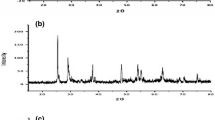
Abstract
Nanoparticles have wide applications in various fields due to their small size. Titanium dioxide nanoparticles are bright with high refractive index (n = 2.4) which makes them suitable for industry dealing with toothpaste, pharmaceuticals, coatings, papers, inks, plastics, food products, cosmetics and textile. Three crystalline phases of titanium dioxide, are anatase (tetragonal), rutile (tetragonal), and brookite (orthorhombic) in which brookite has no commercial value. Due to their self cleaning and antifogging property, they are used in the preparation of cloths, windows, tiles and anti-fogging car mirrors. Titanium dioxide nanoparticles also serve as environment sanitizing agent. Sol–gel route, flame hydrolysis, co-precipitation, impregnation and chemical vapor deposition like techniques are used for the synthesis of TiO2 nanoparticles. Biosynthesis of titanium dioxide nanoparticles has gained wide interest among researchers due to its cost effective, eco-friendly and reproducible approach. The sol–gel route remediation of the titanium dioxide from the environment is an important step and it can be achieved by using physical processes like sedimentation and filtration. The biosynthesis of titanium dioxide nanoparticles can be used in comparison to chemical synthesis. The titanium dioxide nanoparticles have wide applications, viz., reducing toxicity of dyes and pharmaceutical drugs; waste water treatment; reproduction of silkworm; space applications; food industries; etc., and so have immense industrial importance. The applications of nanoparticles synthesized by biological approach will be advantageous for the industries; environment and agriculture.
Similar content being viewed by others
1 Introduction
India is the reservoir of two chief minerals of titanium viz., Ilmenite (FeO.TiO2) and rutile (TiO2). Titanium dioxide (TiO2) exists in rutile, anatase (octahedrite) and brookite form. Brookite is not found in abundance, it is an altered product of some titanium minerals. The reservoirs of TiO2 in different states are shown in Table 1. The TiO2 nanoparticles have many merits viz., high specific-surface area, proper electronic band structure, high quantum efficiency, chemical innerness and stability [1]. The research is gaining immense interest for the synthesis of TiO2 on a large-scale by biological way which will be cost-effective.
There is report on TiO2 nanoparticles synthesized using microbes viz., Lactobacillus sp. and Sachharomyces cerevisae which is low-cost [3, 4]; using Aspergillus flavus TFR7 [5, 6], Chromohalobacter salexigens strain PMT-1 [7]. There is a report on biologically synthesized of TiO2 nanoparticles using Bacillus subtilis (FJ460362) for the study of photo catalytic activity in controlling aquatic biofilm [8]. Nanoparticles have wide applications specially the TiO2 particles viz., cosmaceutical, pharmaceutical, optical, commercial applications [9].
There are reports on applications of TiO2 nanoparticles. Cyanide annual world production is 1.4 million tons and is mainly used for gold mining. But, most of the cyanide from the industry enters the environment and is very toxic. There are various approaches to remove cyanide which is time consuming and costly. Photo catalytic properties of titanium dioxide nanoparticles helps in removal of cyanide from the waste water using the hydrolysis technique [10]. Similarly, there is a report where TiO2-activated carbon composites have application in photo catalytic degradation of β-naphthol from the waste water [11]. The photo catalytic behavior of TiO2 has been studied for the degradation of beta-naphthol using x-ray diffraction and TEM [12].
There are many methods of the synthesis of TiO2 nanoparticles viz., sol–gel technique, solvothermal, hydrothermal, electrochemical process, precipitation method; etc. But they have disadvantages e.g. sol–gel takes hours to days for the formation of TiO2 nanoparticles; hydrothermal method is synthetic method and involves chemical reactions; precipitation method involves difficulty in control of particle size as fast precipitation causes formation of larger particles. Microbial nanoparticle synthesis based method is eco-friendly and cheap as it get operated under mild conditions.
The nanotechnology is gaining immense importance and the researchers are focusing for the new applications of nanoparticles in industries; agriculture; pharmaceuticals; etc. There is a need to study large scale biosynthesis of TiO2 nanoparticles and their applications in unexplored fields.
The novelty of the review here is it mentions specifically the synthesis and applications of TiO2 nanoparticles. Although, there are research papers on TiO2 nanoparticles, but still more work needs to done and the review gives the detail idea of various applications of TiO2 nanoparticles which can be the scope for further research studies on TiO2 nanoparticles.
With this view, the objective of the review here is to focus on the aspects of biosynthesis of TiO2 nanoparticles and also their utility in pharmaceutical, construction, textile, food industries; etc. The applications of TiO2 nanoparticles in aerospace; solar cells and agriculture is also described. The review also highlights the application of TiO2 nanoparticles in waste water treatment dependent on the photo catalytic behavior. The remediation and recycling of TiO2 is also mentioned in the review.
2 Biosynthesis of TiO2 nanoparticles
Living systems are involved in the production of nanoparticles which are more stable as compared with chemically synthesized nanoparticles. Nanoparticles synthesized using microorganisms are found to have less aggregation behavior due to presence of more repulsive forces. Utility of nanoparticles is mainly dependent upon its size, shape as well as stability. Hence, researchers focus on the biogenic synthesis of nanoparticles which can fulfill the criteria. TiO2 nanoparticles which naturally exists in three different crystalline forms anatase, rutile and brookite can be produced using biological agent. Extensive research is carried on the biosynthesis of nanoparticles. Biosynthesis of nanoparticles is one of the growing areas in the field of nanotechnology. Various reports are available on the utilization of bacteria, fungi, algae, plant material and enzymes for the biosynthesis of nanoparticles. The mechanisms involved in the microbial synthesis of nanoparticles are bio absorption, extracellular complexation or precipitation of metals, bioaccumulation, efflux systems, and alteration of solubility and toxicity via reduction or oxidation and lack of specific metal transport systems [13]. Negative electrokinetic potential, bio sorption and bio reduction ability of the microorganisms makes them suitable candidate to synthesize nanoparticles [3, 4]. There is information on synthesis of TiO2 nanoparticles using fungal pathogen Fusarium oxysporium. Saccharomyces cerevisiae and Lactobacillus sp. have been reported to produce 30 and 18 nm TiO2 nanoparticles using TiO (OH)2. Energy source, pH and overall oxidation reduction potential were found to be important factors in the synthesis of TiO2 nanoparticles, while in the case of Saccharomyces cerevisiae, oxidase enzyme plays an important role [3, 4]. TiO2 nanoparticles in anatase form were synthesized using Curcuma longa plant extract due to its content of terpenoids, flavonoids and proteins [14]. Fenugreek (Trigonella foenum graecum (L.)) has been studied to synthesize TiO2 nanoparticles with antimicrobial property [15]. There is a report on the synthesis of rutile TiO2 nanoparticles using custard apple peel extract and precursor TiO (OH)2 [16]. The presence of OH group in the chemical constituents of Annona squamosa was found to be responsible to dehydrate titanyl hydroxide to TiO2 and stabilize the nanoparticles [16]. TiO2 nanoparticles produced using Vigna radiata (green gram) legumes showed antibacterial, antioxidant and cytotoxicity activity against Mg 63 osteosarcoma cell lines [17]. Titanium dioxide nanoparticles due to Lactobacillus crispatus has been found to reduce biofilm formation; hemolysin and also urease which are responsible to develop multidrug resistance ability in the pathogens [18]. Electrostatic interaction between Lactobacillus sp. and metal clusters are responsible for the metal nanoparticles synthesis [19]. The production of anatase TiO2 nanoparticles mediated by Bacillus subtilis has been studied [20].
Guava (Psidium guajava) leaf extract mediated TiO2 nanoparticles showed antioxidant and antimicrobial activities [21]. Guava leaf extract contains alcohol, primary and aromatic amines which assists in the synthesis of TiO2 nanoparticles [21]. TiO2 nanoparticles made by using Aspergillus flavus TFR7 and bulk TiO2, was used as foliar spray (10 mg/l) on mung bean (Vigna radiata L.). This fungal mediated TiO2 found to enhance the vigor index of mung bean [5, 6]. Fungi secrete capping proteins which play the role of encapsulation of nanoparticles while associated proteins contributes in the mineralization of precursor salt [22, 23]. Aspergillus niger and Aspergillus tubingensis TFR-5 have also been reported to have the capability to produce TiO2 nanoparticles [24, 25].
3 Application of titanium dioxide nanoparticles
The applications of titanium dioxide nanoparticles are shown in Fig. 1.
3.1 Industrial applications of titanium dioxide nanoparticles
TiO2 nanoparticles have wide applications in industry; some of them are given in Table 2.
3.2 Role of TiO2 nanoparticles in reducing toxicity of dyes and pharmaceutical drugs
TiO2 work as a nanocatalyst in the degradation of methyl orange dye. TiO2 nanoparticles synthesized using Citrus paradisi peel extract exhibited 72% degradation of methyl orange using sunlight [37]. Antiseptic commonly used in pharmaceutical industry chlorhexidine digluconate (CHD) affects aquatic environment by inducing resistance in microorganisms and also affects human health. Using optimum conditions of UV light intensity of 50 μW cm−2, pH 10.5, 2.5:1 of substrate-to-catalyst ratio 68.2% degradation of chlorhexidine digluconate (CHD) was achieved using titanium dioxide as a photo catalyst. In future, it is possible to reduce the toxic effects of waste water generated from pharmaceutical industry [38]. Researchers tried nanocomposites for the removal of antibiotics from the environment. G-TiO2 composites were found to have 97% removal efficiency of levofloxacin (for 1 mg/l) using 300 W UV power and 45 min irradiation time [39]. Use of TiO2 as a sorbent is limited due to its low surface area which is able to remove contaminants in low concentration. To reduce its agglomeration ability and to increase its surface area along with photo catalytic activity, TiO2 has been supported on zeolite, alumina, activated carbon and stainless steel. Composites of TiO2 with coconut shell powder were prepared using sol–gel method with heat treatment. These composites were reported to remove 98% carbamazepine, antiepileptic drug which is an important organic pollutant [40].
TiO2 nanoparticles can be used for the photo degradation of toxic dyes and other pharmaceutical drugs, thus avoid harmful effects on environment.
3.3 TiO2 nanoparticles in the reproduction of silk worm
It has been reported that low concentrations of TiO2 nanoparticles shows significant changes in the reproduction rate of Bombyx mori. There is a report where feeding Bombyx mori with TiO2 nanoparticles resulted in a rapid development of sex organs and up regulation of reproduction-related genes [41]. After feeding of Bombyx mori average increase was found to be 51 eggs/insect and 0.34 × 10−4 g/egg [41].
Reproduction efficiency of silkworm can be increased by mixing low concentration of TiO2 nanoparticles in their feed.
3.4 Waste water treatment
Low cost, resistance to corrosion and overall stability makes TiO2 nanoparticles suitable for the waste water treatment [42]. Remediation of surface water can be achieved using TiO2 nanoparticles as solid phase extraction (SPE) packing material to preconcentrate and extraction of heavy metals from water [43]. Ultraviolet light and titanium dioxide (TiO2) nanoparticles doped with iron showed 98.58% diazinon removal efficiency, diazinon is one of organophosphate pesticides which it is classified as a relatively dangerous substance (Class II by World Health Organization), found in ground water [44]. Decolorization of waste water is the primary treatment considered in waste water treatment. Dyes like Rhodamine B, methylene blue and malachite green are primary dyes found in the effluent. Dye Rhodamine B is found to degrade up to 95% due to hybrid system combining UV/TiO2 nanoparticles and polyvinylidene fluoride (PVDF) membrane [45].
TiO2 nanoparticles play important role in the removal of the xenobiotic compounds like pesticides; dyes; toxic compounds from the waste water, thus having wide application in waste water treatment.
3.5 Role of TiO2 nanoparticles in agriculture
According to the report given by Chao and Choi, TiO2 nanoparticles increases the photosynthetic rate, immunity of the plant which results in 30% increase in the crop yield [46]. Increased photosynthetic rate was observed in spinach [47]. Zea mays sprayed with nano TiO2 in the reproductive stages of the crop was found to have more pigmentation [48] which is responsible to increase crop yield [49]. TiO2 nanoparticles can be applied either as soil amendment or foliar spray. The nanoparticles get bio distributed through the vascular network. Distribution of nanoparticles is varying among crops. In case of tomato plants, application of TiO2 nanoparticles in aerosol format was found to be more effective to increase photosynthesis and lycopene content as compared with TiO2 nanoparticles as soil amendment [5, 6].
TiO2 nanoparticles can be used to speed the photosynthetic rate of various crops and also enhance the crop yield.
3.5.1 Use of TiO2 nanoparticles in nanomedicine
TiO2 nanoparticles are used in nanotherapeutics [50]; photodynamic therapy (PDT) [51] and for articulating prosthetic implants [52]. TiO2 nanoparticles are also used in cosmetics and in treatment of hyper pigmented skin and other nondermatologic diseases [53].
TiO2 nanoparticles due to their special physical properties have useful applications in nanomedicine.
3.6 In purification of air
Titanium dioxide (TiO2) photo catalyst could oxidize and mineralize compounds in the vapor or liquid phase. Hence, researchers created various filters to get rid of the indoor pollutants. Cigarette smoke (principal indoor air pollutant) can be removed by TiO2-impregnated titanium mesh filter (TMiP) by electrochemical anodization of the titanium mesh, and dip coating into TiO2 anatase solution. This filters can remove volatile and nonvolatile compounds of cigarette smoke like ammonium, nicotine, 3-ethenylpyridine and tar [54]. TiO2 has been employed in Japan as photochemical deodorizer [55]. When TiO2 nanoparticles are stimulated by sunlight, they convert the pollutants and oxides to environmental less toxic products like carbon dioxide and calcium nitrate. Volatile organic compounds (VOC) pose various adverse effects on the health. Toluene is one of the VOC which can be decomposed by coating activated carbon with TiO2 [56].
The oxidation of indoor air pollutants by TiO2 nanoparticles helps in the purification of air, which is very important for all the living beings.
3.7 Use of photo catalytic nano TiO2 for photo catalyst coating
A film of photo catalytic titanium dioxide is a super oxidative, hydrophilic and antistatic material used for self-cleaning of buildings. The film also oxidizes the hydrocarbon emitted as automotive exhaust and rain water washes the dirt and helps the building exterior to remain clean. Nano TiO2 photo catalyst coating will act as an invisible film and can be applied on ceiling, floor carpets, curtains, car interior, toilet seats etc. Due to this nano TiO2 film, the microorganisms can be removed. TiO2 nanoparticles are used in the anti-fogging car mirrors [57].
The photocatalytic film of TiO2 can help to keep the buildings clean.
3.8 TiO2 nanoparticles in treatment of osteosarcoma and chonrosarcoma
Osteosarcoma and chondrosarcoma are malignant bone tumors found in patients of all ages. Surgical amputation of the malignant lesion is generally carried out. Recently, it has been shown that TiO2 nanoparticles kill HeLa cells via photo catalysis in vitro [58]. This indicates the TiO2 nanoparticles induces cytotoxicity to bone tumor cells which will be helpful in minimization of the recurrence of osteosarcoma and chondrosarcoma.
The TiO2 nanoparticles can be used to treat the bone tumors, thus having application in the medical field.
3.9 For dye-sensitized solar cells
TiO2 nanoparticles have applications as semiconductor for dye-sensitized solar cells because it is non-toxic, easily available and cheap [59].
TiO2 nanoparticles have photovoltaic effect and therefore used in solar cells.
3.10 TiO2 in food products
Food products containing TiO2 include white-colored sauces, confectioneries nondairy creamers and cheese. In foods, it restores whiteness of creamy products (e.g. salad dressings) and in confectionery; it forms a blockade for colors (e.g. soft-centered sweets with a crisp shell). India sets the limit on the use of TiO2 nanoparticles up to 1% (w/w) in chewing gum and 0.01% (w/w) in beverages (Food Safety and Standards India [60].
TiO2 nanoparticles has applications in food industries.
3.11 Remediation of nanoparticles
TiO2 nanoparticles have the potential to work as tracer of nanoparticles of similar size and aggregate likeTiO2 [61].
4 Maintenance of historical architecture
Various historically important forts and monuments get dirty over the period of time. Maintenance of cultural heritage from getting dirty is the primary requisite. Strategies applied for the consolidation of restoration materials include application of alkaline earth metal hydroxide nanoparticles or nanostructured SiO2, Al2O3, TiO2 in low and high molecular weight matrices [62, 63]. Self cleaning, depolluting and biocide activity of titania makes them suitable to use in the construction or maintenance of stone surfaces [64]. Photo catalytic activity of TiO2 nanoparticles can exist in the presence of UV light to modify these TiO2 nanoparticles to retain anatase form and photo catalytic potential in the presence of sunlight also. To retain the potential of TiO2 nanoparticles, various approaches like synthesis of TiO2 in benzyl alcohol, water and ethylene glycol dispersions of benzyl-capped anatase nanoparticles have been studied for the retention of photocatalytic activity in presence of sunlight [65]. Spray coating of TiO2 nanoparticles as colloidal suspension on travertine limestone found to be effective to maintain the stone surface [64]. Water and ethylene glycol dispersions of benzyl-capped anatase nanoparticles of TiO2 were applied onto Noto stone and Carrara marble specimens found to retain photo catalytic activity [65].
The photo catalytic potential of TiO2 nanoparticles is very important for the maintenance of historical monuments.
4.1 Titanium for aerospace industry
The application of titanium alloys in the aerospace sector includes airframe, engine, helicopter, and space applications. Light weight, high strength, compatibility with CFRP and external corrosion resistance of titanium and its alloys enables them to be used in aircraft [66]. Broad research has been carried out on the preparation of titanium alloys like, Ti–6Al–4V at low cost and its use in aerospace industry. Titanium alloys have an excellent strength-to-weight ratio.
TiO2 nanoparticles has application in the making of aircraft and aircraft engines.
4.2 Toxic effects of TiO2 on human
Information is available on the toxic effects of TiO2 on human as they can bio concentrate, bio accumulate, and bio magnify in the tissues of mammals and other vertebrates [67]. Microparticles of TiO2 get accumulated in macrophages of human gut-associated lymphoid tissue [68]. There is a report on the in silico study of TiO2 spherical nanoparticles using twenty standard amino acids in aqueous solution [69]. Basic amino acids tend to have direct interaction with TiO2, while acidic amino acids have indirect interaction with TiO2 spherical nanoparticles [69].
4.3 Toxic effects of TiO2 on ecosystem
Wide use of TiO2 nanoparticles in personal care products viz., shaving creams (< 0.01 μg/mg), toothpastes and sunscreens (1% to > 10% titanium by weight), foods and household products leads to its release through human excreta, washed off of surfaces, or disposed of to sewage that enters wastewater treatment plants and in bio solids which will be further applied to soil as fertilizer. As per report on WWTP, raw sewage contained 100 to nearly 3000 μg Ti/l [61]. Treated effluent was found to contain TiO2 particles with 4 and 30 nm particle size [30]. After waste water treatment, effluent was found to contain from less than 5 µg up to 15 µg of nano-TiO2/l [61]. Presence of TiO2 nanoparticles into the environment affects phytoplankton and coastal ecosystems that support fishing and recreational activities. Algae are primarily used as biological marker in aquatic system to study biological toxicity of pollutants. TiO2 nanoparticles inhibit algae by causing membrane structure deformation due to increased lipid peroxidation [70]. TiO2 nanoparticles exhibit more cellular toxicity in anatase due to increased amount of intracellular reactive oxygen species (ROS) [71]. The nano bio interfacial interaction of anatase TiO2-NPs with EPS of algae showed higher algal toxicity than that of rutile TiO2-NPs [71]. Growth rate of freshwater green algae predominant in North America viz., Scenedesmus quadricauda, Chlamydomonas moewusii and Chlorella vulgaris, was found to be inhibited due to the presence of TiO2 nanoparticles in freshwater microcosms [72]. Combination of anatase and rutile showed more toxicity and antagonistic effect on freshwater algae Chlorella [73].
4.4 Recycling of TiO2
Disposal of sludge is very important to avoid its sequential effects. Land filling is one of the methods which are primarily used. But leaching of the heavy metals into the environment limits its use. Another alternative to land filling is restoration or recycling of the sludge. It is possible to prepare TiO2 nanoparticles using sludge. Treatment of the sludge with coagulant TiCl4 and aiding chemicals like Al2(SO4)3, FeCl3 and Ca(OH)2, resulting flocculated sludge after incineration at 600 °C yield TiO2 doped with Fe, Al and Ca [74].
5 Conclusion
Broad spectrum use of titanium dioxide nanoparticles has gained attention among researchers. Extensive research is ongoing on the chemical and biological synthesis of nanoparticles. Rutile TiO2 pigment is generally preferred for the materials which is exposed to outdoor conditions like paints, plastics and inks due to its high resistance to UV light. Anatase TiO2 pigment is the choice for paper, ceramics, rubber and fibers manufacturing industries due to its less abrasive power [75]. Biogenic synthesis is the method of choice due to its cost effectiveness, reproducibility and less time requirement. Microorganisms and plant extracts are used for the biosynthesis of TiO2 nanoparticles. Compared to microorganisms, plant extracts are more feasible to use for nanoparticles synthesis due to less requirement of aseptic conditions [21]. Plants contain flavonoids, citric acid, ascorbic acids, reductases, dehydrogenase and external electron shuttlers which makes them suitable for the nanoparticles synthesis [76]. Biogenic synthesis of nanoparticles requires alcohol, amines, enzymes and proteins for the stabilization and reduction of precursor salts. Titanium can be applied as foliar spray and found to have significant effect on growth, photosynthesis rate and carbohydrate synthesis [5, 6]. TiO2 can sustain in the environment for long period and its antimicrobial activity makes them strong disinfectant which will be 3 times stronger than chlorine, and 1.5 times stronger than ozone. Air purifiers containing TiO2 avoid smoke, pollen, virus, bacteria and harmful gas and grab the free bacteria present in the air by filtering percentage of 99.9% due to the highly oxidizing effect of photo catalyst (TiO2). Protection of lamp houses, walls in tunneling and white tents from becoming sooty and dark, can be achieved due to the self-cleaning and high photo catalytic potential of TiO2.
References
Lai Y, Wang L, Liu D, Chen Z, Lin C (2015) TiO2-based nanomaterials: design, synthesis and applications. J Nanomater 2015:1–3
Indian Minerals Yearbook, Part- III: Mineral Reviews (2013) 52nd edn
Jha A, Prasad K, Kulkarni A (2009) Synthesis of TiO2 nanoparticles using microorganisms. Coll Surf B: Biointerfaces 71:226–229
Jha A, Prasad K, Kulkarni A (2009) Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf B 71:226–229
Raliya R, Biswas P, Tarafdar J (2015) TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiataL.). Biotechnol Rep 5:22–26
Raliya R, Nair R, Chavalmane S, Wang W, Biswas P (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7:1584–1594
Tharanya P, Vadakkan K, Hemapriya J, Kannan V, Vijayanand S (2015) Biogenic approach for the synthesis of titanium dioxide nanoparticles using a halophilic bacterial isolate- Chromohalobacter salexigens strain PMT-1. Int J Curr Res Acad Rev 3:334–342
Dhandapani P, Maruthamuthu S, Rajagopal G (2012) Bio-mediated synthesis of TiO2 nanoparticles and its photocatalytic effect on aquatic biofilm. J Phytochem Photobiol 110:43–49
Banerjee K, Thiagarajan P (2014) A review of titanium dioxide nanoparticles-synthesis, applications and toxicity concerns. Nanosci Nanotechnol Asia 4:132–143
Ijadpanah-Saravi H, Safari M, Khodadadi-Darban A, Rezaei A (2014) Synthesis of titanium dioxide nanoparticles for photocatalytic degradation of cyanide in waste water. Anal Lett 47:1772–1782
Ijadpanah-Saravy H, Dehestaniathar Khodadadi A, Safari M (2016) Optimization of photocatalytic degradation of β-naphthol using nano TiO2-activated carbon composite. Desalin Water Treat 57:4708–4719
Ijadpanah-Saravi H, Sarafi M, Noruzi-Masir B, Darban AK, Bakhshi P (2017) Intelligent tools to model photo-catalytic degradation of titanium dioxide nanoparticles. J Chemom 31:e2907–e2909
Beveridge TJ, Hughes MN, Lee H, Leung KT, Poole RK, Savvaidis I (1997) Metal–microbe interactions: contemporary approaches. Adv Microb Physiol 38:177–243
Abdul R, Nuaman R, Abd A (2016) Biological synthesis of titanium dioxide nanoparticles by Curcuma longa plant extract and study its biological properties. World Sci News 49:204–222
Subhapriya, Gomathipriya (2018) Green synthesis of titaniumdioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb Pathog 116:215–220
Roopan SM, Bharathi A, Prabhakarn A, Rahuman A, Velayutham K, Rajakumar G (2012) Efficient phytosynthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta A Mol Biomol Spectrosc 98:86–90
Chatterjee N, Yang J, Atluri R, Lee W, Hong J, Choi J (2016) Amorphous silica nanopaticle-induced perturbation of cholesterol homeostasis as a function of surface area highlights safe-by-design implementation: an integrated multi-OMICS analysis. RSC Adv 6:68606–68614
Ibrahem KH, Salman JA, Ali FA (2014) Effect of titanium nanoparticles biosynthesis by Lactobacillus crispatus on urease, hemolysin and biofilm forming by some bacteria causing recurrent UTI in Iraqi women. Eur Sci J 10:324–338
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des 2:293–298
Kirthi AV, Rahuman AA, Rajakumar G, Marimuthu S, Santhoshkumar T, Jayaseelan C, Elango G, Zahir A, Kamaraj C, Bagavan A (2011) Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater Lett 65:2745–2747
Santosh M, Sirin C, Philipp S, Andreas H, Carsten S, Marialore S (2016) The role of halide ions in the anisotropic growth of gold nanoparticles: a microscopic, atomistic perspective. Phys Chem Chem Phys 18:13246–13254
Raliya R, Tarafdar J (2012) Novel approach for silver nanoparticle synthesis using Aspergillus terreus CZR-1: mechanism perspective. J Bionanosci 6:12–16
Raliya R, Rathore I, Tarafdar J (2013) Development of microbial nanofactory for zinc, magnesium, and titanium nanoparticles production using soil fungi. J Bionanosci 7:590–596
Durairaj B, Muthu S, Xavier T (2015) Antimicrobial activity of Aspergillus niger synthesized titanium dioxide nanoparticles. Adv Appl Sci Res 6:45–48
Tarafdar A, Raliya R, Wei-Ning W, Pratim B, Tarafdar J (2013) Green synthesis of TiO2 nanoparticle using Aspergillus tubingensis. Adv Sci Eng Med 5:943–949
Wolf R, Matz H, Orion E, Lipozencic J (2003) Sunscreens-the ultimate cosmetic. Acta Dermatovenerol Croat 11:158–162
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:1–33
Fan J, Li Z, Zhou W, Miao Y, Zhang Y, Hu J, Shao S (2014) Dye-sensitized solar cells based on TiO2 nanoparticles/nanobelts double layered film with improved photovoltaic performance. Appl Surf Sci 319:75–82
Zhang W, Zhu Z, Cheng C (2011) A literature review of titanium metallurgical processes. Hydrometallurgy 108:177–188
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N (2012) Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46:2242–2250
Guo S, Wu Z, Zhao W (2009) TiO2-based building materials: above and beyond traditional applications. Chin Sci Bull 54:1137–1142
Mills A, Hodgen S, Lee S (2005) Self-cleaning titania films: an overview of direct, lateral and remote photo-oxidation processes. Res Chem Intermed 31:295–308
Feldman C (2001) Preparation of nano scale pigment particles. Adv Mater 13:1301–1303
Visai L, de Nardo L, Punta C, Melone L, Cigada A, Imbriani M, Arciola C (2011) Titanium oxide antibacterial surfaces in biomedical devices. Int J Artif Organs 34:929–946
Fergus JW (2003) Doping and defect association in oxides for use in oxygen sensors. J Mater Sci 38:4259–4270
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69:8784–8789
Kumar B, Cumbal L (2014) Light harvesting titanium nanocatalyst for remediation of methyl orange. Int J Chem Mol Nucl Mater Metall Eng 8:196–199
Das R, Sarkar S, Chakraborty S, Choi H, Bhattacharjee C (2014) Remediation of antiseptic components in wastewater by photocatalysis using TiO2 nanoparticles. Ind Eng Chem Res 53:3012–3020
Alicanoglu P, Sponza D (2017) Photodegradation of levofloxacin antibiotic via graphene/TiO2 and its reusability. Int J Waste Resour 3(Suppl):7
Khraisheh M, Kim J, Campos L, Al-Muhtaseb A, Walker G, AlGhouti M (2013) Removal of carbamazepine from water by a novel TiO2-coconut shell powder/Uv process: composite preparation and photocatalytic activity. Environ Eng Sci 30:515–526
Ni M, Li F, Wang B, Xu K, Zhang H, Hu J, Tian J, Shen W, Li B (2015) Effect of TiO2 nanoparticles on the reproduction of silkworm. Biol Trace Elem Res 164:106–113
Theron J, Walker J, Cloete T (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34:43–69
Quetel C, Vassileva E, Petrov I, Chakarova K, Hadjiivanov K (2010) First results on Fe solid-phase extraction from coastal seawater using anatase TiO2 nano particles. Anal Bioanal Chem 396:2349–2361
Baneshi M, Rezaei S, Sadat A, Mousavizadeh A, Barafrashtehpour M, Hekmatmanesh H (2017) Investigation of photocatalytic degradation of diazinon using titanium dioxide (TiO2) nanoparticles doped with iron in the presence of ultraviolet rays from the aqueous solution. Biosci Biotech Res Comm 10:60–67
Vatanpour V, Karami Asma, Sheydaei Mohsen (2017) Central composite design optimization of Rhodamine B degradation using TiO2 nanoparticles/UV/PVDF process in continuous submerged membrane photoreactor. Chem Eng Process: Process Intensif 116:68–75
Chao SL, Choi HS (2005) Method for providing enhanced photosynthesis. Korea Res Inst Chem Technol S Korea Bull 11:1–34
Lei Z, Mingyu S, Chao L, Liang C, Hao H, Xiao W, Xiaoqing L, Fan Y, Fengqing G, Fashui H (2007) Effects of nanonatase TiO2 on photosynthesis of spinach chloroplasts under different light Illumination. Biol Trace Elem Res J 119:68–76
Morteza E, Moaveni P, Farahani H, Kiyani M (2013) Study of photosynthetic pigments changes of maize (Zea mays L.) under nano TiO2 spraying at various growth stages. Springerplus 2:1–5
Yang F, Hong FS (2006) Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res 110:179–190
Yuan Y, Ding J, Xu J, Deng J, Guo J (2010) TiO2 nanoparticles co-doped with silver and nitrogen for antibacterial application. J Nanosci Nanotechnol 10:4868–4874
Szacilowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G (2005) Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev 105:2647–2694
Jacobs JJ, Skipor AK, Black J, Urban R, Galante JO (1991) Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J Bone Joint Surg Am 73:1475–1486
Wiesenthal A, Hunter L, Wang S, Wickliffe J, Wilkerson M (2011) Nanoparticles: small and mighty. Int J Dermatol 50:247–254
Slimen H, Ochiai T, Nakata K, Murakami T, Houas A, Morito Y, Fujishima A (2012) Photocatalytic decomposition of cigarette smoke using a TiO2-impregnated titanium mesh filter. Ind Eng Chem Res 51:587–590
Fukuyama J (2004) Odor pollution control for various odor emission sources in Japan, East Asia. In: Odor measurement and control review workshop (Environmental Management Bureau, Ministry of the Environment, Government of Japan), pp 67–77
Rezaee A, Pourtaghi G, Khavanin A, Mamoory R, Ghaneian M, Godini H (2008) Photocatalytic decomposition of gaseous toluene by TiO2 nanoparticles coated on activated carbon. Iran J Environ Health Sci Eng 5:305–310
Montazer M, Seifollahzadeh S (2011) Enhanced self-cleaning, antibacterial and UV protection properties of nano TiO2 treated textile through enzymatic pretreatment. Photochem Photobiol 87:877–883
Sha B, Gao W, Han Y, Wang S, Wu J, Xu F, Lu T (2013) Potential application of titanium dioxide nanoparticles in the prevention of osteosarcoma and chondrosarcoma recurrence. J Nanosci Nanotechnol 13:1208–1211
Mbonyiryivuze A, Zongo S, Diallo A, Bertrand S, Minani E, Yadav L, Mwakikunga B, Dhlamini S, Maaza M (2015) Titanium dioxide nanoparticle biosynthesis for dye sensitized solar cells: review. Phys Mater Chem 3:12–17
Food Safety and Standards [FSS] India (2011) Food safety and standards (food product standards and food additives) regulation. 4:449–529
Kiser M, Westerhoff P, Benn T, Wang Y, Pérez-Rivera J, Hristovski K (2009) Titanium nanomaterial removal and release from wastewater treatment plants. Environ Sci Technol 43:6757–6763
Chelazzi D, Poggi G, Jaidar Y, Toccafondi N, Giorgi R, Baglioni P (2013) Hydroxide nanoparticles for cultural heritage: consolidation and protection of wall paintings and carbonate materials. J Colloids Interf Sci 392:42–49
Miliani C, Velo-Simpson M, Scherer G (2007) Particle-modified consolidants: a study on the effect of particles on sol-gel properties and consolidation effectiveness. J Cult Herit 8:1–6
Goffredo G, Munafo P (2015) Preservation of historical stone surfaces by TiO2 nanocoatings. Coatings 5:222–231
Gherardi F, Colombo A, Goidanich S, Simonutti R, Toniolo L (2014) Innovative nano-TiO2 particles for self-cleaning treatments of historic architecture and sculptures. Restor Build Monum 20:423–432
Inagaki I, Takechi T, Shirai Y, Ariyasu N (2013) Application and features of titanium for aerospace industry. Nippon Steel Sumitomo Met Tech Rep 396:23–28
Jovanovic B (2014) Critical Review of Public Health Regulations of titanium dioxide, a human food additive. Integ Environ Assess Manag 11:10–20
Lomer M, Thompson R, Powell J (2002) Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc Nutr Soc 6:123–130
Liu P, Jin H, Guo Z, Ma J, Zhao J, Li D, Wu H, Gu N (2016) Silver nanoparticles outperform in radiosensitizing U251cells in vitro and in an intracranial mouse model of glioma. Int Nanomed 11:5003–5014
Ozkaleli M, Erdem A (2018) Biotoxicity of TiO2 nanoparticles on Raphidocelis subcapitata microalgae exemplified by membrane deformation. Int J Environ Res Public Health 15:1–12
Gao X, Kaijun Z, Luqing Y, Daohui L (2018) Distinct effects of soluble and bound exopolymeric substances on algal bioaccumulation and toxicity of anatase and rutile TiO2 nanoparticles. Environ Sci Nano 5:720–729
Cardinale BJ, Bier R, Kwan C (2012) Effects of TiO2 nanoparticles on the growth and metabolism of three species of freshwater algae. J Nanopart Res 14:1–8
Iswarya V, Bhuvaneshwari M, Alex S, Iyer S, Chaudhuri G, Chandrasekaran P, Bhalerao G, Chakravarty S, Raichur A, Chandrasekaran N, Mukherjee A (2015) Combined toxicity of two crystalline phases (anatase and rutile) of titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat Toxicol 161:154–169
Shon H, Vigneswaran S, In Kim, Cho J, Kim GJ, Kim JB, Kim JH (2007) Preparation of titanium dioxide (TiO2) from sludge produced by titanium tetrachloride (TiCl4) flocculation of wastewater. Environ Sci Technol 41:1372–1377
Gazquez M, Bolívar J, Garcia-Tenorio R, Vaca F (2014) A Review of the production cycle of titanium dioxide pigment. Mater Sci Appl 5:441–458
Pandey S, Oza G, Mewada A, Sharon M (2012) Green synthesis of highly stable gold nanoparticles using Momordica charantia as nano fabricator. Arch Appl Sci Res 4:1135–1141
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waghmode, M.S., Gunjal, A.B., Mulla, J.A. et al. Studies on the titanium dioxide nanoparticles: biosynthesis, applications and remediation. SN Appl. Sci. 1, 310 (2019). https://doi.org/10.1007/s42452-019-0337-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0337-3