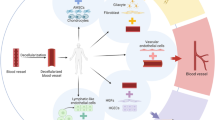
Abstract
Vascular tissue regeneration has gained a lot of interest, especially in addressing the challenges associated with vascular-related diseases and injuries. Hydrogels have shown great promise in the field of vascular tissue regeneration because of their special features, which include biocompatibility and mechanical characteristics that may be adjusted, as well as their likeness to the natural extracellular matrix. The fabrication techniques for vascular scaffolds have been the subject of much investigation due to the effectiveness of scaffold-based tissue engineering in producing new blood vessel tissues. The creation of vascular scaffolds has been greatly aided by recent developments in 3D printing, which presents an encouraging concept for the vascularization of tissues. This review covers the various cutting-edge hydrogel formulations, fabrication techniques, and strategies for the development of functional and biocompatible vascular scaffolds. The review also reveals these novel hydrogel-based techniques’ possible uses, difficulties, and possibilities for the future of vascular tissue regeneration.








Similar content being viewed by others
Data availability
Data will be available upon request.
References
H. Thomas et al., Global Atlas of cardiovascular disease 2000–2016: The path to prevention and control. Global Heart 13(3), 143 (2018). https://doi.org/10.1016/j.gheart.2018.09.511
G.W. Stone et al., A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364(3), 226–235 (2011). https://doi.org/10.1056/nejmoa1002358
S. Stewart, K. MacIntyre, D.J. Hole, S. Capewell, J.J.V. McMurray, More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur. J. Heart Fail. 3(3), 315–322 (2001). https://doi.org/10.1016/S1388-9842(00)00141-0
C. V. C. Bouten, C. Cheng, I. M. Vermue, D. Gawlitta, and R. Passier, “Cardiovascular tissue engineering and regeneration: a plead for further knowledge convergence,” Tissue. Eng. Part A, 28(11–12) 525–541 https://doi.org/10.1089/ten.tea.2021.0231. Mary Ann Liebert Inc
Qiongyu Li, “Authors : Ac ce d M us pt,” 2d Mater, pp. 0–23, 2018.
W. Gu, X. Hong, C. Potter, A. Qu, Q. Xu, Mesenchymal stem cells and vascular regeneration. Microcirculation 24(1), 1–15 (2017). https://doi.org/10.1111/micc.12324
Y. Fang, L. Ouyang, T. Zhang, C. Wang, B. Lu, and W. Sun, “Optimizing bifurcated channels within an anisotropic scaffold for engineering vascularized oriented tissues,” Adv. Healthc. Mater. 9(24) 2020. https://doi.org/10.1002/adhm.202000782.
M. Carrabba, P. Madeddu, Current strategies for the manufacture of small size tissue engineering vascular grafts. Front Bioeng Biotechnol 6, 1–12 (2018). https://doi.org/10.3389/fbioe.2018.00041
F.G. Aoki et al., De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue engineering. Sci. Rep. 9(1), 1–12 (2019). https://doi.org/10.1038/s41598-019-48450-4
H. Yin et al., Dual-encapsulated biodegradable 3D scaffold from liposome and waterborne polyurethane for local drug control release in breast cancer therapy. J. Biomater. Sci. Polym. Ed. 31(17), 2220–2237 (2020). https://doi.org/10.1080/09205063.2020.1796230
H.H.G. Song, R.T. Rumma, C.K. Ozaki, E.R. Edelman, C.S. Chen, Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell 22(3), 340–354 (2018). https://doi.org/10.1016/j.stem.2018.02.009
J. Huang et al., The long-term behaviors and differences in bone reconstruction of three polymer-based scaffolds with different degradability. J Mater Chem B 7(48), 7690–7703 (2019). https://doi.org/10.1039/c9tb02072a
C.V.C. Bouten, P.Y.W. Dankers, A. Driessen-Mol, S. Pedron, A.M.A. Brizard, F.P.T. Baaijens, Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 63(4), 221–241 (2011). https://doi.org/10.1016/j.addr.2011.01.007
S.G. Wise, M.J. Byrom, A. Waterhouse, P.G. Bannon, M.K.C. Ng, A.S. Weiss, A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 7(1), 295–303 (2011). https://doi.org/10.1016/j.actbio.2010.07.022
A. Hasan et al., “Electrospun scaffolds for tissue engineering of vascular grafts,” Acta. Biomater. 2013. https://doi.org/10.1016/j.actbio.2013.08.022.
D.B. Das, T. Liu, Tissue engineering. Asia-Pac. J. Chem. Eng. 6(6), 813–815 (2011). https://doi.org/10.1002/apj.651
J.T. Krawiec, D.A. Vorp, Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials 33(12), 3388–3400 (2012). https://doi.org/10.1016/j.biomaterials.2012.01.014
R. Samuel et al., Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA 110(31), 12774–12779 (2013). https://doi.org/10.1073/pnas.1310675110
C.M. Murphy, F.J. O’Brien, D.G. Little, A. Schindeler, Cell-scaffold interactions in the bone tissue engineering triad. Euro Cells Mater 26, 120–132 (2013). https://doi.org/10.22203/eCM.v026a09. (AO Research Institute Davos)
A.B. Ennett, D. Kaigler, D.J. Mooney, Temporally regulated delivery of VEGF in vitro and in vivo. J. Biomed. Mater. Res. A 79(1), 176–184 (2006). https://doi.org/10.1002/jbm.a.30771
Crispin B. Weinberg, E. Bell, A blood vessel model constructed from collagen and cultured vascular cells. Science 231(4736), 397–400 (1986)
S. Ravi, E.L. Chaikof, Biomaterials for vascular tissue engineering. Regen. Med. 5(1), 107–120 (2010). https://doi.org/10.2217/rme.09.77
W.G. Chang, L.E. Niklason, A short discourse on vascular tissue engineering. NPJ Regen Med 2, 1 (2017). https://doi.org/10.1038/s41536-017-0011-6
E. Ercolani, C. Del Gaudio, A. Bianco, Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach. J Tissue Eng Regen Med 9(8), 861–888 (2015). https://doi.org/10.1002/term.1697
J. Eble, S. Niland, The extracellular matrix of blood vessels. Curr. Pharm. Des. 15(12), 1385–1400 (2009). https://doi.org/10.2174/138161209787846757
C.-Y. Chen et al., Blood flow reprograms lymphatic vessels to blood vessels. J. Clin. Investig. 122(6), 2006–2017 (2012). https://doi.org/10.1172/jci57513
M. Tennant, J.K. McGeachie, Blood vessel structure and function: a brief update on recent advances. Aust. N. Z. J. Surg. 60(10), 747–753 (1990). https://doi.org/10.1111/j.1445-2197.1990.tb07468.x
M.K. Pugsley, R. Tabrizchi, The vascular system. J. Pharmacol. Toxicol. Methods 44(2), 333–340 (2000). https://doi.org/10.1016/s1056-8719(00)00125-8
J.A. Eble, S. Niland, The extracellular matrix of blood vessels. Curr Pharm Des 15(12), 1385–1400 (2009)
E. M. Conway, D. Collen, and P. Carmeliet, “Molecular mechanisms of blood vessel growth,” 2001. [Online]. Available: www.elsevier.com/locate/cardioreswww.elsevier.nl/locate/cardiores
T. Nauta, V. van Hinsbergh, P. Koolwijk, Hypoxic signaling during tissue repair and regenerative medicine. Int. J. Mol. Sci. 15(11), 19791–19815 (2014). https://doi.org/10.3390/ijms151119791
C. López-Camarillo, E. Ruiz-García, N. Starling, and L. A. Marchat, “Editorial: Neovascularization, angiogenesis and vasculogenic mimicry in cancer,” Front Oncol. 10. Frontiers Media S.A., 2020. https://doi.org/10.3389/fonc.2020.01140.
L. Elomaa, Y.P. Yang, Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Eng Part B: Rev 23(5), 436–450 (2017). https://doi.org/10.1089/ten.teb.2016.0348. (Mary Ann Liebert Inc)
S. Negri, P. Faris, R. Berra-Romani, G. Guerra, and F. Moccia, “Endothelial transient receptor potential channels and vascular remodeling: extracellular Ca2 + entry for angiogenesis, arteriogenesis and vasculogenesis,” Front. Physiol. 10. Frontiers Media S.A. 2020. https://doi.org/10.3389/fphys.2019.01618.
H. Saman, S. S. Raza, S. Uddin, and K. Rasul, “Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches,” Cancers. 12(5). MDPI AG 2020. https://doi.org/10.3390/cancers12051172.
S. Y. Tan, Z. Leung, and A. R. Wu, “Recreating physiological environments in vitro: design rules for microfluidic-based vascularized tissue constructs,” Small. 16(9). Wiley-VCH Verlag, 2020. https://doi.org/10.1002/smll.201905055.
J. Lee, K.Y. Lee, Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm. Res. 26(7), 1739–1744 (2009). https://doi.org/10.1007/s11095-009-9884-4
B.M. Baker, B. Trappmann, S.C. Stapleton, E. Toro, C.S. Chen, Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip 13(16), 3246–3252 (2013). https://doi.org/10.1039/c3lc50493j
Y. J. Blinder, A. Freiman, N. Raindel, D. J. Mooney, and S. Levenberg, “Vasculogenic dynamics in 3D engineered tissue constructs,” Sci. Rep. 5 2015. https://doi.org/10.1038/srep17840.
M.W. Laschke, M.D. Menger, Vascularization in tissue engineering: angiogenesis versus inosculation. Eur. Surg. Res. 48(2), 85–92 (2012). https://doi.org/10.1159/000336876
V. Wu et al., “Bone tissue regeneration in the oral and maxillofacial region: a review on the application of stem cells and new strategies to improve vascularization,” Stem. Cells. Intl. 2019. Hindawi Limited, 2019. https://doi.org/10.1155/2019/6279721.
D. Sharma, D. Ross, G. Wang, W. Jia, S.J. Kirkpatrick, F. Zhao, Upgrading prevascularization in tissue engineering: a review of strategies for promoting highly organized microvascular network formation. Acta Biomaterialia. 95, 112–130 (2019). https://doi.org/10.1016/j.actbio.2019.03.016. (Acta Materialia Inc)
M.W. Laschke, M.D. Menger, Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv. 34(2), 112–121 (2016). https://doi.org/10.1016/j.biotechadv.2015.12.004. (Elsevier Inc)
D. Gholobova, L. Terrie, M. Gerard, H. Declercq, and L. Thorrez, “Vascularization of tissue-engineered skeletal muscle constructs,” Biomaterials. 235 2020. https://doi.org/10.1016/j.biomaterials.2019.119708. Elsevier Ltd
D. Kolte, J. A. McClung, and W. S. Aronow, “Vasculogenesis and angiogenesis,” in Translational Research in Coronary Artery Disease: Pathophysiology to Treatment, Elsevier Inc., 2016, pp. 49–65. https://doi.org/10.1016/B978-0-12-802385-3.00006-1.
R.V. Badhe et al., A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. Carbohydr. Polym. 157, 1215–1225 (2017). https://doi.org/10.1016/j.carbpol.2016.09.095
J. Oztemur, S. Ozdemir, H. Sezgin, and I. Yalcin-Enis, Hydrogel-based vascular grafts: state of art, 2023. https://doi.org/10.1016/B978-0-323-91753-7.00011-9.
S. Mohapatra et al., Biomedical application, patent repository, clinical trial and regulatory updates on Hydrogel: an extensive review. Gels 7(4), 207 (2021). https://doi.org/10.3390/gels7040207
S. Cascone and G. Lamberti, “Hydrogel-based commercial products for biomedical applications: a review,” Int. J. Pharm. 573 2020. https://doi.org/10.1016/j.ijpharm.2019.118803.
L.M. Ricles et al., Therapeutic assessment of mesenchymal stem cells delivered within a PEGylated fibrin gel following an ischemic injury. Biomaterials 102, 9–19 (2016). https://doi.org/10.1016/j.biomaterials.2016.06.011
I. Kim, S.S. Lee, S. Bae, H. Lee, N.S. Hwang, Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization. Biomacromol 19(6), 2257–2269 (2018). https://doi.org/10.1021/acs.biomac.8b00331
P.H. Kim et al., Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release 187, 1–13 (2014). https://doi.org/10.1016/j.jconrel.2014.05.010
V. Sacchi et al., Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci USA 111(19), 6952–6957 (2014). https://doi.org/10.1073/pnas.1404605111
I.M. El-Sherbiny, M.H. Yacoub, Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract 2013(3), 38 (2013). https://doi.org/10.5339/gcsp.2013.38
M. R. Singh, S. Patel, and D. Singh, “Natural polymer-based hydrogels as scaffolds for tissue engineering,” in Nanobiomaterials in Soft Tissue Engineering: Applications of Nanobiomaterials, Elsevier Inc., 2016, pp. 231–260. https://doi.org/10.1016/B978-0-323-42865-1.00009-X.
S. Dhingra, R.D. Weisel, R.K. Li, Synthesis of aliphatic polyester hydrogel for cardiac tissue engineering. Methods Mol. Biol. 1181, 51–59 (2014). https://doi.org/10.1007/978-1-4939-1047-2_5
O. Janoušková, Synthetic polymer scaffolds for soft tissue engineering. Physiol Res 67, 335–348 (2018). https://doi.org/10.33549/physiolres.933983. (Czech Academy of Sciences)
A.M.J. Coenen, K.V. Bernaerts, J.A.W. Harings, S. Jockenhoevel, S. Ghazanfari, Elastic materials for tissue engineering applications: natural, synthetic, and hybrid polymers. Acta Biomaterialia 79, 60–82 (2018). https://doi.org/10.1016/j.actbio.2018.08.027
G. Tarun, B. Ajay, K. Bhawna, K. Sunil, J. Ravi, and S. G. L. Bihani, “Scaffold: tissue engineering and regenerative medicine,” 2011. [Online]. Available: www.irjponline.com
F. Y. Hsieh, L. Tao, Y. Wei, and S. H. Hsu, “A novel biodegradable self-healing hydrogel to induce blood capillary formation,” NPG Asia Mater, vol. 9, no. 3, 2017. https://doi.org/10.1038/am.2017.23.
G. Sun, Y.I. Shen, S. Kusuma, K. Fox-Talbot, C.J. Steenbergen, S. Gerecht, Functional neovascularization of biodegradable dextran hydrogels with multiple angiogenic growth factors. Biomaterials 32(1), 95–106 (2011). https://doi.org/10.1016/j.biomaterials.2010.08.091
J.R. Saunders, S. Abu-Salih, T. Khaleque, S. Hanula, W. Moussa, Modeling theories of intelligent hydrogel polymers. J. Comput. Theor. Nanosci. 5(10), 1942–1960 (2008). https://doi.org/10.1166/jctn.2008.1001
A. Nicosia, M. Salamone, S. Costa, M. A. Ragusa, and G. Ghersi, “Mimicking molecular pathways in the design of smart hydrogels for the design of vascularized engineered tissues,” Intl. J. Mol. Sci. 24(15). Multidisciplinary Digital Publishing Institute (MDPI), 2023. https://doi.org/10.3390/ijms241512314.
X. Li, X. Su, Multifunctional smart hydrogels: potential in tissue engineering and cancer therapy. J Mater Chem B 6(29), 4714–4730 (2018). https://doi.org/10.1039/c8tb01078a. (Royal Society of Chemistry)
A. Bordbar-Khiabani and M. Gasik, “Smart hydrogels for advanced drug delivery systems,” Intl. J. Mol. Sci. 23(7) 2022. https://doi.org/10.3390/ijms23073665.
S. Mantha et al., “Smart hydrogels in tissue engineering and regenerative medicine,” Materials. 12(20). MDPI AG 2019. https://doi.org/10.3390/ma12203323.
R. Duncan, Polymer therapeutics as nanomedicines: new perspectives. Curr. Opin. Biotechnol. 22(4), 492–501 (2011). https://doi.org/10.1016/j.copbio.2011.05.507
X. Zheng, P. Zhang, Z. Fu, S. Meng, L. Dai, H. Yang, Applications of nanomaterials in tissue engineering. RSC Adv 11(31), 19041–19058 (2021). https://doi.org/10.1039/d1ra01849c. (Royal Society of Chemistry)
K.A. Rocco, M.W. Maxfield, C.A. Best, E.W. Dean, C.K. Breuer, In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng Part B: Rev 20(6), 628–640 (2014). https://doi.org/10.1089/ten.teb.2014.0123. (Mary Ann Liebert Inc)
M. Vert, Degradable polymers in medicine: Updating strategies and terminology. Inl J Artif Organs 34(2), 76–83 (2011). https://doi.org/10.5301/IJAO.2011.6400. (Wichtig Publishing Srl)
C.S. Hughes, L.M. Postovit, G.A. Lajoie, Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10(9), 1886–1890 (2010). https://doi.org/10.1002/pmic.200900758
O. Smidsrod, G. Skjakbrk, Alginate as immobilization matrix for cells. Trends Biotechnol. 8, 71–78 (1990). https://doi.org/10.1016/0167-7799(90)90139-o
F.A. Johnson, D.Q.M. Craig, A.D. Mercer, Characterization of the block structure and molecular weight of sodium alginates. J. Pharm. Pharmacol. 49(7), 639–643 (1997). https://doi.org/10.1111/j.2042-7158.1997.tb06085.x
M.A. LeRoux, F. Guilak, L.A. Setton, Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J. Biomed. Mater. Res. 47(1), 46–53 (1999). https://doi.org/10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n
P. Eiselt, K.Y. Lee, D.J. Mooney, Rigidity of two-component hydrogels prepared from alginate and poly(ethylene glycol)-diamines. Macromolecules 32(17), 5561–5566 (1999). https://doi.org/10.1021/ma990514m
K.Y. Lee, J.A. Rowley, P. Eiselt, E.M. Moy, K.H. Bouhadir, D.J. Mooney, Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 33(11), 4291–4294 (2000). https://doi.org/10.1021/ma9921347
A. Barre et al., An alginate-based hydrogel with a high angiogenic capacity and a high osteogenic potential. Biores Open Access 9(1), 174–182 (2020). https://doi.org/10.1089/biores.2020.0010
P. Castillo-Briceño Patricia et al., A role for specific collagen motifs during wound healing and inflammatory response of fibroblasts in the teleost fish gilthead seabream. Mol Immunol 4(6–7), 826–834 (2011). https://doi.org/10.1016/j.molimm.2010.12.004
K. Gelse, E. Pöschl, T. Aigner, Collagens - structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55(12), 1531–1546 (2003). https://doi.org/10.1016/j.addr.2003.08.002
B. Alberts, D. Bray, J. Lewis, M. Raff, K. Roberts, JD. Watson Molecular biology of the cell (third edition) Garland Publishing, New York and London 1994 22 164-164
C. H. Lee, A. Singla, and Y. Lee, “Biomedical applications of collagen,” 2001. [Online]. Available: www.elsevier.com/locate/ijpharm
C.R. Lee, A.J. Grodzinsky, M. Spector, The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials 22(23), 3145–3154 (2001). https://doi.org/10.1016/s0142-9612(01)00067-9
G. Chen, T. Ushida, T. Tateishi, Development of biodegradable porous scaffolds for tissue engineering. Mater. Sci. Eng. C. 17(1–2), 63–69 (2001). https://doi.org/10.1016/s0928-4931(01)00338-1
S.-N. Park, J.-C. Park, H.O. Kim, M.J. Song, H. Suh, Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials 23(4), 1205–1212 (2002). https://doi.org/10.1016/s0142-9612(01)00235-6
J.A. Claudio-Rizo, J. Delgado, I.A. Quintero-Ortega, J.L. Mata-Mata, B. Mendoza-Novelo, “Decellularized ECM-derived hydrogels: modification and properties”, in Hydrogels. INTECH (2018). https://doi.org/10.5772/intechopen.78331
J. A. Claudio-Rizo, B. Mendoza-Novelo, J. Delgado, L. E. Castellano, and J. L. Mata-Mata, “A new method for the preparation of biomedical hydrogels comprised of extracellular matrix and oligourethanes,” Biomed. Mater. (Bristol) 11(3) 2016. https://doi.org/10.1088/1748-6041/11/3/035016.
Z. Tian, W. Liu, G. Li, The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym. Degrad. Stab. 130, 264–270 (2016). https://doi.org/10.1016/j.polymdegradstab.2016.06.015
A. Borzacchiello, L. Ambrosio, Network formation of low molecular weight hyaluronic acid derivatives. J. Biomater. Sci. Polym. Ed. 12(3), 307–316 (2001). https://doi.org/10.1163/156856201750180834
E. A. Balazs, Viscoelastic properties of Hyaluronan and its therapeutic use**in this chapter, Vitreus will be used as a noun, designating the connective tissue surrounded by the lens, ciliary body and retina. thus vitreus will replace vitreous humor, vitreous body and vitreous. the two rheological states of the vitreus will be designated as gel vitreus and Liquid Vitreus. vitreous will be used, as an adjective, in the following manner: Vitreous anomalies, vitreous strands, vitreous implants, etc.. from ref. 189. Chemistry and Biology of Hyaluronan. 415–455 (2004). https://doi.org/10.1016/b978-008044382-9/50051-0
A. Gamini et al., Structural investigations of cross-linked hyaluronan. Biomaterials 23(4), 1161–1167 (2002). https://doi.org/10.1016/s0142-9612(01)00231-9
G.D. Prestwich, D.M. Marecak, J.F. Marecek, K.P. Vercruysse, M.R. Ziebell, Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. J. Control. Release 53(1–3), 93–103 (1998). https://doi.org/10.1016/s0168-3659(97)00242-3
S. Oerther et al., High interaction alginate–hyaluronate associations by hyaluronate deacetylation for the preparation of efficient biomaterials. Biopolymers 54(4), 273–281 (2000). https://doi.org/10.1002/1097-0282(20001005)54:4<273::aid-bip40>3.3.co;2-9
K.P. Vercruysse, D.M. Marecak, J.F. Marecek, G.D. Prestwich, Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjug Chem 8(5), 686–694 (1997). https://doi.org/10.1021/bc9701095
G. Miralles et al., Sodium alginate sponges with or without sodium hyaluronate:in vitro engineering of cartilage. J. Biomed. Mater. Res. 57(2), 268–278 (2001). https://doi.org/10.1002/1097-4636(200111)57:2<268::aid-jbm1167>3.0.co;2-l
S. Oerther et al., Hyaluronate-alginate gel as a novel biomaterial: mechanical properties and Formation Mechanism. Biotechnol. Bioeng. 63(2), 206–215 (1999). https://doi.org/10.1002/(sici)1097-0290(19990420)63:2<206::aid-bit9>3.3.co;2-#
K. Tomihata, Y. Ikada, In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 18(7), 567–575 (1997). https://doi.org/10.1016/s0142-9612(96)00167-6
K.Y. Lee, W.S. Ha, W.H. Park, Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 16(16), 1211–1216 (1995). https://doi.org/10.1016/0142-9612(95)98126-y
K. M. Vårum, H. Kristiansen Holme, M. Izume, B. Torger Stokke, O. Smidsrød, Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochimica et Biophysica Acta (BBA) - General Subjects 1291(1), 5–15 (1996). https://doi.org/10.1016/0304-4165(96)00038-4
Y. Zhang, M. Zhang, Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 55(3), 304–312 (2001). https://doi.org/10.1002/1097-4636(20010605)55:3<304::aid-jbm1018>3.0.co;2-j
P.J. VandeVord, H.W.T. Matthew, S.P. DeSilva, L. Mayton, B. Wu, P.H. Wooley, Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 59(3), 585–590 (2002). https://doi.org/10.1002/jbm.1270
J.-K. Francis Suh, H. W. T. Matthew, Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials 21(24), 2589–2598 (2000). https://doi.org/10.1016/s0142-9612(00)00126-5
K.Y. Lee, D.J. Mooney, Hydrogels for tissue engineering. Chem. Rev. 101(7), 1869–1879 (2001). https://doi.org/10.1021/cr000108x
A. Chenite et al., Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 21(21), 2155–2161 (2000). https://doi.org/10.1016/s0142-9612(00)00116-2
K. Ono et al., Photocrosslinkable Chitosan as a biological adhesive. J. Biomed. Mater. Res. 49(2), 289–295 (2000). https://doi.org/10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m
F.-L. Mi, C.-Y. Kuan, S.-S. Shyu, S.-T. Lee, S.-F. Chang, The study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr. Polym. 41(4), 389–396 (2000). https://doi.org/10.1016/s0144-8617(99)00104-6
F. Shen et al., A study on the fabrication of porous chitosan/gelatin network scaffold for tissue engineering. Polym. Int. 49(12), 1596–1599 (2000). https://doi.org/10.1002/1097-0126(200012)49:12<1596::aid-pi553>3.3.co;2-j
V. W. M. Van Hinsbergh, A. Collen, and P. Koolwijk, “Role of fibrin matrix in angiogenesis,” in Annals of the New York Academy of Sciences, New York Academy of Sciences, 2001 426–437. https://doi.org/10.1111/j.1749-6632.2001.tb03526.x.
F.M. Shaikh, A. Callanan, E.G. Kavanagh, P.E. Burke, P.A. Grace, T.M. McGloughlin, Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 188(4), 333–346 (2008). https://doi.org/10.1159/000139772
P.A. Janmey, J.P. Winer, J.W. Weisel, Fibrin gels and their clinical and bioengineering applications. J Royal Soc Inter 6(30), 1–10 (2009). https://doi.org/10.1098/rsif.2008.0327
J. W. Weisel, “Fibrinogen and fibrin,” 2005, https://doi.org/10.1016/S0065-3233(04)70008-X.
A.C. O’Sullivan, Cellulose 4(3), 173–207 (1997). https://doi.org/10.1023/a:1018431705579
N. Petersen, P. Gatenholm, Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol 91(5), 1277–1286 (2011). https://doi.org/10.1007/s00253-011-3432-y
H. Bäckdahl et al., Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 27(9), 2141–2149 (2006). https://doi.org/10.1016/j.biomaterials.2005.10.026
G.M. Cruise, D.S. Scharp, J.A. Hubbell, Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 19(14), 1287–1294 (1998). https://doi.org/10.1016/s0142-9612(98)00025-8
J.L. West, J.A. Hubbell, Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 32(1), 241–244 (1999). https://doi.org/10.1021/ma981296k
S.J. Bryant, K.S. Anseth, The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials 22(6), 619–626 (2001). https://doi.org/10.1016/s0142-9612(00)00225-8
K.M. Huh, Y.H. Bae, Synthesis and characterization of poly(ethylene glycol)/poly(L-lactic acid) alternating multiblock copolymers. Polymer 40(22), 6147–6155 (1999). https://doi.org/10.1016/s0032-3861(98)00822-2
A. Metters, Fundamental studies of a novel, biodegradable PEG-B-pla hydrogel. Polymer 41(11), 3993–4004 (2000). https://doi.org/10.1016/s0032-3861(99)00629-1
M.G. Cascone, M. Laus, D. Ricci, R. Sbarbati Del Guerra, Evaluation of poly(vinyl alcohol) hydrogels as a component of hybrid artificial tissues. J. Mater. Sci. Mater. Med. 6(2), 71–75 (1995). https://doi.org/10.1007/bf00120410
C.R. Nuttelman, D.J. Mortisen, S.M. Henry, K.S. Anseth, Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 57(2), 217–223 (2001). https://doi.org/10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i
I. Orienti, R. Treré, V. Zecchi, Hydrogels formed by cross-linked polyvinylalcohol as colon-specific drug delivery systems. Drug Dev. Ind. Pharm. 27(8), 877–884 (2001). https://doi.org/10.1081/ddc-100107253
J.L. Drury, D.J. Mooney, Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24(24), 4337–4351 (2003). https://doi.org/10.1016/S0142-9612(03)00340-5
S. Bak, T. Ahmad, Y. Bin Lee, J.Y. Lee, E.M. Kim, H. Shin, Delivery of a cell patch of cocultured endothelial cells and smooth muscle cells using thermoresponsive hydrogels for enhanced angiogenesis. Tissue Eng Part A. 22(1–2), 182–193 (2016). https://doi.org/10.1089/ten.tea.2015.0124
S. Yasmeen, M.K. Lo, S. Bajracharya, M. Roldo, Injectable scaffolds for bone regeneration. Langmuir 30(43), 12977–12985 (2014). https://doi.org/10.1021/la503057w
X.L. Hou et al., Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J Mater Chem B 8(37), 8623–8633 (2020). https://doi.org/10.1039/d0tb01370f
S.H. Park, J.Y. Park, Y.B. Ji, H.J. Ju, B.H. Min, M.S. Kim, An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 117, 108–120 (2020). https://doi.org/10.1016/j.actbio.2020.09.013
N. Pettinelli, S. Rodríguez-Llamazares, R. Bouza, L. Barral, S. Feijoo-Bandín, and F. Lago, “Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications,” Int. J. Pharm. 589 2020. https://doi.org/10.1016/j.ijpharm.2020.119828.
C. Hu, F. Zhang, L. Long, Q. Kong, R. Luo, Y. Wang, Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 324, 204–217 (2020). https://doi.org/10.1016/j.jconrel.2020.05.010
R. Tiruvannamalai Annamalai, A.Y. Rioja, A.J. Putnam, J.P. Stegemann, Vascular network formation by human microvascular endothelial cells in modular fibrin microtissues. ACS Biomater Sci Eng 2(11), 1914–1925 (2016). https://doi.org/10.1021/acsbiomaterials.6b00274
Y. Wang et al., “Endothelialized microrods for minimally invasive in situ neovascularization,” Biofabrication. 12(1), 2020. https://doi.org/10.1088/1758-5090/ab47eb.
Quazi, “© 2022 by the author(s). Distributed under a Creative Commons CC BY license.,” 0171119931 1–41, 2022. https://doi.org/10.20944/preprints202109.0104.v1.
Muazzam Nasrullah 2018 et al., “乳鼠心肌提取 HHS Public Access,” Physiol Behav 176 (1)139–148. 2016. https://doi.org/10.1016/j.actbio.2020.03.005.Rapid
A. Serafim et al., Bioinspired hydrogel coating based on methacryloyl gelatin bioactivates polypropylene meshes for abdominal wall repair. Polymers (Basel) 12(8), 1–15 (2020). https://doi.org/10.3390/POLYM12081677
J. Vajda, M. Milojević, U. Maver, B. Vihar, Microvascular tissue engineering—a review. Biomedicines 9(6), 1–27 (2021). https://doi.org/10.3390/biomedicines9060589
T. Hozumi, T. Kageyama, S. Ohta, J. Fukuda, T. Ito, Injectable hydrogel with slow degradability composed of gelatin and hyaluronic acid cross-linked by Schiff’s base formation. Biomacromol 19(2), 288–297 (2018). https://doi.org/10.1021/acs.biomac.7b01133
S.F. Karkan, S. Davaran, R. Rahbarghazi, R. Salehi, A. Akbarzadeh, Electrospun nanofibers for the fabrication of engineered vascular grafts. J. Biol. Eng. 13(1), 1–13 (2019). https://doi.org/10.1186/s13036-019-0199-7
Z. Wang et al., Functionalization of electrospun poly(ε-caprolactone) scaffold with heparin and vascular endothelial growth factors for potential application as vascular grafts. J. Bioact. Compat. Polym. 28(2), 154–166 (2013). https://doi.org/10.1177/0883911512469707
P. Wang, Y. Sun, X. Shi, H. Shen, H. Ning, H. Liu, 3D printing of tissue engineering scaffolds: a focus on vascular regeneration. Biodes Manuf 4(2), 344–378 (2021). https://doi.org/10.1007/s42242-020-00109-0
X. Dong et al., Construction of a bilayered vascular graft with smooth internal surface for improved hemocompatibility and endothelial cell monolayer formation. Biomaterials 181, 1–14 (2018). https://doi.org/10.1016/j.biomaterials.2018.07.027
S.Y. Hann et al., Recent advances in 3D printing: vascular network for tissue and organ regeneration. Transl. Res. 211, 46–63 (2019). https://doi.org/10.1016/j.trsl.2019.04.002
H. Xing, H. Lee, L. Luo, T.R. Kyriakides, Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv 42, 107421 (2020). https://doi.org/10.1016/j.biotechadv.2019.107421
J. Jang et al., 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112, 264–274 (2017). https://doi.org/10.1016/j.biomaterials.2016.10.026
L. Sun et al., Molecularly engineered metal-based bioactive soft materials – neuroactive magnesium ion/polymer hybrids. Acta Biomater. 85, 310–319 (2019). https://doi.org/10.1016/j.actbio.2018.12.040
H. Liu, S. Kitano, S. Irie, R. Levato, M. Matsusaki, Collagen microfibers induce blood capillary orientation and open vascular lumen. Adv Biosyst 4(5), 1–8 (2020). https://doi.org/10.1002/adbi.202000038
L. Shao et al., Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Biodes Manuf 3(1), 30–39 (2020). https://doi.org/10.1007/s42242-020-00062-y
Q. Gao et al., 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng. 3(3), 399–408 (2017). https://doi.org/10.1021/acsbiomaterials.6b00643
F. Liu et al., Poly(l-lactide-co-caprolactone)/tussah silk fibroin nanofiber vascular scaffolds with small diameter fabricated by core-spun electrospinning technology. J. Mater. Sci. 55(16), 7106–7119 (2020). https://doi.org/10.1007/s10853-020-04510-z
S.H. Kim et al., Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 9(1), 1–14 (2018). https://doi.org/10.1038/s41467-018-03759-y
S. Jordahl et al., Engineered fibrillar fibronectin networks as three-dimensional tissue scaffolds. Adv. Mater. 31(46), 1–10 (2019). https://doi.org/10.1002/adma.201904580
W. Wang et al., Fabrication of heterogeneous porous bilayered nanofibrous vascular grafts by two-step phase separation technique. Acta Biomater. 79, 168–181 (2018). https://doi.org/10.1016/j.actbio.2018.08.014
Q. Gao, Y. He, J.Z. Fu, A. Liu, L. Ma, Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61, 203–215 (2015). https://doi.org/10.1016/j.biomaterials.2015.05.031
H. Zhao et al., Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle. Small 14(39), 1–7 (2018). https://doi.org/10.1002/smll.201802630
M.A. Skylar-Scott, J. Mueller, C.W. Visser, J.A. Lewis, Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575(7782), 330–335 (2019). https://doi.org/10.1038/s41586-019-1736-8
K. Christensen, Z. Zhang, C. Xu, Y. Huang, Deformation compensation during buoyancy-enabled inkjet printing of three-dimensional soft tubular structures. J. Manuf. Sci. E. T. ASME 140(1), 1–39 (2018). https://doi.org/10.1115/1.4037996
I. Liashenko, J. Rosell-Llompart, A. Cabot, Ultrafast 3D printing with submicrometer features using electrostatic jet deflection. Nat. Commun. 11(1), 1–9 (2020). https://doi.org/10.1038/s41467-020-14557-w
Y. Du et al., Effect of bi-directional microfabricated topographical cues on cellular behavior of mammalian cell line. Microelectron. Eng. 124, 37–41 (2014). https://doi.org/10.1016/j.mee.2014.04.010
M. Yeo, G.H. Kim, Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 107, 102–114 (2020). https://doi.org/10.1016/j.actbio.2020.02.042
M. Costantini, A. Barbetta, Gas foaming technologies for 3D scaffold engineering. Elsevier Ltd (2018). https://doi.org/10.1016/B978-0-08-100979-6.00006-9
S.A. Poursamar, J. Hatami, A.N. Lehner, C.L. Da Silva, F.C. Ferreira, A.P.M. Antunes, Gelatin porous scaffolds fabricated using a modified gas foaming technique: characterisation and cytotoxicity assessment. Mater. Sci. Eng., C 48, 63–70 (2015). https://doi.org/10.1016/j.msec.2014.10.074
S. Chaudhary, E. Chakraborty, Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni Suef Univ J Basic Appl Sci 11(1), 1–15 (2022). https://doi.org/10.1186/s43088-021-00172-1
A. Eltom, G. Zhong, and A. Muhammad, “Scaffold techniques and designs in tissue engineering functions and purposes: a review,” Adv. Mater. Sci. Eng. 2019, 2019. https://doi.org/10.1155/2019/3429527.
S.K. Norouzi, A. Shamloo, Bilayered heparinized vascular graft fabricated by combining electrospinning and freeze drying methods. Mater. Sci. Eng., C 94, 1067–1076 (2019). https://doi.org/10.1016/j.msec.2018.10.016
S.H. Oh, S.G. Kang, E.S. Kim, S.H. Cho, J.H. Lee, Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 24(22), 4011–4021 (2003). https://doi.org/10.1016/S0142-9612(03)00284-9
H. Cao, L. Duan, Y. Zhang, J. Cao, and K. Zhang, “Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity,” Sig. Transduct. Targeted. Ther. 6(1) 2021. https://doi.org/10.1038/s41392-021-00830-x.
N. Ghane, M.H. Beigi, S. Labbaf, M.H. Nasr-Esfahani, A. Kiani, Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J. Mater. Chem. B 8(47), 10712–10738 (2020). https://doi.org/10.1039/d0tb01842b. (Royal Society of Chemistry)
S.B. Gutekunst et al., 3D hydrogels containing interconnected microchannels of subcellular size for capturing human pathogenic Acanthamoeba castellanii. ACS Biomater. Sci. Eng. 5(4), 1784–1792 (2019). https://doi.org/10.1021/acsbiomaterials.8b01009
W.M. Han, M. Mohiuddin, S.E. Anderson, A.J. García, Y.C. Jang, Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater. 94, 243–252 (2019). https://doi.org/10.1016/j.actbio.2019.06.025
L. Wang et al., Development of a centrally vascularized tissue engineering bone graft with the unique core-shell composite structure for large femoral bone defect treatment. Biomaterials 175, 44–60 (2018). https://doi.org/10.1016/j.biomaterials.2018.05.017
B. Wang, W. Li, D. Dean, M.K. Mishra, K.S. Wekesa, Enhanced hepatogenic differentiation of bone marrow derived mesenchymal stem cells on liver ECM hydrogel. J. Biomed. Mater. Res. A 106(3), 829–838 (2018). https://doi.org/10.1002/jbm.a.36278
S.L. Faley et al., iPSC-derived brain endothelium exhibits stable, long-term barrier function in perfused hydrogel scaffolds. Stem Cell Rep 12(3), 474–487 (2019). https://doi.org/10.1016/j.stemcr.2019.01.009
J.C. Chappell, S.M. Taylor, N. Ferrara, V.L. Bautch, Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell 17(3), 377–386 (2009). https://doi.org/10.1016/j.devcel.2009.07.011
X. Li, L. Claesson-Welsh, M. Shibuya, Chapter 13 VEGF receptor signal transduction. Methods Enzymol 443, 261–284 (2008). https://doi.org/10.1016/S0076-6879(08)02013-2
V. Nehls, D. Drenckhahn, A microcarrier-based cocultivation system for the investigation of factors and cells involved in angiogenesis in three-dimensional fibrin matrices in vitro. Histochem. Cell Biol. 104(6), 459–466 (1995). https://doi.org/10.1007/bf01464336
M. N. Nakatsu, J. Davis, and C. C. W. Hughes, “Optimized fibrin gel bead assay for the study of angiogenesis,” J. Visual. Exp. 3 2007. https://doi.org/10.3791/186.
C.M. Ghajar et al., The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys. J. 94(5), 1930–1941 (2008). https://doi.org/10.1529/biophysj.107.120774
A. Lesman, J. Koffler, R. Atlas, Y.J. Blinder, Z. Kam, S. Levenberg, Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials 32(31), 7856–7869 (2011). https://doi.org/10.1016/j.biomaterials.2011.07.003
D. Hanjaya-Putra et al., Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood 118(3), 804–815 (2011). https://doi.org/10.1182/blood-2010-12-327338
D. Hanjaya-Putra, K.T. Wong, K. Hirotsu, S. Khetan, J.A. Burdick, S. Gerecht, Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials 33(26), 6123–6131 (2012). https://doi.org/10.1016/j.biomaterials.2012.05.027
Y.C. Chen et al., Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 22(10), 2027–2039 (2012). https://doi.org/10.1002/adfm.201101662
T.D. Stocco et al., Carbon nanomaterial-based hydrogels as scaffolds in tissue engineering: a comprehensive review. Intl J Nanomed 18, 6153–6183 (2023). https://doi.org/10.2147/IJN.S436867. (Dove Medical Press Ltd)
R. Bai et al., “Conductive single-wall carbon nanotubes/extracellular matrix hybrid hydrogels promote the lineage-specific development of seeding cells for tissue repair through reconstructing an integrin-dependent niche,” J. Nanobiotechnology. 19 2021. https://doi.org/10.1186/s12951-021-00993-3.
S.C. Neves, L. Moroni, C.C. Barrias, P.L. Granja, Leveling up hydrogels: hybrid systems in tissue engineering. Trends Biotechnol 38, 292–315 (2020). https://doi.org/10.1016/j.tibtech.2019.09.004. (Elsevier Ltd)
E.L. Hopley, S. Salmasi, D.M. Kalaskar, A.M. Seifalian, Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol Adv 32(5), 1000–1014 (2014). https://doi.org/10.1016/j.biotechadv.2014.05.003. (Elsevier Inc)
S.R. Shin et al., Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 6(1), 362–372 (2012). https://doi.org/10.1021/nn203711s
S. Ahadian et al., Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 31, 134–143 (2016). https://doi.org/10.1016/j.actbio.2015.11.047
R.R. Rao, A.W. Peterson, J. Ceccarelli, A.J. Putnam, J.P. Stegemann, Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 15(2), 253–264 (2012). https://doi.org/10.1007/s10456-012-9257-1
R.K. Singh, D. Seliktar, A.J. Putnam, Capillary morphogenesis in PEG-collagen hydrogels. Biomaterials 34(37), 9331–9340 (2013). https://doi.org/10.1016/j.biomaterials.2013.08.016
E.P. Sproul, R.T. Hannan, A.C. Brown, Controlling fibrin network morphology, polymerization, and degradation dynamics in fibrin gels for promoting tissue repair. Methods Mol Biol 1758, 85–99 (2018). https://doi.org/10.1007/978-1-4939-7741-3_7
G. Kim, S. Ahn, Y. Kim, Y. Cho, W. Chun, Coaxial structured collagen-alginate scaffolds: fabrication, physical properties, and biomedical application for skin tissue regeneration. J. Mater. Chem. 21(17), 6165–6172 (2011). https://doi.org/10.1039/c0jm03452e
G. L. Ying et al., “Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels,” Adv. Mater. 30(50) 2018. https://doi.org/10.1002/adma.201805460.
J.S. Jeon et al., Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integrative Biology (United Kingdom) 6(5), 555–563 (2014). https://doi.org/10.1039/c3ib40267c
A. Sobrino et al., “3D microtumors in vitro supported by perfused vascular networks,” Sci. Rep. 6 2016. https://doi.org/10.1038/srep31589.
Q. Pi et al., “Digitally tunable microfluidic bioprinting of multilayered cannular tissues,” Adv. Mater. 30(43) 2018. https://doi.org/10.1002/adma.201706913.
L. Xu et al., “Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step,” Biofabrication. 12(4) 2020. https://doi.org/10.1088/1758-5090/aba2b6.
Funding
The authors would like to thank the Science, Technology, and Innovation Funding Authority (STDF), Egypt (FLUG Call 1—Project ID 46715) for funding a research project relevant to this review topic.
Author information
Authors and Affiliations
Contributions
HAE: literature survey, writing original draft; HAM: literature survey, writing original draft; MHY: writing—review and editing; IME: conceptualization; supervision; writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Ibrahim M. El-Sherbiny.
Google Scholar: https://scholar.google.com/citations?hl=en&user=YkQjnu4AAAAJ&view_op=list_works&sortby=pubdate.
Scopus: https://www.scopus.com/authid/detail.uri?authorId=8681880000.
Hend A. Elshabrawy:.
Google Scholar: https://scholar.google.com/citations?user=TgSMsVgAAAAJ&hl=en.
Magdi H. Yacoub:.
Scopus: https://www.scopus.com/authid/detail.uri?authorId=36041927900.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elshabrawy, H.A., Moustafa, H.A., Yacoub, M.H. et al. Hydrogel advancements in vascular tissue regeneration: a comprehensive review and future prospects. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00678-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00678-1