Abstract
Although osseointegration has proven successful at improving the physical deficits that traditional prostheses leave unfulfilled, future innovation should be systematically guided rather than randomly explored. Therefore, this article attempts to summarise, in a systematic manner, the challenges and prospects of osseointegration limb reconstruction for amputees from an implant design and manufacturing point of view, to provide a template for the development of the next generation of osseointegration implants. A scoping literature review was conducted, and key papers were identified and summarised. To combat osseointegration-related infection, advances such as smart implant coatings, mechanical inactivation of bacteria, biofilm eradication, implant monitoring technologies and nanotechnology were evaluated. Regarding production and biomaterials, the potential of 3D printing to balance supply and demand to achieve cost-effectiveness and sustainability were investigated. Considering the evolution of designs and the goal to provide a sensate limb, the prospects of smart implants, biofeedback and myoelectric pattern recognition were also explored. Osseointegration appears to follow a trajectory like that of total joint arthroplasty, which gained widespread clinical acceptance and adoption over the last 50 years. In our opinion, the future of amputee rehabilitation is bright, and we are optimistic osseointegration will continue to progress and advance as new technologies emerge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
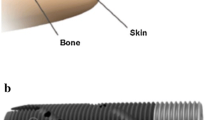
It has been almost 80 years since the word “osseointegration” was first coined by Per-Ingvar Brånemark to describe the direct structural and functional integration of living bone to metal implant [1]. For the past 30 years, this technology has been increasingly applied for the reconstruction and rehabilitation of amputees, after the first osseointegration surgery was performed in Sweden (see Fig. 1) [2, 3]. Recently, a German study of 100 patients with appendicular skeletal tumours reported osseointegration was a viable alternative to limb-salvage mega prostheses [4]. Studies conducted in the US and India have also demonstrated osseointegration, by providing direct skeletal attachment of an artificial limb, represents a promising replacement for the traditional socket-mounted prosthesis [5, 6].
This figure shows the first patient with successful long-term limb osseointegration. The surgery was performed on 15 May 1990 in Sweden, by Rickard Brånemark, son of Per-Ingvar Brånemark, for a young woman who after a road traffic accident had lost both her legs. As shown in the picture, she could stand upright on two prosthetic legs without any need for compressive sockets despite her relatively short residual limb stumps (reproduced with permission from Li Y, Brånemark R. Osseointegrated prostheses for rehabilitation following amputation. Der Unfallchirurg. 2017;120(4):285–92.)
Today, osseointegration has already proven successful as a means to address some of the physical deficits that traditional socket-mounted prostheses leave unfulfilled and there has certainly been increasing interest in the topic area in the UK and throughout the world. However, future innovation should be systematically guided rather than randomly explored; therefore, this review is intended to summarise, in a systematic manner, the challenges and prospects of osseointegration limb reconstruction for amputees from an implant design and manufacturing point of view, to provide a template for the development of the next generation of osseointegration implants.
Methods
A scoping literature search was conducted on PubMed for literature published between 2000 and 2021 using permutations of terms such as “osseointegration”, “amputee”, “infection”, “production”, “sustainability”, “design”, “feedback” and “advances”. Articles discussing topics of advances in infection prevention and control, production and biomaterials, and prosthesis design and feedback were selected. Articles unrelated to potential advances for osseointegration were excluded. Journal articles, books, laboratory-based and experimental studies, conference proceedings and abstracts were included if they fulfilled the inclusion criteria. In total, 90 papers were identified and reviewed, and 31 papers were selected in agreement with all senior authors and summarised under headings of [1] advances in infection prevention and control [2], advances in production and biomaterials and [3] advances in prosthesis design and feedback.
Advances in Infection Prevention and Control
One of the biggest challenges to overcome for osseointegration to be accepted in the wider medical community is infection risk due to the inherent nature of a percutaneously exposed and colonised implant. Almost all orthopaedic surgeons would regard exposed metal as the inherent cause of implant infection, yet this is an integral part of the osseointegration strategy. Over the past two decades, various advances in infection prevention and eradication strategies for arthroplasty implants have been studied, many of which are potentially applicable in osseointegration, including smart implant coatings, mechanical inactivation of infection-causing bacteria, biofilm eradication, implant monitoring technologies and the use of nanotechnology.
Smart Implant Coating
The fundamental dilemma of uncontrolled peri-implant inflammation is that, while it is required at early stages for normal wound healing, it is preferably suppressed at later stages for better recovery and osseointegration. To address this issue, a bio-responsive and endogenously triggered smart coating material, introduced as a “bridge-burning” implant coating, has been equipped with an “on/off switch” for the consecutive harnessing and abolishing of the power of inflammation during the early and later stages of osseointegration, respectively [7].
This “bridge-burning” implant coating consists of macrophage-activating glycans covalently crosslinked by macrophage-eliminating bisphosphonates to the titanium implant surface. Upon implantation, peri-implant inflammation is “switched on” when host macrophages are instructed by glycans to release pro-osteogenic cytokines, encouraging bone cell differentiation. Peri-implant inflammation can then be “switched off” upon the secretion of alkaline phosphatase by the increasingly mature bone cells, to cleave the glycan-bisphosphonate complexes from the implant. These, in turn, selectively kill proinflammatory macrophages that have fulfilled their purpose, in the manner of “burning bridges”, to promote healing and optimise osseointegration [7].
Another potential implant coating that could be used to prevent postoperative implant-related infections is a smart biodegradable antimicrobial implant coating made with poly (ethylene glycol)-poly (propylene sulphide) polymer. It works by providing a controlled and smart local delivery of antibiotics, combining the passive elution of antibiotics with an active release mechanism that targets bacteria to help decrease bacterial burden [8].
Mechanical Inactivation of Staphylococcus aureus and Pseudomonas aeruginosa
Titanium is the preferred material for most orthopaedic implants due to its well-established corrosion resistance and biocompatibility. To counter implant-associated infections, one study proposed the development of a smart mechano-bactericidal surface by tuning the micron-scale surface topology of titanium to inactivate any infection-causing bacteria. Titanium substrata processing micron-scale surface architectures were fabricated using a process of mask-less plasma etching of bulk titanium, and then characterised using two-dimensional Fast-Fourier Transforms (2D-FFT), scanning electron microscopy (SEM) and atomic force microscopy (AFM) to highlight the formation of a two-tier pillared surface topology. Assessments for antibacterial efficacy against Pseudomonas aeruginosa and Staphylococcus aureus bacteria were then carried out on each substratum, attaining maximum antibacterial efficacy of 87.2% and 72.5%, respectively. More importantly, the formation of these three-dimensional (3D) hierarchical features reduced the extent of bacterial attachment, first trapping the bacteria within the micron size pillars and then killing the bacteria within the second tier of pillars. Overall, these results shed new light on the future of smart mechano-bactericidal surfaces and the potential of designing complex hierarchical surfaces for mitigating implant-associated infections [9].
Biofilm Eradication
Biofilm formation is perhaps the single greatest challenge in bone-implant-associated infections due to the predisposition of microbes for tolerance to both the immune system and many antibiotics. As such, a synergistic photothermal/photodynamic therapy (PTT/PDT) strategy aiming for biofilm eradication on titanium (Ti) implants has been developed. The PTT/PDT strategy is integrated with mesoporous polydopamine nanoparticles (MPDA) loading and a photosensitizer, indocyanine green (ICG), by π-π stacking. More precisely, the MPDA is functionalised with an RGD peptide to give the modified titanium sample (Ti-M/I/RGD) better cytocompatibility. Remarkably, on exposure to near-infrared wavelengths in vivo, the Ti-M/I/RGD implant eradicated Staphylococcus aureus biofilm with 95.4% efficacy. These implants still demonstrated excellent osteogenesis and osseointegration performance, indicating PTT/PDT as a feasible strategy to create antibacterial titanium implants for osseointegration [10].
Simultaneous Monitoring of Loosening and Temperature in Orthopaedic Implants
Time to diagnosis can affect subsequent treatment success, yet current diagnostics do not provide adequate early warning, and lack diagnostic sensitivity and specificity. One study proposed an embedded ultrasound system designed to simultaneously monitor for implant loosening by detecting temperature change as an infection prodrome. The system requires only two components to be implanted, a piezoelectric transducer and a coil, for pulse-echo responses to be elicited via a three-coil inductive link. With no need for batteries or microprocessors, the need to modify pre-existing and well-established implant designs is minimised, facilitating mass-market adoption [11].
Realtime Information on Implant Infection
Real-time monitoring of certain physiological parameters, such as pH level, at the tissue-implant interface, can reveal vital information regarding the onset and severity of implant infection to facilitate more timely interventions. A study investigating wireless pH measurement systems has suggested iridium oxide sensors as the most fitting among putative devices due to their low drift, high sensitivity and durability. Any information on implant surface pH levels could also be transferred indirectly to an external device, such as a smartphone or tablet, providing the potential for real-time monitoring [12].
Delivery Options for Adjuvant Therapeutic Agents
Adjuvant therapeutic agents such as recombinant growth factors, lipid mediators, antibiotics, antiphlogistics, proangiogenics and other promising anti-resorptive and anabolic molecules may potentially enhance bone healing and osseointegration, especially when released in a targeted and controlled manner during bone healing. Therefore, the development of smart, biocompatible and biostable polymers such as implant coatings, scaffolds or particle-based materials suitable for drug release will be crucial. Innovative chemical, physical and biochemical strategies for controlled degradation or stimulus-responsive release of substances from these materials, and more, could all prove to be advantageous [13].
Nanotechnology and Nanomaterials
Another prospect which could enhance the clinical applicability of osseointegration is the incorporation of nanotechnology and nanomaterials. Nanotechnology and nanomaterials are promising for orthopaedic applications because of their outstanding tribological properties, resistance to wear and abrasion, sustained drug delivery, tissue regeneration capabilities and most of all, capacity for osseointegration [14].
Nanobiotechnology in the Prevention and Treatment of Orthopaedic Implant-Associated Infections
Nanobiotechnology has progressed remarkably in recent years, particularly with respect to biomaterials, diagnostics and drug delivery systems. Many of these advanced strategies hold genuine promise for the prevention of implant-related infections. By applying nanobiotechnology, novel and smart drug delivery systems that release antibiotics locally upon detection of stimuli such as pH or temperature changes, or the presence of enzymes or antigens to prevent implant-associated infections could be developed. Other promising strategies for preventing implant-related infections include nanoscale modifications on the implant’s surface to inhibit bacterial adhesion and propagation at surgical site, the application of biological approaches such as gene therapy to neutralise bacterial virulence and the use of biomolecules to hinder bacterial quorum sensing and disrupt biofilm formation [15].
Advances in Production and Biomaterials
Another impediment to the widespread adoption of osseointegration is the high cost involved; largely due to the less frequent use of this technology, given the economies of scale. Nonetheless, a natural cost reduction over time is expected to occur as the technique becomes more widely available. Additionally, advances in 3D technology and the development of new biomaterials can further offset future expenses if implants can be printed locally at a lower unit cost. The challenge, however, remains how to best regulate the use of this technology in certain healthcare models where there is less accountability.
3D Printing and Custom-Made Implants
3D printing has already revolutionised many aspects of healthcare [16]. By fusing materials in a layered fashion, 3D printing allows for more flexible designs and more efficient manufacture of both custom-made and patient-specific 3D products. In orthopaedics, 3D printing can equip surgeons with the ability to customise implants, guides and jigs specific to each patient, using existing clinical imaging such as CT and MRI as guides to develop the desired product. 3D-printed orthopaedic implants can transform the way surgery is planned and executed, and can address pathologies that would otherwise be challenging to manage with traditionally manufactured implants (see Figs. 2, 3 and 4) [17,18,19,20].
This figure shows the application of 3D printing in clinical practise. Photos a to c shows the radiographs of a highly comminuted, open, intra-articular, distal femur fracture immediately after injury. Photos d to h show the intra-operative photos and radiographs after first stage reconstruction. Photos i to m show the designing process for a patient-specific 3D printed titanium truss implant. Photo n shows the beginning of the second stage reconstruction. Photo o is the 3D-printed acrylic model of the anticipated final skeletal defect, used to confirm satisfactory fit and alignment of the implant as demonstrated in p. Photos q to t show the final truss implant inserted successfully into defect (reproduced with permission from Tetsworth K, Block S, Glatt V. Putting 3D modelling and 3D printing into practice: virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT-J. 2017;3:16-.)
A shows an example of an osseointegration implant system, OPL, which is manufactured in three models (A), (B) and (C), each designed to accommodate different defects, but options remain limited. 3D printing will allow for more flexible designs and patient-specific implants that would accommodate patient needs better than traditional products. B shows an exploded view of the components of type A OPL implant. C is a radiograph of the implant in a patient’s femur (reproduced with permission from Hoellwarth JS, Tetsworth K, Rozbruch SR, Handal MB, Coughlan A, Al Muderis M. Osseointegration for Amputees. JBJS Reviews. 2020;8(3):e0043-e.)
This figure shows a patient with a successfully osseointegrated transfemoral prosthesis (reproduced with permission from Hoellwarth JS, Tetsworth K, Oomatia A, Akhtar MA, Xu H, Al Muderis M. Association Between Osseointegration of Lower Extremity Amputation and Mortality Among Adults. JAMA Network Open. 2022;5(10):e2235074-e.)
Cost-Effectiveness and Sustainability of 3D-Printed Implants
3D-printed implants have become increasingly popular in resource-constrained countries due to its cost and resource effectiveness in facilitating orthopaedic procedures. 3D printing also opens the door to cheaper demonstration models, customised from complex and unusual cases, which would enhance educational training [21]. 3D printing could also be more sustainable than traditional manufacturing, by constructing patient-specific implants with minimal material wastage. Sustainable 3D printing, along with the use of sustainable biomaterials, could simultaneously make the development of implants more accurate, biocompatible and cost-effective [22].
Natural Medicinal Compounds in Bone Tissue Engineering
Natural medicinal compounds with osteogenic potential can be integrated within 3D-printed implants to enhance bone formation and increase implant performance [23].
Meta-Biomaterials
Meta-biomaterials are designer biomaterials with curious and unprecedented properties resulting from their geometrically designed multi-scale architecture. In orthopaedic surgery, the concept of meta-biomaterials has chiefly been applied and researched in the context of improving bone tissue regeneration and combating implant-related infections. At the macroscale, studies have discussed the concepts of personalised implants, deployable meta-implants and shape-transforming implants. At the microscale, theories of multi-physics meta-biomaterials and the uses of auxetic meta-biomaterials for more durable orthopaedic implants were explored. At the nanoscale, nanopatterned and geometrically surfaced meta-biomaterials designed for simultaneous stimulation of osteogenic stem cell differentiation and bacteria eradication were also studied. The use of origami and self-folding mechanisms in meta-biomaterials has also been proposed [24].
3D-Printed Titanium Alloy Orthopaedic Implants
Titanium alloy orthopaedic implants manufactured by 3D printing have been increasingly popular in the field of orthopaedics because it combines the flexibility for complex and customised designing which 3D printing provides, with the excellent physical and chemical properties which titanium alloys possess, to achieve functional properties desired by orthopaedic surgeons and patients, such as long-term stability, anti-infection and anti-tumour [25].
Advances in Design and Feedback of Prosthetics
Traditional orthopaedic devices do not autonomously communicate with physicians or patients post-operatively. Yet, after implantation, the follow-up of traditional orthopaedic devices is generally limited to episodic monitoring by standard radiography. Fortunately, since the emergence of real-time health monitoring systems in other medical fields, the hopes for developing an orthopaedic device capable of providing direct feedback post-operatively to its user by tracking biological and physiological signals have been optimistic.
SMART Implants
SMART (Sensing, Measuring and Advanced Reporting Technology) orthopaedic implants, introduced in the 2018 FDA public workshop, incorporate technology that enables automated sensing, measuring, processing and reporting of patient or device parameters at or near the implant [26, 27]. Sensors for these implants will be small, simple, robust, reasonably priced and necessitate little to no modification to current implant designs, which is key for seamless integration into our current practice [28].
Technological Advances in Prosthesis Design and Rehabilitation
The complexity of human limbs, whether their form or function, has been a real challenge for prosthetists to recreate in prosthetics. Existing prosthetics often lack properties such as intuitive motor control, light touch sensation and proprioceptive functions typically found in a natural limb. However, new surgical advances are continually being developed to improve upon existing prosthetics, such as targeted muscle reinnervation, regenerative peripheral nerve interfaces, agonist–antagonist myoneural interfaces and targeted sensory reinnervation. Technology designed to restore sensation, such as implanted sensors and haptic devices, have also been invented for integration into prosthetics, and most importantly, the evolution of osseointegration recently shows great promise in improving the livelihoods of amputees. Augmented and virtual reality platforms could also contribute to a better quality of life for amputees by improving prosthesis design, pre-prosthetic education and integration, to achieve the goal of multi-functional, self-identifiable, durable and intuitive prostheses [29].
Biofeedback to Improve Performance of Myoelectric Pattern Recognition
It is expected that next-generation prosthetics will rely extensively on myoelectric pattern recognition-based control for better user dexterity. Myoelectric prostheses utilise electromyogram signals, which are electrical signals generated during residual limb muscle contractions for prosthesis control [30]. A major determinant for the successful incorporation of these prostheses into patients’ lives begins with pre-prosthesis education and their understanding of how these prosthetics work. With the help of an intuitive pattern similarity biofeedback mechanism, it makes training less daunting and allows amputees to optimise and adapt their muscular contractions accordingly to improve prosthesis control, dexterity and overall quality of life [31].
Neurophysiological Evaluation of Haptic Feedback for Myoelectric Prostheses
Haptic feedback in myoelectric prostheses has typically been evaluated based on task performance outcomes, which is crucial, but regrettably fails to fully capture the magnitude of mental effort required for prosthesis control. Cognitive loads, however, have generally been examined using reaction time metrics and secondary task accuracy, which are indirect measures and may not depict the fluctuating nature of mental effort. In this regard, wearable and wireless functional near-infrared spectroscopy (fNIRS) neuroimaging has been proposed, to provide direct and continual assessment of users’ mental effort during prosthesis use. Results suggest that haptic feedback facilitated task performance, reduced the cognitive load required for prosthesis use and demonstrated fNIRS’ potential in providing robust cognitive effort measurements for other human-in-the-loop systems [32].
Conclusion
The application of osseointegration in limb reconstruction for amputees appears to be following a trajectory like that of total joint arthroplasty, which gained universal acceptance and widespread adoption globally over the last 50 years. With the various emerging technological advances to combat the challenges that surgeons currently face in osseointegration, such as infection, manufacturing and implant feedback, the future of amputee rehabilitation is bright in our opinion, and we are optimistic osseointegration will continue to progress and advance as these new technologies continue to develop.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Overmann AL, Aparicio C, Richards JT, Mutreja I, Fischer NG, Wade SM, et al. Orthopaedic osseointegration: Implantology and future directions. J Orthop Res. 2020;38(7):1445–54.
Hoellwarth JS, Tetsworth K, Akhtar MA, Al MM. The clinical history and basic science origins of transcutaneous osseointegration for amputees. Adv Orthop. 2022;2022:1–14.
Li Y, Brånemark R. Osseointegrated prostheses for rehabilitation following amputation. Unfallchirurg. 2017;120(4):285–92.
Hoellwarth JS, Tetsworth K, Akhtar MA, Al MM. Transcutaneous osseointegration for amputees. Bone Joint Res. 2021;10(10):690–2.
Al Muderis MM, Lu WY, Li JJ, Kaufman K, Orendurff M, Highsmith MJ, et al. Clinically relevant outcome measures following limb osseointegration; systematic review of the literature. J Orthop Trauma. 2018;32(2):e64–75.
Gerzina C, Potter E, Haleem AM, Dabash S. The future of the amputees with osseointegration: a systematic review of literature. J Clin Orthop Trauma. 2020;11:S142–8.
Wang Z, Niu Y, Tian X, Yu N, Yin X, Xing Z, et al. Switching on and off macrophages by a “bridge-burning” coating improves bone-implant integration under osteoporosis. Adv Funct Mater. 2021;31(7):2007408.
Stavrakis AI, Zhu S, Hegde V, Loftin AH, Ashbaugh AG, Niska JA, et al. In vivo efficacy of a “smart” antimicrobial implant coating. J Bone Joint Surg. 2016;98(14):1183–9.
Linklater DP, Juodkazis S, Crawford RJ, Ivanova EP. Mechanical inactivation of Staphylococcus aureus and Pseudomonas aeruginosa by titanium substrata with hierarchical surface structures. Materialia. 2019;5:100197.
Yuan Z, Tao B, He Y, Mu C, Liu G, Zhang J, et al. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials. 2019;223:119479.
Hall TAG, Cegla F, van Arkel RJ. Simple smart implants: simultaneous monitoring of loosening and temperature in orthopaedics with an embedded ultrasound transducer. IEEE Trans Biomed Circ Syst. 2021;15(1):102–10.
Fries F, Ghoreishizadeh S (2020) Design methodology of a wireless pH measuring device for orthopaedic implants, pp 1–2. https://doi.org/10.1109/ICECS49266.2020.9294840
Rothe R, Hauser S, Neuber C, Laube M, Schulze S, Rammelt S, et al. Adjuvant drug-assisted bone healing: advances and challenges in drug delivery approaches. Pharmaceutics. 2020;12(5):428.
Pokkalath A, Nadar D, Ravikumar P, Sawarkar SP. Nanomaterials for orthopaedic implants and applications. Handbook on Nanobiomaterials for Therapeutics and Diagnostic Applications: Elsevier; 2021. p. 229–70.
Borse V, Pawar V, Shetty G, Mullaji A, Srivastava R. Nanobiotechnology perspectives on prevention and treatment of ortho-paedic implant associated infection. Curr Drug Deliv. 2016;13(2):175–85.
Tetsworth K, Block S, Glatt V. Putting 3D modelling and 3D printing into practice: virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT-J. 2017;3:16.
Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. https://doi.org/10.5435/JAAOSGlobal-D-20-00230.
Bagaria V, Bhansali R, Pawar P. 3D printing- creating a blueprint for the future of orthopedics: current concept review and the road ahead! J Clin Orthop Trauma. 2018;9(3):207–12.
Andrés-Cano P, Calvo-Haro JA, Fillat-Gomà F, Andrés-Cano I, Perez-Mañanes R. Role of the orthopaedic surgeon in 3D printing: current applications and legal issues for a personalized medicine. Rev Esp Cir Ortopédica y Traumatología (English Edition). 2021;65(2):138–51.
Haleem A, Javaid M, Khan RH, Suman R. 3D printing applications in bone tissue engineering. J Clin Orthop Trauma. 2020;11:S118–24.
Hasan O, Atif M, Jessar MM, Hashmi P (2019) Application of 3D printing in orthopaedic surgery. A new affordable horizon for cost-conscious care. J Pak Med Assoc 69(Suppl 1):S46–S50.
Yadav D, Garg RK, Ahlawat A, Chhabra D. 3D printable biomaterials for orthopedic implants: Solution for sustainable and circular economy. Resour Policy. 2020;68:101767.
Bose S, Sarkar N. Natural medicinal compounds in bone tissue engineering. Trends Biotechnol. 2020;38(4):404–17.
Zadpoor AA. Meta-biomaterials. Biomater Sci. 2020;8(1):18–38.
Jing Z, Zhang T, Xiu P, Cai H, Wei Q, Fan D, et al. Functionalization of 3D-printed titanium alloy orthopedic implants: a literature review. Biomed Mater. 2020;15(5):052003.
Baumann AP, O’Neill C, Owens MC, Weber SC, Sivan S, D’Amico R, et al. FDA public workshop: orthopaedic sensing, measuring, and advanced reporting technology (SMART) devices. J Orthop Res. 2021;39(1):22–9.
Ramakrishna VAS, Chamoli U, Rajan G, Mukhopadhyay SC, Prusty BG, Diwan AD. Smart orthopaedic implants: a targeted approach for continuous postoperative evaluation in the spine. J Biomech. 2020;104:109690.
Ledet EH, Liddle B, Kradinova K, Harper S. Smart implants in orthopedic surgery, improving patient outcomes: a review. Innov Entrep Health. 2018;5:41–51.
Bates TJ, Fergason JR, Pierrie SN. Technological advances in prosthesis design and rehabilitation following upper extremity limb loss. Curr Rev Musculoskelet Med. 2020;13(4):485–93.
Hargrove LJ, Miller LA, Turner K, Kuiken TA. Myoelectric pattern recognition outperforms direct control for transhumeral amputees with targeted muscle reinnervation: a randomized clinical trial. Sci Rep. 2017;7(1):13840.
de Montalivet E, Bailly K, Touillet A, Martinet N, Paysant J, Jarrasse N. Guiding the training of users with a pattern similarity biofeedback to improve the performance of myoelectric pattern recognition. IEEE Trans Neural Syst Rehabil Eng. 2020;28(8):1731–41.
Thomas N, Ung G, Ayaz H, Brown JD. Neurophysiological evaluation of haptic feedback for myoelectric prostheses. IEEE Trans Hum-Mach Syst. 2021;51(3):253–64.
Funding
None.
Author information
Authors and Affiliations
Contributions
M.A.A. contributed to study conception and design, literature search, writing of draft manuscripts, reading and approving final manuscript for publication. C.L. contributed to study conception and design, writing of draft manuscripts, reading and approving final manuscript for publication, and submission of manuscript to publisher. C.T. contributed to study conception and design, literature search, review of draft manuscript. J.S.H. contributed to study conception and design, literature search, review of draft manuscript. M.A.M. contributed to study conception and design, literature search, review of draft manuscript. K.T. contributed to study conception and design, literature search, review of draft manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No ethical approval was required for this study as this is a scoping literature review of ethically approved papers.
Consent to Participate
No participant consent was required to obtain for this study as this is a scoping literature review of papers that received informed consent to participate from all individual participants included in their study.
Consent for Publication
No participant consent was required to obtain for this study as this is a scoping literature review of published papers that received informed consent to publish from all individual participants included in their study.
Competing Interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Munjed Al Muderis is the sole beneficiary of Osseointegration Holdings Pty Ltd. (OH) and Osseointegration International Pty Ltd. (OI). OI exclusively distributes the OPL implant system worldwide. OH owns the rights and patents to the OPL implant system. The other authors have no declarations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akhtar, M.A., Low, C., Tiemessen, C. et al. Current Challenges and Future Prospects of Osseointegration Limb Reconstruction for Amputees. SN Compr. Clin. Med. 6, 4 (2024). https://doi.org/10.1007/s42399-023-01629-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01629-3