Measurement (2012) Working environment measurement guidebook Vol. 5 Organic solvents (4th edition). The Japan Association for Working Environment Measurement
Tokumura M, Hatayama R, Tatsu K, Naito T, Takeda T, Raknuzzaman M, Habibullah-Al-Mamun M, Masunaga S (2016) Car indoor air pollution by volatile organic compounds and aldehydes in Japan. AIMS Environmental Science 3:362–381
Tunsaringkarn T, Prueksasit T, Morknoy D, Sawatsing R, Chinveschakitvanich V, Rungsiyothin A, Zapaung K (2015) Indoor air assessment, health risks, and their relationship among elderly residents in urban warrens of Bangkok, Thailand. Air Quality, Atmosphere & Health 8:603–615
Uang S-N, Shih T-S, Chang C-H, Chang S-M, Tsai C-J, Deshpande CG (2006) Exposure assessment of organic solvents for aircraft paint stripping and spraying workers. Sci Total Environ 356:38–44
US EPA (2012) Estimation programs interface suite™ for microsoft® windows, v 4.11. United States Environmental Protection Agency, Washington, DC, USA
Vitali M, Ensabella F, Stella D, Guidotti M (2006) Exposure to organic solvents among handicraft car painters: a pilot study in Italy. Ind Health 44:310–317
Acknowledgement
This study was supported by a Health Labor Sciences Research Grant of the Ministry of Health, Labor, and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 1172 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Miyake, Y., Tokumura, M., Wang, Q. et al. Comparison of the volatile organic compound recovery rates of commercial active samplers for evaluation of indoor air quality in work environments. Air Qual Atmos Health 10, 737–746 (2017). https://doi.org/10.1007/s11869-017-0465-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-017-0465-0