Abstract
The multidisciplinary research field of bioprinting combines additive manufacturing, biology and material sciences to create bioconstructs with three-dimensional architectures mimicking natural living tissues. The high interest in the possibility of reproducing biological tissues and organs is further boosted by the ever-increasing need for personalized medicine, thus allowing bioprinting to establish itself in the field of biomedical research, and attracting extensive research efforts from companies, universities, and research institutes alike. In this context, this paper proposes a scientometric analysis and critical review of the current literature and the industrial landscape of bioprinting to provide a clear overview of its fast-changing and complex position. The scientific literature and patenting results for 2000–2020 are reviewed and critically analyzed by retrieving 9314 scientific papers and 309 international patents in order to draw a picture of the scientific and industrial landscape in terms of top research countries, institutions, journals, authors and topics, and identifying the technology hubs worldwide. This review paper thus offers a guide to researchers interested in this field or to those who simply want to understand the emerging trends in additive manufacturing and 3D bioprinting.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioprinting is a collection of additive manufacturing (AM) technologies, whose aim is to fabricate parts imitating real tissue and organ functionalities by combining both living and non-living materials in a specific three-dimensional (3D) spatial organization structure. As in traditional 3D printing or AM, the target is achieved through the use of computer-aided design (CAD) that represents the fundamental configuration of the target tissue or organ, in order to produce bioengineered structures that have various applications in regenerative medicine, tissue engineering, reconstructive surgery, drug discovery, pharmacokinetics, medical and basic cell-biology research [1]. Compared to traditional 3D printing or AM processes, bioprinting brings a main innovative feature, namely the printing of living cells within a specific medium called bioink, which adds many different challenges, such as how to avoid the deterioration of living cells while printing constructs that have a 3D volumetric shape similar to the ones of natural tissues and organs.
In light of the application of such manifolds and the growing interest towards personalized medicine, bioprinting methods have attracted increasing attention in recent years from both academia and industry, which has translated into extensive research efforts. During the last decade, many novel procedures and technologies related to biomanufacturing have emerged, ranging from dedicated 3D bioprinters [2] to specific “raw biomaterials” named bioinks [3, 4].
A bioprinter is a 3D printer that realizes biological tissue constructs by the layerwise deposition of living cells. To achieve this aim, bioprinters generally use bioinks, which are soft biomaterials loaded with living cells manipulated according to specific protocols to build biological constructs. The use of secondary dissolvable materials is an additional option to vertically support and protect cells during the printing process.
Although many bioprinting review papers focusing on describing techniques or bioink classifications have been published in recent years [3, 5,6,7], a systematic and quantitative investigation of the actual landscape has not been performed, including the analysis of papers, patents and companies with the aim of highlighting the actual distribution of key players in academia and industry, as well as the main topics currently under study. To the best of our knowledge, the first and only scientometric review on 3D bioprinting cannot be considered up-to-date including the latest scientific innovations in this area, as it was published in 2017 [8] based on data retrieved from 2000 to mid-2016. In fact, two-thirds of the total publications related to bioprinting to date have been published since 2016.
Given the rapid growth of this special field, the present work is aimed at stimulating the interest of scientists and experts already involved in traditional 3D printing or AM by highlighting the emerging trends and the most recent advancements [1, 9,10,11,12]. This review presents a rational roadmap to the scientific and patenting results produced to date, which can be especially useful for researchers new to the field, as they can quickly obtain the geographical distribution of laboratories and companies actively involved in 3D bioprinting combined with a critical analysis of their output in terms of publications, patents, new tools and manufacturing techniques.
The paper is organized as follows: the literature review results are presented and discussed in “The academic research trends” section with a detailed analysis of the most productive authors and active research networks worldwide. “Market and patent landscape” section describes the market and patent landscape to identify both emerging and established technology hubs. Finally, the main conclusions are drawn in “Conclusions” section.
The academic research trends
Trends in the relevant scientific literature: critical data analysis and classification of applications and trends
Following previous scientometric studies and AM [8, 12], we based our literature analysis considering all research and review papers published in scientific journals included in Scopus (Elsevier) and Web of Science (WoS) in the past 20 years (from 2000 to 2020). We also used SciVal (https://www.scival.com/) as a supporting tool in our query. The latter was focused on bioprinting processes, materials and bioapplications according to the latest definition of bioprinting, and is a modified version of the one used by Rodríguez-Salvador et al. (details in the Supplementary Information). In order to better highlight the most recent trends, a detailed analysis was further performed with reference to scientific results published in the last four years, i.e., since 2016.
A total number of 13,111 papers (11,683 research articles and 2537 review papers) were initially collected using both the Scopus and WoS databases. An extensive cleaning and deduplication process was subsequently performed through EndNote (X9, Clarivate Analytics, Philadelphia, USA), leading to 9314 unique documents, consisting of 7574 research articles and 1740 review papers).
It is worth noting that 79% of these papers were published after 2014 and nearly 53% of total publications were published after 2017. Specifically, 61% (4620 out of 7574) of research articles and 74% (1288 out of 1740) of review papers have been published since 2016, showing an exponential growth of attention on this topic in the scientific literature. Figure 1 shows the total number of publications retrieved from Scopus for the last 20 years, where the steady rise during the past 10 years is clearly visible. This growing number of scientific papers led to a 143% increase in the number of review papers in a single year for 2016. Since then, due to the continuous evolution and rapid innovation in this field, a constant annual growth rate of (28 ± 9)% in review papers has been reported.
In order to select the most relevant venues for 3D bioprinting papers, SciVal (https://www.scival.com/) was used to research on the topic T.8060 (Bioprinting; Printability; Tissue Engineering) together with InCites Journal Citation Reports to include information on Impact Factor, Article Citation Median and Review Citation Median focusing on 2018 and 2019 (details are also given in Table S1 of the Supplementary Information).
The number of papers published (usually referred to as ‘scholarly output’Footnote 1) in the past five years was specifically used to select the twenty most productive journals in the bioprinting field. Figure 2 presents the main results of this ranking. As clearly seen in the figure, Biofabrication (with 319 publications, namely 297 articles and 22 review papers), Biomaterials (with 184 publications, namely 166 articles and 18 review papers), and Acta Biomaterialia (with 162 publications, consisting of 124 articles and 38 review papers) are the most prolific journals in this field. Moreover, the percentage of publications focusing on bioprinting with respect to the overall number of papers from 2000 to 2020 was used as an additional indicator of the level of attention to this topic (data retrieved from Scopus), and are shown as dots in Fig. 2. As expected, Bioprinting (66%), Biofabrication (43%), International Journal of Bioprinting (42%), and Bio-Design and Manufacturing (26%) are the top-focalized journals. Most of these are young journals (founded in 2009, 2015, 2016, and 2018, respectively) focusing on this novel field, with impact factors (IF) revealing their age and their specific field of focus (IF values ranging from 4.10 for Bio-Design and Manufacturing to 8.21 of Biofabrication, compared with older and more generic journals such as Advanced Materials with IF equal to 27.4Footnote 2).
The top twenty journals focusing on 3D bioprinting (SciVal-Scopus). The bars represent the number of publications (blue: articles, light blue: reviews) retrieved from Scopus, while the yellow dots represent the percentage of publications focusing on 3D bioprinting with regards to the total number of publications. The examined time interval is 2000–2020
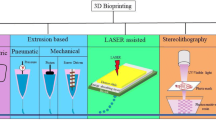
With regard to review papers, a different classification can be outlined depending on the specific 3D bioprinting technology each paper refers to [13, 14]. As for traditional AM processes, different bioprinting techniques vary in the technique of layerwise deposition of biomaterial. Even if the bioprinting literature does not assume the proper terminology defined in the AM standards (ISO/ASTM 52900), AM technologies similar to the ones used for polymers are often adopted. The first class of technologies is based on nozzle-deposition [11, 15,16,17,18,19], which can have different printing resolutions and speed depending on the precision of the bioprinting head, the nozzle diameter size and the droplet formation mechanism (Fig. 3a). A second main class of technologies are optical-based, namely the vat photopolymerization (always referred to as stereolithography in the literature on bioprinting [11, 20, 21]) both in its traditional setting and the two-photon polymerization version.
Figure 3b shows that extrusion-based bioprinting is the most studied approach in the literature, potentially because it is the most affordable solution for an entry-level bioprinter, and the least expensive technology that allows the use of a wide range of printable biomaterials [2]. The second and third most widespread techniques are vat photopolymerization and inkjet bioprinting. The former is characterized by many benefits, i.e., higher resolution, a wide variety of bioink viscosities and higher cell density [24, 25]. Eventually, thanks to the drop-on-demand (DOD) patterning method available in most bioprinters, jetting is often used for printing smaller features.
The extrusion-based technique is rapidly becoming popular likely because of the great number of entry-level bioprinters that have entered the market in recent years. Meanwhile, vat photopolymerization 3D bioprinting is emerging as a prominent bioprinting method for complex tissues.
Bioprinting research landscape: main applications and emerging topics
The main utilities of 3D bioprinting are in basic medical/cell biology research, the production of pathology models, mini-tissue production for drug screening, and the field of regenerative medicine for the future replacement of tissues and organs [5]. Within this framework, the ideal workflow of bioprinting should start from retrieving patient-specific cells through biopsy, designing the morphology of the organ or tissue to be replaced, and going back to the patient at the end for the transplantation of a functional organ [26,27,28,29,30,31]. To the best of our knowledge, this ideal workflow cannot be yet completed from end to end, as different challenges [1, 32] need to be overcome. Among the most important ones, vascularization and multi-material printing are the most relevant. Vascularization consists of printing tiny vessels and capillaries that are specifically designed to enable the survival of living cells by the delivery of nutrients and oxygen. Multiple materials are needed to allow different types of cells and hydrogels to be combined in the 3D structure, as it occurs in real biological tissues.
Considering the long-term goals and driving factors, research on 3D bioprinting is now progressing in three major areas:
-
1.
Application-driven research focusing on specific utilities of 3D bioprinting, i.e., distinct tissues, pathology models or organ-on-a-chip for drug discovery.
-
2.
Biomaterials research to develop novel bioink formulations that improve printability or support tissue differentiation and maturation, and allow the study of cells to be bioprinted in the construct.
-
3.
Process-driven research focusing on the printing technology to improve the resolution and accuracy of 3D bioprinting while avoiding cell damage, support the design of complex shapes, reduce printing time and costs, and allow specific functionalities, i.e., multi-material printing.
In order to highlight the main trends in the literature, we clustered papers published since 2000 based on text analytics keywords. The number of articles related to each topic is shown together with its evolution over time in Fig. 4.
A considerable number of publications, especially review papers, are focused at the fundamental aspects of 3D bioprinting, and are included within the class of process-driven papers. For instance, a basic theme such as biomimicry shows steady growth from 2010, while there are newer ideas, including four-dimensional (4D) bioprinting that first appeared in 2016 and is already the subject of 28 papers [33,34,35,36,37,38,39,40,41]. Some publications show the bioprinting workflow [27,28,29] and areas [42], while the ethical aspects of bioprinting are still relatively underrepresented [43].
Regarding the applications of 3D bioprinting, about 40% of all publications refer to a specific tissue or organ starting with their title (as shown in Table 1 and the Supplementary Information). Many review papers are directed at the bone, cartilage (in particular, articular cartilage), vascularized tissue, cardiac tissues, liver, neural tissue, skin, pancreas, cornea, kidney and muscle, where the first classes mentioned are also the most frequently studied ones (see Fig. 5). On the other hand, some emerging topics have received increased attention in the last few years, such as dental tissue, nerve regeneration, lung, intestine, thyroid gland [44], urethra [45], and encapsulated T-cells [46]. This trend might continue in the near future.
Catalogue of all publications based on the automatic assignment of keywords extracted from the titles relative to the tissues and organs (others: articulation, nerve regeneration, kidney, adipose tissue, lung, dental, trachea, ear, pancreas, cornea, aortic valve, esophagus, retina, neural tissue, thyroid gland, urethra, intestine, eye, T-cells). The sum is not equivalent to the total number of publications, since each paper can focus on more than one tissue
Among other applications, graft and implants, pathology models, and organs-on-a-chip are also addressed, with a relative role (i.e., percentage of reviews over the total number of publications) showing an upward trend for the past 10 years. In this area, we can observe studies on traditional topics, such as bioglues, grafts and implants, but also new solutions including the BioPen (which is a handheld device invented by Wallace and co-workers [72] for printing cartilage in vivo) or the application of bioprinting to cryopreservation.
Since the beginning (the first papers date back to 2002), 3D bioprinting has also been subject to pathology models for in vitro studies of diseases. In particular, 3D-bioprinted cancer models have been described for breast cancer [115,116,117,118,119,120,121], mammary ductal carcinoma [115], appendiceal cancer [122], mesothelioma [123], glioblastoma and metastasis. Other types of diseases that have been modeled through bioprinting include epilepsy [124], diabetes [110, 125], degenerative diseases, immune-enhanced organoids for immunotherapy screening [126], and wound healing [127, 128]. In all these applications, 3D bioprinting has been utilized for drug discovery, drug screening, and pharmaceutical applications, especially after 2011. On the one hand, the production of pathological tissues and organs using cells from patients leads to a personalized approach on drug discovery [129]. On the other hand, the serial production of mini-tissues in a standardized manner can be highly useful for the high-throughput screening of large libraries of drugs already available on the market (drug screening [130, 131] or novel drug discovery [132]). In the future, the main target is to 3D print patient-specific models using the patient’s own cells to test different chemotherapeutic drugs in vitro for selecting the most efficient patient-specific therapy. Translational medicine and the implications of 3D bioprinting in regenerative medicine, as well as the clinical translation of 3D bioprinted constructs [50, 133, 134], are certainly becoming hot topics in the near future.
Compared to other applications, publications on translational medicine occurred fairly lately (starting in 2009), adding up to 117 publications with more than 60% classified as review papers. In fact, the application of 3D-bioprinted tissues in medicine is still being implemented; to the best of our knowledge, no tissues or organs produced by 3D bioprinting have been implanted in vivo in real patients. However, the 3D printing of biomaterials [135,136,137] is increasingly common in medicine, especially for the production of bone and dental implants and grafts, but also in surgery for the production of patient-specific 3D models on which surgeons can train before the actual procedure.
Microfluidics and organs-on-a-chip are some of the latest areas in 3D bioprinting, and, even though the first occurrence dates back to 2004, most of the relevant publications have been published after 2010. At present, only about 100 publications refer to this topic by the title. Publications on organ-on-a-chip models focus either on modeling healthy or pathologic organs [138], where bioprinting can be useful for studying gene expression and cell differentiation in different healthy conditions by controlling the microfluidics and the microenvironment, or can be used to realize in vitro models for drug screening in pathology studies.
Concerning biomaterials, one of the most exciting field of research relates to bioinks, with about 25% of the whole number of publications on bioprinting focusing on the development of novel bioinks to obtain specific biological, mechanical, and chemical characteristics. This stream of research is fairly new, as research on bioinks was rather limited before the rise of 3D bioprinting. Nowadays, the number of reviews on bioprinting is growing together with the rising need of information to standardize tests on 3D cultures. On this subtopic, the literature focuses on imaging (73 publications), biological characterization (726 publications), resolution (49 publications) and printability (32 publications), with an increasing interest in rheology (21 publications) and structural integrity (9 articles).
Most of the recent papers on bioinks outline the need to find the best compromise between printability and specialization for the specific cell or tissue under study [139, 140]. In fact, each cell type requires highly specific conditions in addition to a number of standard requirements (e.g., aqueous environment, sufficient oxygen and nutrient diffusion, appropriate pH, physiological osmolarity of key vitamins and minerals). For example, certain cell types require appropriate sites for attachment, specific substrate properties and space in order to proliferate and produce their extracellular matrix (ECM) [141]. Bioinks can be classified depending on their origin (natural or synthetic), the type of 3D printing process they can be used in (e.g., bioinks for material extrusion, jetting or photopolymerization differ in their rheological characteristics, shape fidelity and printability features) or the gelation kinetics: ionic, stereocomplex, thermal, photocrosslinking, enzymatic and click chemistry [142].
Overall, about 15% of all publications focus on innovative cell types in 3D bioprinting, such as stem cells, spheroids, and organoids. This rate is yet to increase mostly because innovative cell types are still under investigation in biology with the aim to overcome open challenges concerning differentiation and maturation. With reference to stem cells in 3D bioprinting [143, 144], Skeldon et al. outlined that the main types of stem cells used in this context are mesenchymal stem cells, neural stem cells, and human induced Pluripotent Stem Cells (iPSCs) [143]. However, our search found that general multipotent human Adipose Stem Cells (hASCs), as well as nasal and bone marrow stem cells, have also been used. Spheroids have been used in 3D bioprinting since 2003, mostly as the living components of bioinks. Finally, organoids have become one of the latest cell sources used in 3D bioprinting since their first occurrence in 2017 [145].
Surprisingly, the characterization or development of new process technologies for 3D bioprinting has received rather limited attention in the literature. The rate of publications on this topic decreased from around 30% in 2010 to 15% in 2019. This can be mainly ascribed to the increasing focus on biology, medicine, or material science rather than engineering driving the increase of attention to bioprinting. Secondly, most of the processes used in this field are those borrowed from the traditional 3D printing of polymers with modifications to achieve the desired results. However, a lot of research is lacking, especially for most of the complex technologies. This is clearly visible in the literature, where most of the studied techniques are the laser-based ones (144 articles and 14 reviews) and stereolithography (83 articles and 18 reviews). Inkjet was introduced in 2006 and is among the oldest techniques, while extrusion 3D bioprinting first appeared in 2001, but expanded especially after 2015 with the entry of commercial bioprinters to the market.
Moreover, the application categories include printing techniques that simply exploit existing printing technologies and processes in innovative ways to meet the needs of a specific application (e.g., creating channels to form vascularized tissue). Such is the case of bioprinting in a suspension bath, primarily developed to create vascularized tissues. Among others, one of the most recent techniques is called freeform reversible embedding of suspended hydrogels (FRESH), which has now progressed to its second version and consists of extruding a bioink in a dissolvable suspension bath usually made of a gelatin microparticle slurry, which enables the 3D bioprinting of constructs with higher resolution and is useful for the production of vessels of very small diameters (5 to 10 µm) [146]. This technique has been used very recently for the 3D bioprinting of a full-size human heart [147]. An alternative utility of this type of technique is sacrificial writing in functional tissues (SWIFT), which enables the production of small vessels and vascularization through extrusion bioprinting directly inside a functional and vital tissue, which simultaneously acts as a suspension bath [63].
Moreover, a further highly innovative branch of applications is the magnetic levitation approach, introduced in 2020 by Mironov et al. (also affiliated to the company 3D Bioprinting Solutions [148]). However, the first experiments with magnetic-based bioprinters showed a limitation that the bioinks have to withstand the pull of Earth’s gravity. Regarding this aspect, space agencies like ESA or NASA are also investigating the idea of using microgravity to improve the 3D printing of soft human tissues, such as blood vessels and muscles. This means using a scaffold-free, nozzle-free and label-free approach (i.e., without magnetic nanoparticles). Enabling in-space bioprinting may not only help improve bioprinting research to face organ shortage on Earth but would also have repercussions in long-term/long-distance human space missions (including Moon and Mars programs). The increased risk of injuries in such distant missions impose the need to develop the ability to print replacement tissues or organs for astronauts in emergency situations. In this context, 3D bioprinting could be considered as a mission enabler for such kinds of projects (i.e., space exploration and planet colonization) [149].
Worldwide distribution of the most prolific academic institutions
In order to highlight countries and institutions currently involved in 3D bioprinting research, the geographical distribution of affiliations declared in the publications were analyzed. A preliminary analysis was performed on the aggregated data retrieved from SciVal. The United States (USA), China, South Korea, Germany, United Kingdom (UK), and Canada scored as the most relevant countries where research on 3D bioprinting is currently ongoing. Similar results were obtained by ranking the countries depending on the authors’ affiliations (see Fig. 6aFootnote 3 for further details). As seen in Fig. 6b, the US has an obvious leading role in terms of absolute performance (number of authors and institutions involved in bioprinting research), which shows a more diffused attention to this topic (with an average of 4.6 top authors in each of the leading institutions). Meanwhile, China has a second leading position but is characterized by a more focused profile, where only a handful of institutions are currently hosting the most prolific authors on 3D bioprinting (with 7.5 authors on average in each of the top institutions).
a Geographic localization of the current affiliation of the 100 most relevant authors (blue), and the most relevant affiliations (green) according to SciVal based on the Scholarly output. The ten most relevant universities are highlighted. The interactive map can be viewed at https://ggle.io/3kuZ. Map data ©2021 Google. b Number of the most prolific universities (retrieved by considering the affiliations in papers) and top authors per country. The number of the most relevant authors, in blue, and the number of the most relevant institutions per country, in green, were retrieved from SciVal on the topic T.8060 (Bioprinting; Printability; Tissue Engineering). The countries are listed following the SciVal ranking based on the Scholarly output. China, South Korea, and Germany have the highest number of authors per affiliation. The fraction of authors over the number of institutions per country is represented in yellow, and the data are shown on the secondary y axis on the right
In Table 2, the number in the parentheses after the research institute refers to the relative position of the institution/author in the worldwide ranking obtained by considering the number of published products (called ‘scholarly output’ in SciVal). In particular, products are associated to the institution depending on the affiliation of the authors of each product.
The table lists the top ten affiliations; it can be observed that the University of California at San Diego (1) and Harvard University (2) in the USA, and Nanyang Technological University (3) in Singapore are the three leading institutions in this field (see also Table S2 for a complete list of top affiliations and authors per country). A similar geographical distribution is shown for the most prolific authors (shown in blue in Fig. 6b).
A more complete analysis of the top-leading laboratories and scientists is presented in Table 3, with specific attention to the investigated topics. For the most inclusive analysis possible, these authors were selected as the 20 researchers with the highest scholarly output and/or citation count within the topic of 3D bioprinting according to SciVal. Moreover, the network of collaborations between universities defined by considering co-authorships is shown in Fig. 7, from which it can be inferred that, despite global collaborations, the highest number of publications in collaborations are also geographically clustered. The clusters identified from this graph are also discussed in Table 4.
Network graph showing collaborations between the most prolific authors; the authors’ names and relative affiliations are presented in color and black, respectively. The size of the node (circle) is directly proportional to the number of publications on 3D bioprinting retrieved from that author, while the color indicates the country of affiliation. The links between the nodes denote the number of collaborations (only collaborations on at least 10 publications are shown); the thickness of a link is proportional to the number of articles produced in collaboration between the two authors. Twelve clusters of collaborations can be identified from this graph, in which 5 are prominent in terms of the number of publications of authors and the number of collaborations
Within the US, three clusters of collaborations can be recognized. The most relevant group in the USA per number of publications can be referred to as the “Harvard cluster” in which a strong collaboration between PIs affiliated to Harvard can be seen; the PIs involved are Khademhosseini, Ali, whose current first affiliation is Terasaki Institute for Biomedical Innovation, and Zhang, Yu Shrike, who is currently affiliated to Harvard Medical School. Considering authors’ multiple affiliations, this cluster also has a connection with Massachusetts Institute of Technology (6). Within this cluster, vascularization and heart [75, 150,151,152,153] are the types of tissue attracting the greatest interest. In the US, another group of collaborations can be identified as the “Wake Forest cluster”, in which a network of connections can be recognized within the university with the affiliations of Atala, Anthony, Yoo, James, and Lee, Sangjin. Within this cluster, the focus is mainly on process [154], cartilage [155] and articulations [156].
A further research facility worth mentioning is the UC San Diego (1), which is the leading university in the world on 3D bioprinting, to which Chen, Shaochen is affiliated. Publications by this university are mainly focused on the optimization of the bioprinting process, particularly inkjet [157,158,159,160], and the evaluation of printability [161, 162]; regarding the type of tissues, the recurrent topic is the creation of tubular structures and vasculature [163].
Within Asia, China is ranked second in terms of the number of publications (1036 papers), with leading institutions such as Zhejiang University (5), Tsinghua University (8) and the Chinese Academy of Sciences (9). Notably, while the USA has mainly academic players, among the 14 top institutions in China, two are government-run and one is a medical institution (see Fig. S1 in the Supplementary Information for further details). Interestingly, most of the collaborations in Asia occur within universities.
Within Zhejiang University (5), a strong collaboration can be noticed between Fu, Jianzhong, He, Yong, and Gao, Qing, with the main focus of publications on vascularization [164,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,183]. Other universities worth mentioning are Tsinghua University and the Ministry of Education, where Sun, Wei and Li, Xinda are the most prolific authors, respectively. The focus of these collaborations is on topics such as the inkjet process [184, 185], biomaterials [186], with targeted efforts on tumor model preparation [187], especially regarding glioma [188] and lung cancer [189], the use of stem cells [190], and the formation of vasculature [191].
South Korea is ranked third in terms of published products (scholarly output from SciVal). The main academic institutions here are Pohang University of Science and Technology (7), Konkuk University (15), and Sungkyunkwan University (14). The Pohang University of Science and Technology (7) can be considered as the center of a relative cluster to which the Korea Polytechnic University also belongs. To the first affiliation, Cho, Dongwoo and Jang, Jinah are active and mainly focused on the liver [192, 193], cardiac repair [194, 195], cartilage [196], vascularization [197, 198], and cornea [111, 199].
Within Asia, further notable institutions are located in Singapore (6), which is globally ranked the sixth in terms of number of publications, with the main participating institutions of Agency for Science, Technology and Research (40), to which Naing, May Win is affiliated, and the Singapore University of Technology and Design, to which Chua, Chee Kai is affiliated. Moreover, the most prolific institution in Russia is the Sechenov First Moscow State Medical University, to which Mironov, Vladimir A. is affiliated.
In Europe, Germany (4) and the UK (5) are the two leading countries in terms of publications, number of top authors and top institutions. However, the most productive institution on bioprinting in Europe is Utrecht University in the Netherlands (10). Four clusters of collaborations can be identified within Europe, one being the “Utrecht University cluster”, which primarily links Malda, Jos and Levato, Riccardo from Utrecht University (10), and Groll, Jürgen from University of Würzburg in Germany (4), with a main focus on the general aspects of 3D bioprinting [14, 31, 200,201,202]. Two additional clusters of collaborations can be identified in Germany within the Technische Universität Dresden with researchers Lode, Anja and Ahlfeld, Tilman, and Friedrich-Alexander University Erlangen-Nürnberg to which Boccaccini, Aldo R. and Detsch, Rainer are affiliated. In addition, a cluster of collaboration can be identified in Poland with a collaboration between the Warsaw University of Technology (Święszkowski, Wojciech) and the Polish Academy of Sciences (Costantini, Marco).
Finally, it is worth noting that some leading universities are also located in Oceania; the University of Wollongong in Australia, to which Wallace, Gordon G. and Yue, Zhilian are affiliated, and the University of Otago in New Zealand, to which Woodfield, T. B.F. and Lim, K. S. are affiliated.
Market and patent landscape
In recent years, interest in 3D bioprinting has been gathering momentum not only in academia, but also in the industry. Between 2014 and 2015, numerous 3D bioprinting companies have entered the market, and new start-ups, spin-offs and subsidiaries continue to emerge. Bioprinting could become a new standard for the biofabrication of tissues in the field of regenerative medicine; many bioprinter manufacturers have started to commercialize their proposals and services in research or other professional fields. Most of these companies sell materials (bioinks and cells), bioprinters and consulting services.
According to the latest market research by Mordor Intelligence [260], the global bioprinting industry was valued at USD 586.13 million in 2019 and is expected to reach USD 1,949.94 million by 2025, which is equivalent to a compound annual growth rate (CAGR) of 21.91% for the period of 2020–2025 [261]. These values were confirmed by another report, in which the value of 3D bioprinting market was projected to reach USD 1,647.4 million by 2024 at a CAGR of 20.4% for 2019–2024.
The growth of the 3D bioprinting industry, which is mainly driven by technological improvements on biomaterials and 3D bioprinters, has pushed business players to develop and enhance their existing manufacturing and distribution capabilities.
To review and analyze the companies and start-ups currently on the market, we used commercial magazines, newsletters and specialized blogs to retrieve 70 legally claimed bioprinting companies (latest update in July 2020). The analysis excluded 3D printing or biotechnology companies which announced their entrance into the market with no actual 3D bioprinting-related commercial products or services offered. The list of these companies, together with the available basic information regarding their business and their bioprinter models are reported in Table S3.
Based on the analysis, the business models of such companies could be classified as follows: (a) those selling commercial bioprinters and/or bioinks (63% of the whole market), (b) those providing bioprinting services (such as CAD modelling, specific tissue or cell culture constructs, scaffolds, grafts, or only consulting) with their own proprietary technology or commercially unavailable bioprinters (37% of the industry) and/or starting custom tissue partnership with clients (usually cosmetics or pharmaceutics industries) that have specific requests, as well as granting technology access partnerships (Table 5).
Around 80% of the market is composed of established companies, while 20% are start-ups with strong economic growth, mainly stemming from university spin-offs.
Table 6 reports the bioprinter market composition classified by technique, based only on the available information from manufacturer’s websites. Once again, it is possible to see that extrusion-based models are the most widespread ones, as their popularity is guaranteed by the lower cost and ease of use. Inkjet-based bioprinters consist the second most common technology. Nowadays, the inkjet technology is included in most of the extrusion-based bioprinters commercially available as an additional printing head. Despite the fact that stereolithography was the first technology in AM, stereolithography-based bioprinters are a new addition to the bioprinting industry, some of which only appeared at the time of writing of this paper or have yet to be announced. Laser-assisted bioprinters are among the most expensive bioprinters, which are usually part of more sophisticated systems. These are among devices capable of reaching the highest resolutions on the market. Only two-photon stereolithography has even better resolution, but it is not always categorized as a pure bioprinter, as this system is mainly useful for printing scaffolds for cells to attach to rather than printing cells and using bioink at the same time.
Based on the previous analysis, the industry is obviously growing at a fast rate not only in terms of quantity, but also in terms of diversification of the technologies developed and offered. Even though there are some polarizing countries, the companies that develop and commercialize bioprinting technologies are relatively dispersed across nearly all continents (Fig. 8a).
a Worldwide distribution of 3D bioprinting companies. The interactive map can be viewed at https://ggle.io/3kuZ. Map data ©2021 Google. b 3D bioprinting market composition by continent
Mapping the companies making up this industry is essential to find potential technology hubs.
Considering single countries, the retrieved data suggests that USA remains the most significant player with 39% of all companies, exceeding all the other countries by one order of magnitude, whose percentages vary between 7 and 1%. In terms of continents, apart from the 40% share of North America, consisting basically of USA and Canada, Europe harbors 36% of all companies, with countries like Germany, UK and France representing nearly half of all European companies. The continents that follow are Asia (14%), Latin America (8%) and Oceania (1%) (Fig. 8b).
As far as we are concerned, there is a multitude of university start-ups, especially in China and in Latin America, that prefer to use their own custom-designed bioprinting technologies.
Emerging technological trends
The fact that several 3D bioprinting companies across the globe currently manufacture commercially available 3D bioprinters is a clear indication that the field of AM and the bioengineering industry are evolving at a rapid pace. Along with the number of companies, the abundance of technological innovations associated with bioprinters and bioinks is also growing rapidly. In fact, the main leading bioprinting companies are trying to break into the market with increasingly peculiar technologies.
Most of the companies try to produce all-in-one extrusion-based bioprinting platforms with support for multi-materials (viscous pastes, gels and hydrogels, ABS/PLA and other filaments or polymer powders, liquids, ceramics and foods), multi-tools (laser system for ultra-high-precision cutting and engraving, CNC milling machine, photo-crosslinking UV LED, microscope, HD cameras for monitoring, autocalibration tools, 3D electronics printer, built-in incubator) and custom-made software (e.g. AI powered automatic organ and tissue segmentation software), often available in different versions according to customer requirements [153, 220, 244, 245, 262,263,264,265].
This panorama also includes firms that invest their resources in developing more refined solutions that aim to solve specific problems. A possible starting trend is to develop methods capable of using tissue spheroids and managing them, for example, through magnetic bioprinting such as the Organ.Aut, a magnetic bioprinter from the Russian company 3D Bioprinting Solutions [266], also delivered to the ISS on board the Soyuz MS-11 spacecraft. Furthermore, the Japanese company Cyfuse Biomedical [267] developed a platform that allows to create scaffold-free tissues using the Kenzan bioprinting method to manipulate spheroids. In this method, the production of 3D constructs is achieved by placing cellular spheroids in a temporary array of needles through a cell-dispensing robotic mechanism. On the other hand, there are companies, such as the Germany-based Cellbricks, that prefer to produce complex 3D-printed cell culture structures with a proprietary non-commercial stereolithography-based bioprinting platform [268].
Moreover, some enterprises try to propose bioprinters with more degrees of freedom to increase system flexibility and the range of printable features, like the American company Advanced Solutions [269], which patented a six-axis robotic extrusion-based bioprinter arm capable of loading up to ten independent biomaterials during a single print run. Other companies decided to focus on unusual features of their 3D bioprinters, such as the Rollovesselar™ module of the Chinese company Revotek for printing scaffold-free 3D cylindrical structures with a proprietary bio-ink to create vessels. This company claimed to have successfully replaced a short segment of the abdominal artery in 30 rhesus monkeys [270].
The bioprinting industry is not only driven by extrusion-based platforms. Other technologies to achieve the single cell deposition accuracy are under development, such as the Image Based Single Cell Isolation (IBSCI) developed by the French company Cellenion [271], which is a high-resolution-based technology consisting of automated image acquisition, processing and advanced algorithms to automatically isolate single cells from a cell suspension. Another French company, Poietis [272] focuses on laser-assisted bioprinting combined with extrusion-based and inkjet technologies supported via a proprietary PIA™ software to reconstitute the 3D representation of an entire tissue, layer after layer. Yet other companies, such as the Canada-based Aspect Biosystems, attempt to achieve improved accuracy in the development of microfluidic platforms equipped with an on-printhead crosslinking system that is able to print bioinks with a coaxial shell.
Some new business entities aim to increase their market share by widening the offer, producing affordable systems and collaborating with other entities. This is the case of CELLINK [273] that provides a wide range of solutions, both in terms of affordable bioprinters (extrusion-based and DLP-based) and various specific bioinks. In connection with Prellis Biologics, they have just released one of the first systems using two-photons stereolithography to the market, named the Holograph X™, with a special solution to increase the 3D printing speed by using a parallel set of photons, i.e., a multiphoton technology, in order to simultaneously cure millions of points in the bioink, and in turn achieve bioprinting speeds of up to 250,000 voxels per second.
Pioneering bioprinting companies like Organovo [274] instead prefer to provide services or products (like liver and kidney tissue models histologically and functionally similar to the native ones [241, 275]) along with their proprietary technology.
It is also worth mentioning BIOLIFE4D, an upcoming biotech firm founded in 2015, with headquarters in Illinois (USA). The company is dedicated to produce a patient-specific, fully functioning heart through 3D bioprinting and with a patient’s own cells. In 2018, BIOLIFE4D successfully constructed a 3D-bioprinted vascularized and contractile cardiac patch made of iPSC. In 2019, they claimed that their next milestone would be to produce a human mini-heart, which would constitute the 3D-bioprinted mini version of a full-sized heart [276].
Evolution of patent trends
The industrial interest toward 3D bioprinting can be quantified in terms of number of deposited patents, which reflects the propensity of a company to protect its ideas and solutions. In this work, the Espacenet website [277] was used to identify the patents submitted in this field.
A new version of the global query matching the syntax and other specifications of this different database was made. A patent search was conducted in July 2020, and a total of 309 patent abstracts were found since the year of 2000. The abstracts of all patent records were carefully reviewed and grouped into the following categories: “bioprinting method”, “bioink”, “scaffold”, “bioprinter technology”, and “marginal involvement of 3D printing”.
At first glance, it is apparent that the number of patents published shows exponential growth, just as the number of scientific publications. Two-thirds of all patents found were published in the last 3 years (Fig. 9a). This further confirms the growing number of companies and researchers entering this market.
Despite the fact that, as the previous analysis has highlighted, nearly all of the main bioprinting-related companies are based in the USA and Europe, more than two-third of the patents originate from Asia (Fig. 9b). It is important to underline that most of these patents were published recently, which is a good sign that Asian companies are expected to soon break into the market. Among the Asian countries, China is leading the field of 3D bioprinting with 58% of all patents published so far (against 19% of USA), followed by South Korea (14%).
Another interesting aspect concerns the topic of patents (Table 7). Nearly half of them are about new bioprinting methods for specific functions (bone, vascular, trachea graft), for describing novel 3D bioprinting techniques, or to patent new bioprinter technologies. One-third is instead relative to biomaterials: novel bioink formulations rather than specific applications for specific bioink.
Intriguingly, patents regarding scaffold production or bioprinter technologies were more common in the early years, while those concerning bioinks or specific applications became more prevalent later. This is probably an indication that current technologies have been somewhat established, and new solutions in this area can more easily concern new material developments for organ- or tissue-specific customization.
Figure 10 demonstrates that over two-thirds of the considered patents came from universities or unaffiliated scientists. It is clear that, in recent years, the number of academic applicants (i.e., universities, hospitals and research centers) is growing much faster than those coming from the industrial sector, whose number stays fairly constant. A more in-depth analysis of the patent origin (Figs. 10, 11) indicates that about 56% of those in the academic field and 61% of those in the industry come from China, which means that research output on bioprinting in this country is still booming. It is thus possible to justify the huge discrepancy between the high number of Chinese patents and the low number of Chinese companies. The next few years will probably see the birth of a growing number of Chinese companies focused on bioprinting.
Conclusions
The field of 3D bioprinting, which represents a novel area within AM technologies, shows a great potential for future expansion. In the last few years, this discipline has received an impressive level of interest in the scientific literature, attracting many innovators and creating new exciting markets. All these signals outline that we are possibly observing the expansion of a long-term research direction. Instead of preparing an additional review paper, the aim of this study was to provide the reader with a comprehensive overview of the academic and industry landscape of 3D bioprinting, in order that unfamiliar researchers have a compass to venture into exciting emerging technologies, and experienced academics are provided with an updated snapshot of the current status of this fast-changing field.
In the first part, a scientometric review of the literature was provided, with an analysis of all of the impressive literature (almost 10,000 papers, with most of them published in the last few years) to highlight the globally most relevant applications and key actors in terms of laboratories and research networks.
In the second part, the associated companies and emerging technologies were described to highlight the upcoming innovations and the most relevant players that consider the technology for new market developments.
It was confirmed that both paper and patent publications exhibited exponential growth in this sector, with the USA leading the level of scientific output while China showing an impressive growth in the whole number of patents, which clearly highlights its possible future position as a leading country in the bioprinting industry.
Many open challenges highlighted in this study call for new technological solutions that can be possibly borrowed from traditional AM research. The enhancement of printing resolution and speed, as well as cost reduction are common challenges to be faced in the near future. Remarkably though, bioprinting has certain unique features, such as the requirement of avoiding the mistreatment of cells during printing, and taking multi-material printing as a key asset for future technological developments.
To achieve this aim, multidisciplinary research should combine engineering expertise in AM, biological knowledge on cell growth and differentiation, material science for biomaterial developments, and expertise in biomedicine and pharmaceutics to highlight and solve relevant research questions. With such a multidisciplinary approach, we might see a flourishing area that can have a relevant impact on successful future technologies aimed at the improvement of human wellbeing.
Notes
The Scholarly Output measures the number of research outputs [278].
IF data refer to 2019.
Data from SciVal, map created using Google MyMaps.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- 4D:
-
Four-dimensional
- ABS:
-
Acrylonitrile butadiene styrene
- AI:
-
Artificial intelligence
- AM:
-
Additive manufacturing
- CAD:
-
Computer-aided design
- CAGR:
-
Compound annual growth rate
- CNC:
-
Computerized numerical control
- DLP:
-
Digital light processing
- DOD:
-
Drop-on-demand
- ECM:
-
Extracellular matrix
- GelMA:
-
Gelatin methacryloyl
- hASCs:
-
Human adipose stem cells
- HD:
-
High-definition
- IBSC:
-
Image-based single cell isolation
- IF:
-
Impact factor
- iPSC:
-
Induced pluripotent stem cell
- ISS:
-
International space station
- PCL:
-
Polycaprolactone
- PLA:
-
Polylactic acid
- PLGA:
-
Poly (lactic-co-glycolic acid)
- UK:
-
United Kingdom
- USA:
-
United States
- USD:
-
United States Dollar
- UV LED:
-
Ultraviolet light emitting diode
- WoS:
-
Web of science
References
Ng WL, Chua CK, Shen YF (2019) Print me an organ! Why we are not there yet. Prog Polym Sci 97:101145. https://doi.org/10.1016/j.progpolymsci.2019.101145
Choudhury D, Anand S, Naing MW (2018) The arrival of commercial bioprinters—towards 3D bioprinting revolution! Int J Bioprint 4(2). https://doi.org/10.18063/IJB.v4i2.139
Hölzl K, Lin S, Tytgat L et al (2016) Bioink properties before, during and after 3D bioprinting. Biofabrication 8(3):032002. https://doi.org/10.1088/1758-5090/8/3/032002
Hospodiuk M, Dey M, Sosnoski D et al (2017) The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv 35(2):217–239. https://doi.org/10.1016/j.biotechadv.2016.12.006
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. https://doi.org/10.1038/nbt.2958
Li J, Chen M, Fan X et al (2016) Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 14(1):271. https://doi.org/10.1186/s12967-016-1028-0
Ozbolat IT, Moncal KK, Gudapati H (2017) Evaluation of bioprinter technologies. Addit Manuf 13:179–200. https://doi.org/10.1016/j.addma.2016.10.003
Rodríguez-Salvador M, Rio-Belver RM, Garechana-Anacabe G (2017) Scientometric and patentometric analyses to determine the knowledge landscape in innovative technologies: the case of 3D bioprinting. PLoS ONE 12(6). https://doi.org/10.1371/journal.pone.0180375
Agarwala S, Lee JM, Ng WL et al (2018) A novel 3D bioprinted flexible and biocompatible hydrogel bioelectronic platform. Biosens Bioelectron 102:365–371. https://doi.org/10.1016/j.bios.2017.11.039
Munoz-Abraham AS, Rodriguez-Davalos MI, Bertacco A et al (2016) 3D printing of organs for transplantation: Where are we and where are we heading? Curr Transpl Rep 3(1):93–99. https://doi.org/10.1007/s40472-016-0089-6
Antoshin AA, Churbanov SN, Minaev NV et al (2019) LIFT-bioprinting, is it worth it? Bioprinting 15(May):e00052. https://doi.org/10.1016/j.bprint.2019.e00052
Jin Y, Ji S, Li X et al (2017) A scientometric review of hotspots and emerging trends in additive manufacturing. J Manuf Technol Manag 28(1):18–38. https://doi.org/10.1108/JMTM-12-2015-0114
Ramos T, Moroni L (2020) Tissue engineering and regenerative medicine 2019: the role of biofabrication—a year in review. Tissue Eng Part C Methods 26(2):91–106. https://doi.org/10.1089/ten.tec.2019.0344
Moroni L, Boland T, Burdick JA et al (2018) Biofabrication: a guide to technology and terminology. Trends Biotechnol 36(4):384–402. https://doi.org/10.1016/j.tibtech.2017.10.015
Gao G, Kim BS, Jang J et al (2019) Recent strategies in extrusion-based three-dimensional cell printing toward organ biofabrication. ACS Biomater Sci Eng 5(3):1150–1169. https://doi.org/10.1021/acsbiomaterials.8b00691
Panwar A, Tan LP (2016) Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 21(6). https://doi.org/10.3390/molecules21060685
Davoodi E, Sarikhani E, Montazerian H et al (2020) Extrusion and microfluidic-based bioprinting to fabricate biomimetic tissues and organs. Adv Mater Technol 5(8). https://doi.org/10.1002/admt.201901044
Boularaoui S, Al Hussein G, Khan KA et al (2020) An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 20:e00093. https://doi.org/10.1016/j.bprint.2020.e00093
Gudapati H, Dey M, Ozbolat I (2016) A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102:20–42. https://doi.org/10.1016/j.biomaterials.2016.06.012
Ng WL, Lee JM, Zhou M et al (2020) Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges. Biofabrication 12(2). https://doi.org/10.1088/1758-5090/ab6034
Kumar H, Kim K (2020) Stereolithography 3D bioprinting. Methods Mol Biol 2140:93–108
Derakhshanfar S, Mbeleck R, Xu K et al (2018) 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 3(2):144–156. https://doi.org/10.1016/j.bioactmat.2017.11.008
Loai S, Kingston BR, Wang Z et al (2019) Clinical perspectives on 3D bioprinting paradigms for regenerative medicine. Regen Med Front 1(1). https://doi.org/10.20900/rmf20190004
Zhang J, Xiao P (2018) 3D printing of photopolymers. Polym Chem 9(13):1530–1540. https://doi.org/10.1039/c8py00157j
Pawar AA, Saada G, Cooperstein I et al (2016) High-performance 3D printing of hydrogels by water-dispersible photoinitiator nanoparticles. Sci Adv 2(4):e1501381. https://doi.org/10.1126/sciadv.1501381
Datta P, Barui A, Wu Y et al (2018) Essential steps in bioprinting: from pre- to post-bioprinting. Biotechnol Adv 36(5):1481–1504. https://doi.org/10.1016/j.biotechadv.2018.06.003
Sun W, Starly B, Daly AC et al (2020) The bioprinting roadmap. Biofabrication 12(2):022002. https://doi.org/10.1088/1758-5090/ab5158
Kengla C, Renteria E, Wivell C et al (2017) Clinically relevant bioprinting workflow and imaging process for tissue construct design and validation. 3D Print Addit Manuf 4(4):239–247. https://doi.org/10.1089/3dp.2017.0075
Hunsberger J, Harrysson O, Shirwaiker R et al (2015) Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl Med 4(2):130–135. https://doi.org/10.5966/sctm.2014-0254
Rezende RA, Selishchev SV, Kasyanov VA et al (2013) An organ biofabrication line: enabling technology for organ printing. Part I: from biocad to biofabricators of spheroids. Biomed Eng 47(3):116–120. https://doi.org/10.1007/s10527-013-9348-3
Levato R, Jungst T, Scheuring RG et al (2020) From shape to function: The next step in bioprinting. Adv Mater 32(12). https://doi.org/10.1002/adma.201906423
Gao G, Huang Y, Schilling AF et al (2018) Organ bioprinting: Are we there yet? Adv Healthc Mater 7(1). https://doi.org/10.1002/adhm.201701018
Miao S, Cui H, Nowicki M et al (2018) Stereolithographic 4D bioprinting of multiresponsive architectures for neural engineering. Adv Biosyst 2(9):9. https://doi.org/10.1002/adbi.201800101
An J, Chua CK, Mironov V (2016) A perspective on 4D bioprinting. Int J Bioprint 2(1):3–5. https://doi.org/10.18063/IJB.2016.01.003
Miao S, Zhu W, Castro NJ et al (2016) 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep 6. https://doi.org/10.1038/srep27226
Lukin I, Musquiz S, Erezuma I et al (2019) Can 4D bioprinting revolutionize drug development? Expert Opin Drug Discov 14(10):953–956. https://doi.org/10.1080/17460441.2019.1636781
Gao B, Yang Q, Zhao X et al (2016) 4D bioprinting for biomedical applications. Trends Biotechnol 34(9):746–756. https://doi.org/10.1016/j.tibtech.2016.03.004
Esworthy TJ, Miao S, Lee SJ et al (2019) Advanced 4D-bioprinting technologies for brain tissue modeling and study. Int J Smart Nano Mater 10(3):177–204. https://doi.org/10.1080/19475411.2019.1631899
Li YC, Zhang YS, Akpek A et al (2017) 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 9(1). https://doi.org/10.1088/1758-5090/9/1/012001
Yang GH, Yeo M, Koo YW et al (2019) 4D bioprinting: technological advances in biofabrication. Macromol Biosci 19(5). https://doi.org/10.1002/mabi.201800441
Castro NJ, Meinert C, Levett P et al (2017) Current developments in multifunctional smart materials for 3D/4D bioprinting. Curr Opin Biomed Eng 2:67–75. https://doi.org/10.1016/j.cobme.2017.04.002
Ozbolat IT, Peng W, Ozbolat V (2016) Application areas of 3D bioprinting. Drug Discov Today 21(8):1257–1271. https://doi.org/10.1016/j.drudis.2016.04.006
Vijayavenkataraman S (2016) A perspective on bioprinting ethics. Artif Organs 40(11):1033–1038. https://doi.org/10.1111/aor.12873
Bulanova EA, Koudan EV, Degosserie J et al (2017) Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication 9(3):3. https://doi.org/10.1088/1758-5090/aa7fdd
Zhang K, Fu Q, Yoo J et al (2017) 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater 50:154–164. https://doi.org/10.1016/j.actbio.2016.12.008
Kim J, Hope CM, Gantumur N et al (2020) Encapsulation of Human Natural and Induced Regulatory T-Cells in IL-2 and CCL1 Supplemented Alginate-GelMA Hydrogel for 3D Bioprinting. Adv Funct Mater 30(15). https://doi.org/10.1002/adfm.202000544
Murphy SV, De Coppi P, Atala A (2019) Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng 4(4):370–380. https://doi.org/10.1038/s41551-019-0471-7
Matai I, Kaur G, Seyedsalehi A et al (2020) Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226. https://doi.org/10.1016/j.biomaterials.2019.119536
Singh M, Jonnalagadda S (2020) Advances in bioprinting using additive manufacturing. Eur J Pharm Sci 143:105167. https://doi.org/10.1016/j.ejps.2019.105167
Heinrich MA, Liu W, Jimenez A et al (2019) 3D bioprinting: from benches to translational applications. Small 15(23):1–47. https://doi.org/10.1002/smll.201805510
Tasnim N, De la Vega L, Anil Kumar S et al (2018) 3D bioprinting stem cell derived tissues. Cell Mol Bioeng 11(4):219–240. https://doi.org/10.1007/s12195-018-0530-2
Chameettachal S, Yeleswarapu S, Sasikumar S et al (2019) 3D bioprinting: recent trends and challenges. J Indian Inst Sci 99(3):375–403. https://doi.org/10.1007/s41745-019-00113-z
Shahabipour F, Ashammakhi N, Oskuee RK et al (2020) Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res 216:57–76. https://doi.org/10.1016/j.trsl.2019.08.010
Nowicki M, Zhu W, Sarkar K et al (2020) 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting 17. https://doi.org/10.1016/j.bprint.2019.e00066
Dhawan A, Kennedy PM, Rizk EB et al (2019) Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. J Am Acad Orthop Surg 27(5):E215–E226. https://doi.org/10.5435/JAAOS-D-17-00632
Ashammakhi N, Hasan A, Kaarela O et al (2019) Advancing Frontiers in bone bioprinting. Adv Healthcare Mater 8(7). https://doi.org/10.1002/adhm.201801048
Yun BG, Lee SHS-H, Jeon JH et al (2019) Accelerated bone regeneration via three-dimensional cell-printed constructs containing human nasal turbinate-derived stem cells as a clinically applicable therapy. ACS Biomater Sci Eng 5:6171–6185
Datta P, Dhawan A, Yu Y et al (2017) Bioprinting of osteochondral tissues: a perspective on current gaps and future trends. Int J Bioprint 3(2):109–120. https://doi.org/10.18063/IJB.2017.02.007
Yang J, Zhang YS, Yue K (2017) Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater 57:1–25. https://doi.org/10.1016/j.actbio.2017.01.036
Chimene D, Miller L, Cross LM et al (2020) Nanoengineered osteoinductive bioink for 3D bioprinting bone tissue. ACS Appl Mater Interf 12(14):15976–15988. https://doi.org/10.1021/acsami.9b19037
Bendtsen ST, Quinnell SP, Wei M (2017) Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res Part A 105(5):1457–1468. https://doi.org/10.1002/jbm.a.36036
Xing F, Xiang Z, Rommens PM et al (2020) 3D bioprinting for vascularized tissue-engineered bone fabrication. Materials 13(10). https://doi.org/10.3390/ma13102278
Skylar-Scott MA, Uzel SGM, Nam LL et al (2019) Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv 5(9). https://doi.org/10.1126/sciadv.aaw2459
Sarker MD, Naghieh S, Sharma NK et al (2019) Bioprinting of vascularized tissue scaffolds: influence of biopolymer, cells, growth factors, and gene delivery. J Healthc Eng 2019. https://doi.org/10.1155/2019/9156921
Koduru SV, Leberfinger AN, Pasic D et al (2019) Cellular based strategies for microvascular engineering. Stem Cell Rev Rep 15(2):218–240. https://doi.org/10.1007/s12015-019-09877-4
Datta P, Ayan B, Ozbolat IT (2017) Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater 51:1–20. https://doi.org/10.1016/j.actbio.2017.01.035
Mouser VHM, Levato R, Mensinga A et al (2020) Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect Tissue Res 61(2):137–151. https://doi.org/10.1080/03008207.2018.1553960
Yi HG, Choi YJ, Jung JW et al (2019) Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J Tissue Eng 10. https://doi.org/10.1177/2041731418824797
Duchi S, Onofrillo C, O’Connell CD et al (2017) Handheld co-axial bioprinting: application to in situ surgical cartilage repair. Sci Rep 7(1):1. https://doi.org/10.1038/s41598-017-05699-x
Francis SL, Di Bella C, Wallace GG et al (2018) Cartilage tissue engineering using stem cells and bioprinting technology—barriers to clinical translation. Front Surg 5. https://doi.org/10.3389/fsurg.2018.00070
Di Bella C, Duchi S, O’Connell CD et al (2018) In situ handheld three-dimensional bioprinting for cartilage regeneration. J Tissue Eng Regen Med 12(3):611–621. https://doi.org/10.1002/term.2476
O’Connell CD, Di Bella C, Thompson F et al (2016) Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 8(1). https://doi.org/10.1088/1758-5090/8/1/015019
Wang Z, Lee SJ, Cheng HJ et al (2018) 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater 70:48–56. https://doi.org/10.1016/j.actbio.2018.02.007
Cui H, Miao S, Esworthy T et al (2018) 3D bioprinting for cardiovascular regeneration and pharmacology. Adv Drug Deliv Rev 132:252–269. https://doi.org/10.1016/j.addr.2018.07.014
Zhang YS, Arneri A, Bersini S et al (2016) Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110:45–59. https://doi.org/10.1016/j.biomaterials.2016.09.003
Duan B (2017) State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng 45(1):195–209. https://doi.org/10.1007/s10439-016-1607-5
Lee W, Hong Y, Dai G (2019) 3D bioprinting of vascular conduits for pediatric congenital heart repairs. Transl Res 211:35–45. https://doi.org/10.1016/j.trsl.2019.03.007
Mao Q, Wang Y, Li Y et al (2020) Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C 109. https://doi.org/10.1016/j.msec.2020.110625
Lee H, Chae S, Kim JJY et al (2019) Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 11(2):2. https://doi.org/10.1088/1758-5090/aaf9fa
Schneeberger K, Spee B, Costa P et al (2017) Converging biofabrication and organoid technologies: The next frontier in hepatic and intestinal tissue engineering? Biofabrication 9(1). https://doi.org/10.1088/1758-5090/aa6121
Lee SJ, Esworthy T, Stake S et al (2018) Advances in 3D bioprinting for neural tissue engineering. Adv Biosyst 2(4). https://doi.org/10.1002/adbi.201700213
Knowlton S, Cho Y, Li XJ et al (2016) Utilizing stem cells for three-dimensional neural tissue engineering. Biomater Sci 4(5):768–784. https://doi.org/10.1039/c5bm00324e
Hsieh FY, Lin HH, Hui Hsu S (2015) 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 71:48–57. https://doi.org/10.1016/j.biomaterials.2015.08.028
Yan WC, Davoodi P, Vijayavenkataraman S et al (2018) 3D bioprinting of skin tissue: from pre-processing to final product evaluation. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2018.07.016
Vijayavenkataraman S, Lu WF, Fuh JYH (2016) 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 8(3). https://doi.org/10.1088/1758-5090/8/3/032001
Seol YJ, Lee H, Copus JS et al (2018) 3D bioprinted biomask for facial skin reconstruction. Bioprinting 10. https://doi.org/10.1016/j.bprint.2018.e00028
Ng WL, Wang S, Yeong WY et al (2016) Skin bioprinting: Impending reality or fantasy? Trends Biotechnol 34(9):689–699. https://doi.org/10.1016/j.tibtech.2016.04.006
Kim WJ, Lee H, Lee JU et al (2020) Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 230:119632. https://doi.org/10.1016/j.biomaterials.2019.119632
Laternser S, Keller H, Leupin O et al (2018) A novel microplate 3D bioprinting platform for the engineering of muscle and tendon tissues. SLAS Technol 23(6):599–613. https://doi.org/10.1177/2472630318776594
Arrigoni C, Petta D, Bersini S et al (2019) Engineering complex muscle-tissue interfaces through microfabrication. Biofabrication 11(3):032004. https://doi.org/10.1088/1758-5090/ab1e7c
Ostrovidov S, Salehi S, Costantini M et al (2019) 3D bioprinting in skeletal muscle tissue engineering. Small 15(24). https://doi.org/10.1002/smll.201805530
Onofrillo C, Duchi S, O’Connell CD et al (2018) Biofabrication of human articular cartilage: a path towards the development of a clinical treatment. Biofabrication 10(4):4. https://doi.org/10.1088/1758-5090/aad8d9
Mouser VHM, Levato R, Bonassar LJ et al (2017) Three-dimensional bioprinting and its potential in the field of articular cartilage regeneration. Cartilage 8(4):327–340. https://doi.org/10.1177/1947603516665445
Piluso S, Li Y, Abinzano F et al (2019) Mimicking the articular joint with in vitro models. Trends Biotechnol 37(10):1063–1077. https://doi.org/10.1016/j.tibtech.2019.03.003
Groen WM, Diloksumpan P, van Weeren PR et al (2017) From intricate to integrated: biofabrication of articulating joints. J Orthop Res 35(10):2089–2097. https://doi.org/10.1002/jor.23602
Levato R, Webb WR, Otto IA et al (2017) The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater 61:41–53. https://doi.org/10.1016/j.actbio.2017.08.005
O’Connell G, Garcia J, Amir J (2017) 3D bioprinting: new directions in articular cartilage tissue engineering. ACS Biomater Sci Eng 3(11):2657–2668. https://doi.org/10.1021/acsbiomaterials.6b00587
Daly AC, Freeman FE, Gonzalez-Fernandez T et al (2017) 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthc Mater 6(22):1700298. https://doi.org/10.1002/adhm.201700298
Antich C, de Vicente J, Jiménez G et al (2020) Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater 106:114–123. https://doi.org/10.1016/j.actbio.2020.01.046
Bedir T, Ulag S, Ustundag CB et al (2020) 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater Sci Eng C 110:110741. https://doi.org/10.1016/j.msec.2020.110741
Singh NK, Han W, Nam SA et al (2020) Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials 232. https://doi.org/10.1016/j.biomaterials.2019.119734
Nam H, Mi Choi Y, Jang J (2020) Vascularized lower respiratory-physiology-on-a-chip. Appl Sci 10(3). https://doi.org/10.3390/app10030900
Park JY, Ryu H, Lee B et al (2019) Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 11(1):1. https://doi.org/10.1088/1758-5090/aae545
Fishman JM, Wiles K, Lowdell MW et al (2014) Airway tissue engineering: an update. Expert Opin Biol Ther 14(10):1477–1491. https://doi.org/10.1517/14712598.2014.938631
Carter SSD, Costa PF, Vaquette C et al (2017) Additive biomanufacturing: an advanced approach for periodontal tissue regeneration. Ann Biomed Eng 45(1):12–22. https://doi.org/10.1007/s10439-016-1687-2
Ke D, Yi H, Est-Witte S et al (2020) Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication 12(1):015022. https://doi.org/10.1088/1758-5090/ab5354
Lee JS, Kim BS, Seo D et al (2017) Three-dimensional cell printing of large-volume tissues: application to ear regeneration. Tissue Eng Part C Methods 23(3):136–145. https://doi.org/10.1089/ten.tec.2016.0362
Hakobyan D, Médina C, Dusserre N et al (2020) Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 12(3):035001. https://doi.org/10.1088/1758-5090/ab7cb8
Yue Z, Liu X, Coates PT et al (2016) Advances in printing biomaterials and living cells: Implications for islet cell transplantation. Curr Opin Organ Transpl 21(5):467–475. https://doi.org/10.1097/MOT.0000000000000346
Kim J, Kang K, Drogemuller CJ et al (2019) Bioprinting an artificial pancreas for type 1 diabetes. Curr Diab Rep 19(8). https://doi.org/10.1007/s11892-019-1166-x
Kim HKH-KH, Park MNM-N, Kim J et al (2019) Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J Tissue Eng 10. https://doi.org/10.1177/2041731418823382
Shi P, Edgar TYS, Yeong WY et al (2017) Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int J Bioprint 3(2):138–146. https://doi.org/10.18063/IJB.2017.02.008
Tan YSE, Shi PJ, Choo CJ et al(2018) Tissue engineering of retina and Bruch’s membrane: a review of cells, materials and processes. Br J Ophthalmol 102(9):1182–1187. https://doi.org/10.1136/bjophthalmol-2017-311390
Duan B, Hockaday LA, Kang KH et al (2013) 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res Part A 101A(5):1255–1264. https://doi.org/10.1002/jbm.a.34420
Duchamp M, Liu T, van Genderen AM et al (2019) Sacrificial bioprinting of a mammary ductal carcinoma model. Biotechnol J 14(10):10. https://doi.org/10.1002/biot.201700703
Wang Y, Shi W, Kuss M et al (2018) 3D bioprinting of breast cancer models for drug resistance study. ACS Biomater Sci Eng 4(12):4401–4411. https://doi.org/10.1021/acsbiomaterials.8b01277
Bahcecioglu G, Basara G, Ellis BW et al (2020) Breast cancer models: engineering the tumor microenvironment. Acta Biomater 106:1–21. https://doi.org/10.1016/j.actbio.2020.02.006
Roberts S, Peyman S, Speirs V (2019) Current and emerging 3D models to study breast cancer. Adv Exp Med Biol 1152:413–427. https://doi.org/10.1007/978-3-030-20301-6_22
Cui H, Esworthy T, Zhou X et al (2020) Engineering a novel 3D printed vascularized tissue model for investigating breast cancer metastasis to bone. Adv Healthc Mater 9(15). https://doi.org/10.1002/adhm.201900924
Leonard F, Godin B (2016) 3D in vitro model for breast cancer research using magnetic levitation and bioprinting method. In: Cao J (ed) Breast Cancer. Methods in molecular biology, vol 1406. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3444-7_21
Zhou X, Zhu W, Nowicki M et al (2016) 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl Mater Interf 8(44):30017–30026. https://doi.org/10.1021/acsami.6b10673
Votanopoulos KI, Mazzocchi A, Sivakumar H et al (2019) Appendiceal cancer patient-specific tumor organoid model for predicting chemotherapy efficacy prior to initiation of treatment: a feasibility study. Ann Surg Oncol 26(1):139–147. https://doi.org/10.1245/s10434-018-7008-2
Mazzocchi AR, Rajan SAP, Votanopoulos KI et al(2018) In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci Rep 8(1). https://doi.org/10.1038/s41598-018-21200-8
van Tienderen GS, Berthel M, Yue Z et al (2018) Advanced fabrication approaches to controlled delivery systems for epilepsy treatment. Expert Opin Drug Deliv 15(9):915–925. https://doi.org/10.1080/17425247.2018.1517745
Ravnic DJ, Leberfinger AN, Ozbolat IT (2017) Bioprinting and cellular therapies for type 1 diabetes. Trends Biotechnol 35(11):1025–1034. https://doi.org/10.1016/j.tibtech.2017.07.006
Votanopoulos KI, Forsythe S, Sivakumar H et al (2020) Model of patient-specific immune-enhanced organoids for immunotherapy screening: feasibility study. Ann Surg Oncol 27(6):1956–1967. https://doi.org/10.1245/s10434-019-08143-8
Albanna M, Binder KW, Murphy SV et al (2019) In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 9(1):1. https://doi.org/10.1038/s41598-018-38366-w
Van Kogelenberg S, Yue Z, Dinoro JN et al (2018) Three-dimensional printing and cell therapy for wound repair. Adv Wound Care 7(5):145–155. https://doi.org/10.1089/wound.2017.0752
Mazzocchi A, Votanopoulos K, Skardal A (2018) Personalizing cancer treatments empirically in the laboratory: patient-specific tumor organoids for optimizing precision medicine. Curr Stem Cell Rep 4(2):97–104. https://doi.org/10.1007/s40778-018-0122-z
Nie J, Gao Q, Fu J et al (2020) Grafting of 3D bioprinting to in vitro drug screening: a review. Adv Healthc Mater 9(7). https://doi.org/10.1002/adhm.201901773
Mazzocchi A, Soker S, Skardal A (2019) 3D bioprinting for high-throughput screening: drug screening, disease modeling, and precision medicine applications. Appl Phys Rev 6(1). https://doi.org/10.1063/1.5056188
Satpathy A, Datta P, Wu Y et al (2018) Developments with 3D bioprinting for novel drug discovery. Expert Opin Drug Discov 13(12):1115–1129. https://doi.org/10.1080/17460441.2018.1542427
Wu Y, Ravnic DJ, Ozbolat IT (2020) Intraoperative bioprinting: repairing tissues and organs in a surgical setting. Trends Biotechnol 38(6):594–605. https://doi.org/10.1016/j.tibtech.2020.01.004
Gu Q, Zhu H, Li J et al (2016) Three-dimensional bioprinting speeds up smart regenerative medicine. Natl Sci Rev 3(3):331–344. https://doi.org/10.1093/nsr/nww037
Zhao H, Yang F, Fu J et al (2017) Printing@Clinic: from medical models to organ implants. ACS Biomater Sci Eng 3(12):3083–3097. https://doi.org/10.1021/acsbiomaterials.7b00542
Shafiee A, Atala A (2016) Printing technologies for medical applications. Trends Mol Med 22(3):254–265. https://doi.org/10.1016/j.molmed.2016.01.003
Martelli N, Serrano C, Van Den Brink H et al (2016) Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery 159(6):1485–1500. https://doi.org/10.1016/j.surg.2015.12.017
Yi HG, Lee H, Cho DW (2017) 3D printing of organs-on-chips. Bioengineering 4(1). https://doi.org/10.3390/bioengineering4010010
Gungor-Ozkerim PS, Inci I, Zhang YS et al (2018) Bioinks for 3D bioprinting: an overview. Biomater Sci 6(5):915–946. https://doi.org/10.1039/c7bm00765e
Cui X, Li J, Hartanto Y et al (2020) Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater 9(15). https://doi.org/10.1002/adhm.201901648
Moroni L, Burdick JA, Highley C et al (2018) Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater 3(5):21–37. https://doi.org/10.1038/s41578-018-0006-y
Prendergast ME, Solorzano RD, Cabrera D (2017) Bioinks for biofabrication: current state and future perspectives. J 3D Print Med 1(1):49–62. https://doi.org/10.2217/3dp-2016-0002
Skeldon G, Lucendo-Villarin B, Shu W (2018) Three-dimensional bioprinting of stem-cell derived tissues for human regenerative medicine. Philos Trans R Soc B Biol Sci 373(1750). https://doi.org/10.1098/rstb.2017.0224
Leberfinger AN, Ravnic DJ, Dhawan A et al (2017) Concise review: bioprinting of stem cells for transplantable tissue fabrication. Stem Cells Transl Med 6(10):1940–1948. https://doi.org/10.1002/sctm.17-0148
Zhang YS, Pi Q, van Genderen AM (2017) Microfluidic bioprinting for engineering vascularized tissues and organoids. J Vis Exp 126:2017. https://doi.org/10.3791/55957
Corbett DC, Olszewski E, Stevens K (2019) A FRESH take on resolution in 3D bioprinting. Trends Biotechnol 37(11):1153–1155. https://doi.org/10.1016/j.tibtech.2019.09.003
Mirdamadi E, Tashman JW, Shiwarski DJ et al (2020) FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater Sci Eng 6(11):6453–6459. https://doi.org/10.1021/acsbiomaterials.0c01133
Parfenov VA, Khesuani YD, Petrov SV et al (2020) Magnetic levitational bioassembly of 3D tissue construct in space. Sci Adv 6(29). https://doi.org/10.1126/sciadv.aba4174
Ghidini T (2018) Regenerative medicine and 3D bioprinting for human space exploration and planet colonisation. J Thorac Dis 10(Suppl 20):S2363–S2375. https://doi.org/10.21037/jtd.2018.03.19
Jia W, Gungor-Ozkerim PS, Zhang YS et al (2016) Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106:58–68. https://doi.org/10.1016/j.biomaterials.2016.07.038
Zhang YS, Davoudi F, Walch P et al (2016) Bioprinted thrombosis-on-a-chip. Lab Chip 16(21):4097–4105. https://doi.org/10.1039/c6lc00380j
Rezaei Nejad H, Goli Malekabadi Z, Kazemzadeh Narbat M et al (2016) Laterally confined microfluidic patterning of cells for engineering spatially defined vascularization. Small 12(37):5132–5139. https://doi.org/10.1002/smll.201601391
Massa S, Sakr MA, Seo J et al (2017) Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 11(4):4. https://doi.org/10.1063/1.4994708
Skardal A, Devarasetty M, Kang H-WHW et al (2016) Bioprinting cellularized constructs using a tissue-specific hydrogel bioink. J Vis Exp 2016(110):110. https://doi.org/10.3791/53606
Xu T, Binder KW, Albanna MZ et al (2013) Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5(1). https://doi.org/10.1088/1758-5082/5/1/015001
Merceron TK, Burt M, Seol YJ et al (2015) A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 7(3). https://doi.org/10.1088/1758-5090/7/3/035003
Xu C, Zhang M, Huang Y et al (2014) Study of droplet formation process during drop-on-demand inkjetting of living cell-laden bioink. Langmuir 30(30):9130–9138. https://doi.org/10.1021/la501430x
Christensen K, Xu C, Chai W et al (2015) Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng 112(5):1047–1055. https://doi.org/10.1002/bit.25501
Xiong R, Christensen K, Xu C et al (2014) Jet-based 3D printing of biological constructs. In: 25th Annual International Solid Freeform Fabrication Symposium—an Additive Manufacturing Conference, pp 1069–1075
Xu C, Zhang Z, Fu J et al (2013) Time-resolved study of droplet formation process during inkjetting of alginate solution. In: 24th International Solid Freedom Fabrication Symposium—an Additive Manufacturing Conference, pp 253–259
Zhang Z, Jin Y, Yin J et al (2018) Evaluation of bioink printability for bioprinting applications. Appl Phys Rev 5(4). https://doi.org/10.1063/1.5053979
Zhang Z, Xu C, Xiong R et al (2017) Effects of living cells on the bioink printability during laser printing. Biomicrofluidics 11(3). https://doi.org/10.1063/1.4985652
Xu C, Zhang Z, Christensen K et al (2014) Freeform vertical and horizontal fabrication of alginate-based vascular-like tubular constructs using inkjetting. J Manuf Sci Eng Trans ASME 136(6). https://doi.org/10.1115/1.4028578
Gao Q, He Y, Zhong Fu J et al (2015) Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61:203–215. https://doi.org/10.1016/j.biomaterials.2015.05.031
Gao Q, Liu Z, Lin Z et al (2017) 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater Sci Eng 3(3):399–408. https://doi.org/10.1021/acsbiomaterials.6b00643
Shao H, Yang X, He Y et al (2015) Bioactive glass-reinforced bioceramic ink writing scaffolds: sintering, microstructure and mechanical behavior. Biofabrication 7(3). https://doi.org/10.1088/1758-5090/7/3/035010
Gao Q, Gu H, Zhao P et al (2018) Fabrication of electrospun nanofibrous scaffolds with 3D controllable geometric shapes. Mater Des 157:159–169. https://doi.org/10.1016/j.matdes.2018.07.042
Zhao H, Chen Y, Shao L et al (2018) Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle. Small 14(39):39. https://doi.org/10.1002/smll.201802630
Shao L, Gao Q, Zhao H et al (2018) Fiber-based mini tissue with morphology-controllable GelMA microfibers. Small 14(44). https://doi.org/10.1002/smll.201802187
Gao Q, Niu X, Shao L et al (2019) 3D printing of complex GelMA-based scaffolds with nanoclay. Biofabrication 11(13). https://doi.org/10.1088/1758-5090/ab0cf6
Xie M, Gao Q, Zhao H et al (2019) Electro-assisted bioprinting of low-concentration GelMA microdroplets. Small 15(4):4. https://doi.org/10.1002/smll.201804216
Shao H, Liu A, Ke X et al (2017) 3D robocasting magnesium-doped wollastonite/TCP bioceramic scaffolds with improved bone regeneration capacity in critical sized calvarial defects. J Mater Chem B 5(16):2941–2951. https://doi.org/10.1039/c7tb00217c
Wang X, Zhang L, Ke X et al (2015) 45S5 Bioglass analogue reinforced akermanite ceramic favorable for additive manufacturing mechanically strong scaffolds. RSC Adv 5(124):102727–102735. https://doi.org/10.1039/c5ra19272b
Gao Q, He Y, Zhong Fu J et al (2016) Fabrication of shape controllable alginate microparticles based on drop-on-demand jetting. J Sol Gel Sci Technol 77(3):610–619. https://doi.org/10.1007/s10971-015-3890-2
Shao L, Gao Q, Xie C et al (2019) Bioprinting of cell-laden microfiber: Can it become a standard product? Adv Healthc Mater 8(9). https://doi.org/10.1002/adhm.201900014
Xie M, Gao Q, Qiu J et al (2020) 3D biofabrication of microfiber-laden minispheroids: a facile 3D cell co-culturing system. Biomater Sci 8(1):109–117. https://doi.org/10.1039/c9bm01189g
Shao L, Gao Q, Xie C et al (2020) Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Bio-Design Manuf 3(1):30–39. https://doi.org/10.1007/s42242-020-00062-y
Xie M, Yu K, Sun Y et al (2019) Protocols of 3D bioprinting of gelatin methacryloyl hydrogel based bioinks. J Vis Exp 2019(154). https://doi.org/10.3791/60545
Xie C, Gao Q, Wang Pet al (2019) Structure-induced cell growth by 3D printing of heterogeneous scaffolds with ultrafine fibers. Mater Des 181. https://doi.org/10.1016/j.matdes.2019.108092
Gao Q, Zhao P, Zhou R et al (2019) Rapid assembling organ prototypes with controllable cell-laden multi-scale sheets. Bio-Des Manuf 2(1):1–9. https://doi.org/10.1007/s42242-019-00032-z
Gao Q, Xie C, Wang P et al (2020) 3D printed multi-scale scaffolds with ultrafine fibers for providing excellent biocompatibility. Mater Sci Eng C 107. https://doi.org/10.1016/j.msec.2019.110269
Jin Y, Gao Q, Xie C et al (2020) Fabrication of heterogeneous scaffolds using melt electrospinning writing: design and optimization. Mater Des 185. https://doi.org/10.1016/j.matdes.2019.108274
Shao L, Gao Q, Xie C et al (2020) Synchronous 3D bioprinting of large-scale cell-laden constructs with nutrient networks. Adv Healthc Mater 9(15). https://doi.org/10.1002/adhm.201901142
Li X, Liu B, Pei B et al (2020) Inkjet bioprinting of biomaterials. Chem Rev 120(19):10793–10833. https://doi.org/10.1021/acs.chemrev.0c00008
Zhou D, Chen J, Liu B et al (2019) Bioinks for jet-based bioprinting. Bioprinting 16(May):e00060. https://doi.org/10.1016/j.bprint.2019.e00060
Zhang X, Kim GJ, Kang MG et al (2018) Marine biomaterial-Based bioinks for generating 3D printed tissue constructs. Mar Drugs 16(12):12. https://doi.org/10.3390/md16120484
Dai X, Liu L, Ouyang J et al (2017) Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci Rep 7(1):1–12. https://doi.org/10.1038/s41598-017-01581-y
Wang X, Li X, Ding J et al (2021) 3D bioprinted glioma microenvironment for glioma vascularization. J Biomed Mater Res Part A 109(6):915–925. https://doi.org/10.1002/jbm.a.37082
Wang X, Zhang X, Dai X et al (2018) Tumor-like lung cancer model based on 3D bioprinting. 3 Biotech 8(12). https://doi.org/10.1007/s13205-018-1519-1
Zhou D, Chen L, Ding J et al (2020) A 3D engineered scaffold for hematopoietic progenitor/stem cell co-culture in vitro. Sci Rep 10(1):1–11. https://doi.org/10.1038/s41598-020-68250-5
Li X, Liu L, Zhang X et al (2018) Research and development of 3D printed vasculature constructs. Biofabrication 10(3). https://doi.org/10.1088/1758-5090/aabd56
Park JY, Jang J, Kang HW (2018) 3D Bioprinting and its application to organ-on-a-chip. Microelectron Eng 200:1–11. https://doi.org/10.1016/j.mee.2018.08.004
Lee H, Han W, Kim H et al (2017) Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromol 18(4):1229–1237. https://doi.org/10.1021/acs.biomac.6b01908
Jang J, Park HJ, Kim SW et al (2017) 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112:264–274. https://doi.org/10.1016/j.biomaterials.2016.10.026
Das S, Kim SW, Choi YJ et al (2019) Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater 95:188–200. https://doi.org/10.1016/j.actbio.2019.04.026
Kundu J, Shim JH, Jang J et al (2015) An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 9(11):1286–1297. https://doi.org/10.1002/term.1682
Gao G, Lee JH, Jang J et al (2017) Tissue engineered bio-blood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: a novel therapy for ischemic disease. Adv Funct Mater 27(33):33. https://doi.org/10.1002/adfm.201700798
Gao G, Park JY, Kim BS et al (2018) Coaxial cell printing of freestanding, perfusable, and functional in vitro vascular models for recapitulation of native vascular endothelium pathophysiology. Adv Healthc Mater 7(23). https://doi.org/10.1002/adhm.201801102
Park J, Lee K-PKP, Kim HKHK et al (2019) Biocompatibility evaluation of bioprinted decellularized collagen sheet implanted in vivo cornea using swept-source optical coherence tomography. J Biophotonics 12(11):11. https://doi.org/10.1002/jbio.201900098
Groll J, Boland T, Blunk T et al (2016) Biofabrication: reappraising the definition of an evolving field. Biofabrication 8(1). https://doi.org/10.1088/1758-5090/8/1/013001
Groll J, Burdick JA, Cho DW et al (2019) A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11(1):013001. https://doi.org/10.1088/1758-5090/aaec52
Malda J, Visser J, Melchels FP et al (2013) 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater 25(36):5011–5028. https://doi.org/10.1002/adma.201302042
Nichol JW, Koshy ST, Bae H et al (2010) Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31(21):5536–5544. https://doi.org/10.1016/j.biomaterials.2010.03.064
Yue K, Trujillo-de Santiago G, Alvarez MM et al (2015) Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73:254–271. https://doi.org/10.1016/j.biomaterials.2015.08.045
Pati F, Jang J, Ha DH et al (2014) Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5. https://doi.org/10.1038/ncomms4935
Ozbolat IT, Yu Y (2013) Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng 60(3):691–699. https://doi.org/10.1109/TBME.2013.2243912
Ozbolat IT (2015) Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 33(7):395–400. https://doi.org/10.1016/j.tibtech.2015.04.005
Yeong WY, Chua CK, Leong KF et al (2007) Comparison of drying methods in the fabrication of collagen scaffold via indirect rapid prototyping. J Biomed Mater Res Part B Appl Biomater 82(1):260–266. https://doi.org/10.1002/jbm.b.30729
Lee JM, Yeong WY (2016) Design and printing strategies in 3D bioprinting of cell-hydrogels: a review. Adv Healthc Mater 5(22):2856–2865. https://doi.org/10.1002/adhm.201600435
Xu C, Chai W, Huang Y et al (2012) Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioeng 109(12):3152–3160. https://doi.org/10.1002/bit.24591
Riggs BC, Dias AD, Schiele NR et al (2011) Matrix-assisted pulsed laser methods for biofabrication. MRS Bull 36(12):1043–1050. https://doi.org/10.1557/mrs.2011.276
Tan KH, Chua CK, Leong KF et al (2003) Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials 24(18):3115–3123. https://doi.org/10.1016/S0142-9612(03)00131-5
Suntornnond R, An J, Chua CK (2017) Bioprinting of thermoresponsive hydrogels for next generation tissue engineering: a review. Macromol Mater Eng 302(1). https://doi.org/10.1002/mame.201600266
Izadifar Z, Chang T, Kulyk W et al (2016) Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng Part C Methods 22(3):173–188. https://doi.org/10.1089/ten.tec.2015.0307
Li MG, Tian XY, Chen XB (2009) A brief review of dispensing-based rapid prototyping techniques in tissue scaffold fabrication: role of modeling on scaffold properties prediction. Biofabrication 1(3). https://doi.org/10.1088/1758-5082/1/3/032001
He Y, Wu Y, Fu JZ et al (2016) Developments of 3D printing microfluidics and applications in chemistry and biology: a review. Electroanalysis 28(8):1658–1678. https://doi.org/10.1002/elan.201600043
Woodfield TBF, Malda J, De Wijn J et al (2004) Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 25(18):4149–4161. https://doi.org/10.1016/j.biomaterials.2003.10.056
Kang HW, Lee SJ, Ko IK et al (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319. https://doi.org/10.1038/nbt.3413
Hochleitner G, Jüngst T, Brown TD et al (2015) Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 7(3). https://doi.org/10.1088/1758-5090/7/3/035002
Jang J, Park JY, Gao G et al (2018) Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 156:88–106. https://doi.org/10.1016/j.biomaterials.2017.11.030
Kim GH, Son JG, Park S et al (2008) Hybrid process for fabricating 3D hierarchical scaffolds combining rapid prototyping and electrospinning. Macromol Rapid Commun 29(19):1577–1581. https://doi.org/10.1002/marc.200800277
Hong N, Yang GH, Lee J et al (2018) 3D bioprinting and its in vivo applications. J Biomed Mater Res Part B Appl Biomater 106(1):444–459. https://doi.org/10.1002/jbm.b.33826
Mironov V, Visconti RP, Kasyanov V et al (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30(12):2164–2174. https://doi.org/10.1016/j.biomaterials.2008.12.084
Skardal A, Mack D, Kapetanovic E et al (2012) Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1(11):792–802. https://doi.org/10.5966/sctm.2012-0088
Devarasetty M, Mazzocchi AR, Skardal A (2018) Applications of bioengineered 3D tissue and tumor organoids in drug development and precision medicine: current and future. BioDrugs 32(1):53–68. https://doi.org/10.1007/s40259-017-0258-x
Cui H, Zhu W, Holmes B et al (2016) Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv Sci 3(8). https://doi.org/10.1002/advs.201600058
Cui H, Nowicki M, Fisher JP et al (2017) 3D bioprinting for organ regeneration. Adv Healthc Mater 6(1). https://doi.org/10.1002/adhm.201601118
Chung JHY, Naficy S, Yue Z et al (2013) Bio-ink properties and printability for extrusion printing living cells. Biomater Sci. https://doi.org/10.1039/c3bm00012e
Ferris CJ, Gilmore KG, Wallace GG et al (2013) Biofabrication: an overview of the approaches used for printing of living cells. Appl Microbiol Biotechnol 97(10):4243–4258. https://doi.org/10.1007/s00253-013-4853-6
Colosi C, Shin SR, Manoharan V et al (2016) Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater 28(4):677–684a. https://doi.org/10.1002/adma.201503310
Bertassoni LE, Cecconi M, Manoharan V et al (2014) Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14(13):2202–2211. https://doi.org/10.1039/c4lc00030g
Skardal A, Devarasetty M, Kang HW et al (2015) A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater 25:24–34. https://doi.org/10.1016/j.actbio.2015.07.030
Seol YJ, Kang HW, Lee SJ et al (2014) Bioprinting technology and its applications. Eur J Cardiothorac Surg 46(3):342–348. https://doi.org/10.1093/ejcts/ezu148
Zhu W, Webster TJ, Zhang LG (2019) 4D printing smart biosystems for nanomedicine. Nanomedicine 14(13):1643–1645. https://doi.org/10.2217/nnm-2019-0134
Shafiee A, Ghadiri E, Ramesh H et al (2019) Physics of bioprinting. Appl Phys Rev 6(2). https://doi.org/10.1063/1.5087206
Kolesky DB, Truby RL, Gladman AS et al (2014) 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26(19):3124–3130. https://doi.org/10.1002/adma.201305506
Highley CB, Rodell CB, Burdick JA (2015) Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater 27(34):5075–5079. https://doi.org/10.1002/adma.201501234
Gauvin R, Chen YC, Lee JW et al (2012) Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 33(15):3824–3834. https://doi.org/10.1016/j.biomaterials.2012.01.048
Zorlutuna P, Annabi N, Camci-Unal G et al (2012) Microfabricated biomaterials for engineering 3D tissues. Adv Mater 24(14):1782–1804. https://doi.org/10.1002/adma.201104631
Ouyang L, Highley CB, Sun W et al (2017) A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv Mater 29(8). https://doi.org/10.1002/adma.201604983
Bhise NS, Manoharan V, Massa S et al (2016) A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8(1):1. https://doi.org/10.1088/1758-5090/8/1/014101
Zhang YS, Khademhosseini A (2015) Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine. https://doi.org/10.2217/nnm.15.18
Hou X, Zhang YS, De Santiago GT et al (2017) Interplay between materials and microfluidics. Nat Rev Mater. https://doi.org/10.1038/natrevmats.2017.16
Miri AK et al (2018) Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv Mater 30(27):27. https://doi.org/10.1002/adma.201800242
Pi Q, Maharjan S, Yan X et al (2018) Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv Mater 30(43):43. https://doi.org/10.1002/adma.201706913
Ali M, AnilKumar PR, Yoo JJ et al (2019) A photo-crosslinkable kidney ECM-derived bioink accelerates renal tissue formation. Adv Healthc Mater 8(7). https://doi.org/10.1002/adhm.201800992
Skardal A, Murphy SV, Crowell K et al (2017) A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res Part B Appl Biomater 105(7):1986–2000. https://doi.org/10.1002/jbm.b.33736
Skardal A, Devarasetty M, Forsythe S et al (2016) A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol Bioeng 113(9):2020–2032. https://doi.org/10.1002/bit.25950
Murphy SV, Skardal A, Atala A (2013) Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res Part A 101A(1):272–284. https://doi.org/10.1002/jbm.a.34326
Skardal A, Atala A (2015) Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng 43(3):730–746. https://doi.org/10.1007/s10439-014-1207-1
Xu T, Olson J, Zhao W et al (2008) Characterization of cell constructs generated with inkjet printing technology using in vivo magnetic resonance imaging. J Manuf Sci Eng Trans ASME 130(2):210131–210137. https://doi.org/10.1115/1.2902857
Li Y et al (2019) High-fidelity and high-efficiency additive manufacturing using tunable pre-curing digital light processing. Addit Manuf 30. https://doi.org/10.1016/j.addma.2019.100889
Kim BS, Kim H, Gao G et al (2017) Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication 9(3). https://doi.org/10.1088/1758-5090/aa7e98
Jang J, Kim TG, Kim BS et al (2016) Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater 33:88–95. https://doi.org/10.1016/j.actbio.2016.01.013
Yi HG, Jeong YH, Kim Y et al (2019) A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat Biomed Eng 3(7):509–519
Castilho M, Feyen D, Flandes-Iparraguirre et al (2017) Melt electrospinning writing of poly-hydroxymethylglycolide-co-ε-caprolactone-based scaffolds for cardiac tissue engineering. Adv Healthc Mater 6(18):18. https://doi.org/10.1002/adhm.201700311
Stichler S, Böck T, Paxton N et al (2017) Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ϵ-caprolactone) for MSC chondrogenesis. Biofabrication 9(4). https://doi.org/10.1088/1758-5090/aa8cb7
Castilho M, Hochleitner G, Wilson W et al (2018) Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci Rep 8(1):1. https://doi.org/10.1038/s41598-018-19502-y
de Ruijter M, Hrynevich A, Haigh JN et al (2018) Out-of-plane 3D-printed microfibers improve the shear properties of hydrogel composites. Small 14(8). https://doi.org/10.1002/smll.201702773
3D bioprinting market by component (3D bioprinters (microextrusion, inkjet, laser), bioink (natural, synthetic, hybrid)), material (hydrogel, living cells), application (skin, drug research), end user (biopharma, academia)—global forecast to 2024
3D bioprinting market—growth, trends and forecasts (2020–2025)
Aether 2020. https://discoveraether.com/
Liu W, Zhang YS, Heinrich MA et al (2017) Rapid continuous multimaterial extrusion bioprinting. Adv Mater 29(3):3. https://doi.org/10.1002/adma.201604630
Zhu K, Shin SR, van Kempen T et al (2017) Gold nanocomposite bioink for printing 3D cardiac constructs. Adv Funct Mater 27(12):12. https://doi.org/10.1002/adfm.201605352
Liu W, Zhong Z, Hu N et al (2018) Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10(2):2. https://doi.org/10.1088/1758-5090/aa9d44
3D Bioprinting Solutions (2020) https://bioprinting.ru/
Cyfuse Biomedical (2020) https://www.cyfusebio.com/en/
Cellbricks (2020) https://cellbricks.com/
Advanced Solutions (2020) https://www.advancedsolutions.com/
Revotek (2020) http://www.revotekhealth.com/index.aspx
Cellenion (2020) https://www.cellenion.com/
Poietis (2020) https://poietis.com/
CELLINK (2020) https://cellink.com/global/
Organovo (2020) https://organovo.com/
Grundy C, Smith R, Nickel J et al (2011) Utilization of human liver tissues for the evaluation of valproic acid-induced liver injury. 92121
BIOLIFE4D (2020) https://biolife4d.com/
Colledge L (2017) Snowball metrics recipe book standardised research metrics—by the sector, for the sector. Snowball Metrics
Acknowledgements
This study was partially supported by the collaboration agreement between the Italian Space Agency and Politecnico di Milano, “Attività di Ricerca e Innovazione” Agreement n. 2018-5-HH.0.
Funding
Open access funding provided by Politecnico di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
SS, SGG, MS, DM and BMC were involved in conceptualization; SS, SGG were involved in data collection, analysis and writing—original draft; MS, DM and BMC contributed to formal analysis, supervision, validation and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santoni, S., Gugliandolo, S.G., Sponchioni, M. et al. 3D bioprinting: current status and trends—a guide to the literature and industrial practice. Bio-des. Manuf. 5, 14–42 (2022). https://doi.org/10.1007/s42242-021-00165-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-021-00165-0