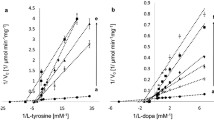
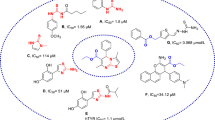
Abstract
Besides being used in the treatment of melanin hyperpigmentation, inhibition of tyrosinase (TYR) is a promising approach to prevent economic losses. This study focuses on several natural tyrosinase inhibitors based on the literature published, and subsequently molecular docking studies have brought further perspective to predict binding affinities of these compounds for supply useful and potential tyrosinase inhibitors. In this work, a series of natural origin tyrosinase inhibitors were selected and according to values of their KI and IC50, the potential tyrosinase inhibitory activity was analyzed. The results revealed that natural sources such as flavonoids and aromatic carboxylic acids and their aldehydes analogues serve as updated database tyrosinase inhibitors, and their IC50 values were comparable to that of the positive control tyrosinase inhibitor (kojic acid). It was speculated that aldehyde substituted carboxylic group of cumic and anisic acid exhibit potent tyrosinase inhibitory activity. According to this study, understanding tyrosinase inhibitors from natural compounds and their structural modification is very important to development and discovery of novel tyrosinase inhibitors that can help to health promoting. Also, theoretical techniques could be used to identify new and potent tyrosinase inhibitors based on their structural properties.
Graphical Abstract
The scheme of biosynthesis of melanins in melanocytes catalysed by tyrosinase.





Similar content being viewed by others
References
Bagherzadeh K, Shirgahi Talari F, Sharifi A, Ganjali MR, Saboury AA, Amanlou M (2015) A new insight into mushroom tyrosinase inhibitors: docking, pharmacophore-based virtual screening, and molecular modeling studies. J Biomol Struct Dyn 33(3):487–501. https://doi.org/10.1080/07391102.2014.893203
Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, Saboury AA (2019) A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem 34(1):279–309. https://doi.org/10.1080/14756366.2018.1545767
Fernandes MS, Kerkar S (2017) Microorganisms as a source of tyrosinase inhibitors: a review. Ann Microbiol 67(4):343–358. https://doi.org/10.1007/s13213-017-1261-7
Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M (2006) Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J Biol Chem 281(13):8981–8990. https://doi.org/10.1074/jbc.M509785200
Olivares C, Solano F (2009) New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melan Res 22(6):750–760. https://doi.org/10.1111/j.1755-148X,2009.00636.x
Deeth RJ, Diedrich C (2010) Structural and mechanistic insights into the oxy form of tyrosinase from molecular dynamics simulations. JBIC J Biol Inorg Chem 15(2):117–129. https://doi.org/10.1007/s00775-009-0577-6
Gardelly M, Trimech B, Horchani M, Znati M, Jannet HB, Romdhane A (2021) Anti-tyrosinase and anti-butyrylcholinesterase quinolines-based coumarin derivatives: synthesis and insights from molecular docking studies. Chem Afr 4(3):491–501. https://doi.org/10.1007/s42250-021-00235-x
Sugumaran M (2002) Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res 15(1):2–9. https://doi.org/10.1034/j.1600-0749.2002.00056.x
Hridya H, Amrita A, Sankari M, Doss CGP, Gopalakrishnan M, Gopalakrishnan C, Siva R (2015) Inhibitory effect of brazilein on tyrosinase and melanin synthesis: Kinetics and in silico approach. Int J Biol Macromol 81:228–234. https://doi.org/10.1016/j.ijbiomac.2015.07.064
Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109(7):3012–3043. https://doi.org/10.1021/cr900019j
Masum MN, Yamauchi K, Mitsunaga T (2019) Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev Agric Sci 7:41–58. https://doi.org/10.7831/ras.7.41
Karakaya G, Ercan A, Oncul S, Aytemir MD (2018) Synthesis and cytotoxic evaluation of kojic acid derivatives with inhibitory activity on melanogenesis in human melanoma cells. Anti Cancer Agents Med Chem (Form Curr Med Chem Anti Cancer Agents 18(15): 2137–2148. https://doi.org/10.2174/1871520618666180402141714
Blaut M, Braune A, Wunderlich S, Sauer P, Schneider H, Glatt H (2006) Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria. Food Chem Toxicol 44(11):1940–1947. https://doi.org/10.1016/j.fct.2006.06.015
Zheng ZP, Tan HY, Chen J, Wang M (2013) Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structure–activity relationship study. Fitoterapia, 84: 242–247. https://doi.org/10.1016/j.fitote.2012.12.006
Si YX, Wang ZJ, Park D, Chung HY, Wang SF, Yan L, Park YD (2012) Effect of hesperetin on tyrosinase: inhibition kinetics integrated computational simulation study. Int J Biol Macromol 50(1):257–262. https://doi.org/10.1016/j.ijbiomac.2011.11.001
Thilakarathna SH, Rupasinghe HV (2013) Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5(9):3367–3387. https://doi.org/10.3390/nu5093367
Hu JN, Zou XG, He Y, Chen F, Deng ZY (2016) Esterification of quercetin increases its transport across human caco‐2 cells. J Food Sci 81(7), H1825–H1832. https://doi.org/10.1111/1750-3841.13366
Vaezi M (2021) Evaluation of quercetin omega-6 and-9 esters on activity and structure of mushroom tyrosinase: spectroscopic and molecular docking studies. J Food Biochem 45(11):e13953. https://doi.org/10.1111/jfbc.13953
Sova M (2012) Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem 12(8):749–767. https://doi.org/10.2174/138955712801264792
Muhammad JS, Zaidi SF, Shaharyar S, Refaat A, Usmanghani K, Saiki I, Sugiyama T (2015) Anti-inflammatory effect of cinnamaldehyde in Helicobacter pylori induced gastric inflammation. Biol Pharm Bull 38(1):109–115. https://doi.org/10.1248/bpb.b14-00609
Chen CY, Lin LC, Yang WF, Bordon J, Wang DHM (2015) An updated organic classification of tyrosinase inhibitors on melanin biosynthesis. Curr Org Chem 19(1):4–18. https://doi.org/10.2174/1385272819666141107224806
Panzella L, Ebato A, Napolitano A, Koike K (2018) The late stages of melanogenesis: exploring the chemical facets and the application opportunities. Int J Mol Sci 19(6):1753. https://doi.org/10.3390/ijms19061753
Niu C, Aisa HA (2017) Upregulation of melanogenesis and tyrosinase activity: potential agents for vitiligo. Molecules 22(8):1303. https://doi.org/10.3390/molecules22081303
Chang TS (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10(6):2440–2475. https://doi.org/10.3390/ijms10062440
Zhao DY, Zhang MX, Dong XW, Hu YZ, Dai XY, Wei X, Zhou T (2016) Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg Med Chem Lett 26(13):3103–3108. https://doi.org/10.1016/j.bmcl.2016.05.006
Poma A, Pacioni G, Colafarina S, Miranda M (1999) Effect of tyrosinase inhibitors on Tuber borchii mycelium growth in vitro. FEMS Microbiol Lett 180(1): 69–75. https://doi.org/10.1111/j.1574-6968.1999.tb08779.x
Jakimiuk K, Sari S, Milewski R, Supuran CT, Şöhretoğlu D, Tomczyk M (2022) Flavonoids as tyrosinase inhibitors in in silico and in vitro models: basic framework of SAR using a statistical modelling approach. J Enz Inhib Med Chem 37(1): 427–436. https://doi.org/10.1080/14756366.2021.2014832
Xue YL, Miyakawa T, Hayashi Y, Okamoto K, Hu F, Mitani N, Tanokura M (2011) Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J Agric Food Chem 59(11):6011–6017. https://doi.org/10.1021/jf200940h
Wang Y, Zhang G, Yan J, Gong D (2014) Inhibitory effect of morin on tyrosinase: Insights from spectroscopic and molecular docking studies. Food Chem 163: 226–233. https://doi.org/10.1016/j.foodchem.2014.04.106
Schurink M (2007) Peptides as inhibitors of lipoxygenase and tyrosinase. Wageningen University and Research.
Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, Bae H (2006) Survey and mechanism of skin depigmenting and lightening agents. Phytother Res Int J Dev Pharmacol Toxicol Eval Natl Prod Derivat 20(11):921–934. https://doi.org/10.1002/ptr.1954
Lee HS (2002) Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem 50(6):1400–1403. https://doi.org/10.1021/jf011230f
Kim YJ, Uyama H (2005) Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci CMLS 62(15):1707–1723. https://doi.org/10.1007/s00018-005-5054-y
Cabanes J, Garcia-Canovas F, Tudela J, Lozano JA, García-Carmona F (1987) L-mimosine a slow-binding inhibitor of mushroom tyrosinase. Phytochemistry 26(4):917–919. https://doi.org/10.1016/S0031-9422(00)82317-1
Şöhretoğlu D, Sari S, Barut B, Özel A (2018) Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg Chem 81:168–174. https://doi.org/10.1016/j.bioorg.2018.08.020
Yu L (2003) Inhibitory effects of (S)-and (R)-6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acids on tyrosinase activity. J Agric Food Chem 51(8):2344–2347. https://doi.org/10.1021/jf0208379
Xie LP, Chen QX, Huang H, Wang HZ, Zhang RQ (2003) Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochem (Mos) 68(4):487–491. https://doi.org/10.1023/A:1023620501702
Kim D, Park J, Kim J, Han C, Yoon J, Kim N, Lee C (2006) Flavonoids as mushroom tyrosinase inhibitors: a fluorescence quenching study. J Agric Food Chem 54(3):935–941. https://doi.org/10.1021/jf0521855
Kubo I, Kinst-Hori I (1998) Tyrosinase inhibitors from anise oil. J Agric Food Chem 46(4):1268–1271. https://doi.org/10.1021/jf9708958
Conrad JS, Dawso SR, Hubbard ER, Meyers TE, Strothkamp KG (1994) Inhibitor binding to the binuclear active site of tyrosinase: temperature, pH, and solvent deuterium isotope effects. Biochemistry 33(19):5739–5744. https://doi.org/10.1021/bi00185a010
Hassani S, Haghbeen K, Fazli M (2016) Non-specific binding sites help to explain mixed inhibition in mushroom tyrosinase activities. Eur J Med Chem 122:138–148. https://doi.org/10.1016/j.ejmech.2016.06.013
Kubo I, Chen QX, Nihei KI, Calderón JS, Céspedes CL (2003) Tyrosinase inhibition kinetics of anisic acid. Zeitschrift für Naturforschung C 58(9–10):713–718. https://doi.org/10.1515/znc-2003-9-1021
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93. https://doi.org/10.3390/medicines5030093
Vaezi M (2022) Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: review and new insight. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2022.2069157
Obaid RJ, Mughal EU, Naeem N, Sadiq A, Alsantali RI, Jassas RS, Ahmed SA (2021) Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: a systematic review. RSC Adv 11(36):22159–22198. https://doi.org/10.1039/D1RA03196A
Fan M, Zhang G, Hu X, Xu X, Gong D (2017) Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res Int 100:226–233. https://doi.org/10.1016/j.foodres.2017.07.010
Povinelli APR, Zazeri G, Cornélio ML (2019) Molecular mechanism of flavonoids using fluorescence spectroscopy and computational tools. Flavon A Color Model Cheer Up Life. https://doi.org/10.5772/intechopen.84480
Nazir Y, Rafique H, Roshan S, Shamas S, Ashraf Z, Rafiq M, Asad MHHB (2022) Molecular docking, synthesis, and tyrosinase inhibition activity of acetophenone amide: potential inhibitor of melanogenesis. Biomed Res Int. https://doi.org/10.1155/2022/1040693
Chen JS, Wei CI, Rolle RS, Otwell WS, Balaban MO, Marshall MR (1991) Inhibitory effect of kojic acid on some plant and crustacean polyphenol oxidases. J Agric Food Chem 39(8):1396–1401. https://doi.org/10.1021/jf00008a008
Morrison JF, Walsh CT (1988) The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol 61:201–301. https://doi.org/10.1002/9780470123072.ch5
Kocer M, Istifli ES (2022) Chemical composition and cholinesterase, tyrosinase, alpha-amylase and alpha-glucosidase inhibitory activity of the essential oil of Salvia tomentosa. Int J Plant Based Pharm 2(1):1–16. https://doi.org/10.5281/zenodo.6386394
Nofal A, Ibrahim ASM, Nofal E, Gamal N, Osman S (2019) Topical silymarin versus hydroquinone in the treatment of melasma: a comparative study. J Cosmet Dermatol 18(1):263–270. https://doi.org/10.1111/jocd.12769
Singh BK, Park SH, Lee HB, Goo YA, Kim HS, Cho SH, Kim EK (2016) Kojic acid peptide: a new compound with anti-tyrosinase potential. Ann Dermatol 28(5):555–561. https://doi.org/10.5021/ad.2016.28.5.555
Higa Y, Kawabe M, Nabae K, Toda Y, Kitamoto S, Hara T, Takahashi M (2007) Kojic acid-absence of tumor-initiating activity in rat liver, and of carcinogenic and photo-genotoxic potential in mouse skin. J Toxicol Sci 32(2):143–159. https://doi.org/10.2131/jts.32.143
Arulmozhi V, Pandian K, Mirunalini S (2013) Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Coll Surf B 110:313–320. https://doi.org/10.1016/j.colsurfb.2013.03.039
Yin SJ, Si YX, Qian GY (2011) Inhibitory effect of phthalic acid on tyrosinase: the mixed-type inhibition and docking simulations. Enzyme Res. https://doi.org/10.4061/2011/294724
Adams TB, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ, Wagner BM (2005) The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food Chem Toxicol 43(8):1207–1240. https://doi.org/10.1016/j.fct.2004.11.014
Jiménez M, Chazarra S, Escribano J, Cabanes J, García-Carmona F (2001) Competitive inhibition of mushroom tyrosinase by 4-substituted benzaldehydes. J Agric Food Chem 49(8), 4060–4063. https://doi.org/10.1021/jf010194h
Rafiee M, Javaheri M (2015) A theoretical study of benzaldehyde derivatives as tyrosinase inhibitors using Ab initio calculated NQCC parameters. Mol Biol Res Commun 4(3): 151. https://doi.org/10.22099/mbrc.2015.3118
Kubo I, Kinst-Hori I (1998) Tyrosinase inhibitors from cumin. J Agric Food Chem 46(12):5338–5341. https://doi.org/10.1021/jf980226+
Kong YH, Jo YO, Cho CW, Son D, Park S, Rho J, Choi SY (2008) Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol Pharm Bull 31(5):946–948. https://doi.org/10.1248/bpb.31.946
Singh K (2020) Evaluation of antifungal activity of cinnamaldehyde against Cryptococcus neoformans var. grubii. Folia Microbiol 65(6): 973–987. https://doi.org/10.1007/s12223-020-00806-4
Bang HB, Lee YH, Kim SC, Sung CK, Jeong KJ (2016) Metabolic engineering of Escherichia coli for the production of cinnamaldehyde. Microb Cell Fact 15(1): 1–12. https://doi.org/10.1186/s12934-016-0415-9
Parvez S, Kang M, Chung HS, Bae H (2007) Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res Int J Dev Pharmacol Toxicol Eval Natl Prod Derivat 21(9):805–816. https://doi.org/10.1002/ptr.2184
Funding
No financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vaezi, M. In Silico and In Vitro Studies of Naturally Occurring Tyrosinase Inhibitors: Structure–Activity Relationship. Chemistry Africa 5, 1873–1887 (2022). https://doi.org/10.1007/s42250-022-00466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00466-6