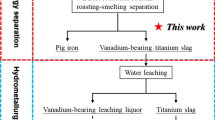
Abstract
Titanomagnetite concentrate is one of the important titanium resources. The apparent activation energy (\({E}_{\mathrm{a}}\)) of the direct reduction of titanomagnetite concentrate was composed of two parts (average activation energy: \({\overline{E} }_{\mathrm{a}}={\overline{E} }_{\mathrm{a}-\mathrm{L}}+{E}_{\mathrm{a}-\mathrm{Step }1}\), where \({E}_{\mathrm{a}-\mathrm{L}}\) is the lattice energy of titanomagnetite concentrate, and \({E}_{\mathrm{a}-\mathrm{Step\ }1}\) is the activation energy of step 1 for the reduction of titanomagnetite concentrate in the route of Fe3+ \(\mathop{\longrightarrow}\limits^{\rm{Step}1}\) Fe2+ \(\mathop{\longrightarrow}\limits^{\rm{Step}2}\) Fe2O2+ \(\mathop{\longrightarrow}\limits^{\rm{Step}3}\) Fe0). \({\overline{E} }_{\mathrm{a}}\) (583.43 kJ/mol), \({\overline{E} }_{\mathrm{a}-\mathrm{L}}\) (426.4 kJ/mol), and \({E}_{\mathrm{a}-\mathrm{Step}1}\) (157.0 kJ/mol) were calculated by the model-free methods based on thermogravimetry and Dmol3 module. Combined with the analysis of activation energy fluctuation and the shifting trend of related mechanism functions, the reduction kinetic system with three main characteristics, namely nucleation, diffusion and concentration fluctuation, was established. In addition, the scanning electron microscopy comparison analysis of the samples from microwave reduction and conventional reduction shows that microwave heating could realize the microstructure Ti–Fe separation and reduce the lattice energy of the titanomagnetite concentrate, thus enhancing the reduction process by 7.68% from the perspective of activation energy.
Graphical abstract














Similar content being viewed by others
References
M.H. Lee, Y. Kalcheim, J. del Valle, I.K. Schuller, ACS Appl. Mater. Interfaces 13 (2021) 887–896.
J. Ye, D. Yuan, M. Ding, Y. Long, T. Long, L. Sun, C. Jia, J. Power Sources 482 (2021) 229032.
Q. Sun, M. Li, X.L. Shi, S.D. Xu, W.D. Liu, M. Hong, W.Y. Lyu, Y. Yin, M. Dargusch, J. Zou, Z.G. Chen, Adv. Energy Mater. 11 (2021) 2100544.
N. Kesharwani, N. Chaudhary, C. Haldar, Catal. Lett. 151 (2021) 3562–3581.
R.R. Moskalyk, A.M. Alfantazi, Miner. Eng. 16 (2003) 793–805.
M. Anpo, M. Takeuchi, J. Catal. 216 (2003) 505–516.
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, Y. Taga, Science 293 (2001) 269–271.
X. Nie, W. He, S. Zang, X. Wang, J. Zhao, Surf. Coat. Technol. 253 (2014) 68–75.
Y. Xie, W. Zhai, L. Chen, J. Chang, X. Zheng, C. Ding, Acta Biomater. 5 (2009) 2331–2337.
L. Benea, E. Mardare-Danaila, M. Mardare, J.P. Celis, Corros. Sci. 80 (2014) 331–338.
H. Sun, A. Ajala, Z. Wang, F. Pan, Q. Zhu, in: Seminar on Clean Utilization of Non-ferrous Metals Resources and Energy Conservation and Emission Reduction, The Nonferrous Metals Society of China, Kunming, China, 2016, pp. 15–19.
W. Li, G.Q. Fu, M.S. Chu, M.Y. Zhu, Steel Res. Int. 88 (2017) 1600120.
M. Zhou, T. Jiang, S. Yang, X. Xue, Int. J. Miner. Process. 142 (2015) 125–133.
Z. Yu, G. Li, T. Jiang, Y. Zhang, F. Zhou, Z. Peng, ISIJ Int. 55 (2015) 907–909.
D. Chen, H. Zhao, G. Hu, T. Qi, H. Yu, G. Zhang, L. Wang, W. Wang, J. Hazard. Mater. 294 (2015) 35–40.
S. Wang, Y.F. Guo, T. Jiang, F. Chen, F.Q. Zheng, China Metallurgy 26 (2016) No. 10, 40–44.
W. Yu, X. Wen, J. Chen, J. Kuang, Q. Tang, Y. Tian, J. Fu, W. Huang, T. Qiu, Minerals 7 (2017) 220.
P. Liu, L. Zhang, B. Liu, G. He, J. Peng, M. Huang, Int. J. Miner. Metall. Mater. 28 (2021) 88–97.
C. Lv, K. Yang, S. Wen, S. Bai, Q. Feng. JOM 69 (2017) 1801–1805.
T. Jiang, S. Wang, Y. Guo, F. Chen, F. Zheng. Metals 6 (2016) 107.
S. Wang, Y. Guo, T. Jiang, L. Yang, F. Chen, F. Zheng, X. Xie, M. Tang, JOM 69 (2017) 1646–1653.
S. Wang, Y. Guo, T. Jiang, F. Chen, F. Zheng, M. Tang, L. Yang, G. Qiu, Trans. Nonferrous Met. Soc. China 28 (2018) 2528–2537.
Y. Zhao, T. Sun, H. Zhao, C. Xu, S. Wu, ISIJ Int. 59 (2019) 981–987.
J. Chen, W. Chen, L. Mi, Y. Jiao, X. Wang, Metals 9 (2019) 95.
D. Chen, B. Song, L. Wang, T. Qi, Y. Wang, W. Wang, Miner. Eng. 24 (2011) 864–869.
D. Huang, New process and comparative study of utilization of vanadium-titanium magnetite, Central South University, Changsha, China, 2011.
A.A. Adetoro, H. Sun, S. He, Q. Zhu, H. Li, Metall. Mater. Trans. B 49 (2018) 846–857.
S.S. Liu, Y.F. Guo, G.Z. Qiu, T. Jiang, Rare Met. 39 (2020) 1348–1352.
E. Cruz-Sánchez, J.F. Álvarez-Castro, J.A. Ramirez Picado, J.A. Matutes-Aquino, J. Alloy. Compd. 369 (2004) 265–268.
R.R. Mishra, A.K. Sharma, Composites: Part A 81 (2016) 78–97.
M.C. Biesinger, B.P. Payne, A.P. Grosvenor, L.W.M. Lau, A.R. Gerson, R.S.C. Smart, Appl. Surf. Sci. 257 (2011) 2717–2730.
H.Z. Zhang, Nonferrous Metals 46 (1994) No. 1, 41–44.
P.M. Guo, P. Zhao, J. Iron Steel Res. 19 (2007) No. 5, 25–28+33.
P. Liu, C. Liu, T. Hu, J. Shi, L. Zhang, B. Liu, J. Peng, Chem. Eng. J. 408 (2021) 127355.
M.J. Starink, Thermochim. Acta 404 (2003) 163–176.
J.H. Flynn, L.A. Wall. Polym. Lett. 4 (1966) 323–328.
H.E. Kissinger, J. Res. Natl. Bur. Stand. 57 (1956) 217–221.
A.W. Coats, J.P. Redfern, Nature 201 (1994) 68–69.
A.W. Coats, J.P. Redfern, Polym. Lett. 3 (1965) 917–920.
S. Vyazovkin, A.K. Burnham, J.M. Criado, L.A. Pérez-Maqueda, C. Popescu, N. Sbirrazzuoli, Thermochim. Acta 520 (2011) 1–19.
S. Vyazovkin, K. Chrissafis, M.L. Di Lorenzo, N. Koga, M. Pijolat, B. Roduit, N. Sbirrazzuoli, J.J. Suñol, Thermochim. Acta 590 (2014) 1–23.
I. Oluwoye, Z. Zeng, S. Mosallanejad, M. Altarawneh, J. Gore, B.Z. Dlugogorski, Chem. Eng. J. 411 (2021) 128427.
P. Liu, C. Liu, S. Li, B. Liu, J. Shi, L. Zhang, J. Peng, Environ. Prog. Sustain. Energy 38 (2019) 13201.
A.I. Rusanov, Russ. Chem. Rev. 33 (1964) 385–399.
C. Liu, J. Peng, A. Ma, L. Zhang, J. Li, J. Hazard. Mater. 322 (2017) 325–333.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2018YFC1900500), Yunnan Province Special Key Project of Basic Research (202101as070014), and Scientific Research Fund of Panzhihua University (XJ2022001301).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, P., Gong, Sy., Chao, Yw. et al. Kinetic study on microwave-enhanced direct reduction of titanomagnetite concentrate with coal. J. Iron Steel Res. Int. 30, 429–445 (2023). https://doi.org/10.1007/s42243-022-00888-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-022-00888-z