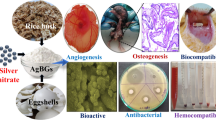
Abstract
Hydroxyapatite (HA) nanoparticles and silver (Ag) nanoparticles are expected to enable desirable bioactivity and antibacterial properties on biopolymer scaffolds. Nevertheless, interfacial adhesion between HA/Ag and the biopolymer is poor due to the large physicochemical differences between these components. In this study, poly L-lactic acid (PLLA) powder was first surface-modified with bioactive polydopamine (PDA) in an alkaline environment. Next, HA and Ag nanoparticles were grown in situ on the PDA-coated PLLA powder, which was then adhered to the porous bone scaffold using a selective laser-sintering process. Results showed that HA and Ag nanoparticles were homogenously distributed in the matrix, with enhanced mechanical properties. Simulated body fluid bioactivity tests showed that the in situ grown HA-endowed scaffold shows excellent bioactivity. In vitro tests confirmed that the scaffold exhibits favorable biocompatibility with human umbilical cord mesenchymal stem cells, as well as strong antibacterial activity against Gram-negative Escherichia coli. Furthermore, in vivo assays indicated that the scaffold promoted bone generation, with a new bone area fraction of 71.8% after 8 weeks’ implantation, without inflammation.










Similar content being viewed by others
References
Oladapo BI, Zahedi SA, Ismail SO et al (2021) 3D printing of PEEK/HAp scaffold for medical bone implant. Bio-des Manuf 4:44–59. https://doi.org/10.1007/s42242-020-00098-0
Liu H, Lin M, Liu X et al (2020) Doping bioactive elements into a collagen scaffold based on synchronous self-assembly/mineralization for bone tissue engineering. Bioact Mater 5(4):844–858. https://doi.org/10.1016/j.bioactmat.2020.06.005
Reiter T, Panick T, Schuhladen K et al (2019) Bioactive glass based scaffolds coated with gelatin for the sustained release of icariin. Bioact Mater 4:1–7. https://doi.org/10.1016/j.bioactmat.2018.10.001
Bigham A, Foroughi F, Ghomi ER et al (2020) The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-des Manuf 3:281–306. https://doi.org/10.1007/s42242-020-00094-4
Didekhani R, Sohrabi MR, Seyedjafari E et al (2018) Electrospun composite PLLA/Oyster shell scaffold enhances proliferation and osteogenic differentiation of stem cells. Biologicals 54:33–38. https://doi.org/10.1016/j.biologicals.2018.04.006
Poh PS, Hutmacher DW, Holzapfel BM et al (2016) In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater 30:319–333. https://doi.org/10.1016/j.actbio.2015.11.012
de Siqueira L, Ribeiro N, Paredes M et al (2019) Influence of PLLA/PCL/HA scaffold fiber orientation on mechanical properties and osteoblast behavior. Materials 12(23):3879. https://doi.org/10.3390/ma12233879
Skadiņš I, Kroiča J, Salma I et al (2019) Influence of antibiotic-impregnated biomaterials on inflammatory cytokines. Proc Latvian Acad Sci Sect B Nat Exact Appl Sci 73(2):177–184. https://doi.org/10.2478/prolas-2019-0028
Zheng K, Wu J, Li W et al (2018) Incorporation of Cu-containing bioactive glass nanoparticles in gelatin-coated scaffolds enhances bioactivity and osteogenic activity. ACS Biomater Sci Eng 4(5):1546–1557. https://doi.org/10.1021/acsbiomaterials.8b00051
Poorraeisi M, Afshar A (2019) Synthesizing and comparing HA–TiO2 and HA–ZrO2 nanocomposite coatings on 316 stainless steel. SN Appl Sci 1(2):155. https://doi.org/10.1007/s42452-019-0168-2
Khan ME, Han TH, Khan MM et al (2018) Environmentally sustainable fabrication of Ag@ g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts. ACS Appl Nano Mater 1(6):2912–2922. https://doi.org/10.1021/acsanm.8b00548
Wang Y, Yan L, Cheng R et al (2018) Multifunctional HA/Cu nano-coatings on titanium using PPy coordination and doping via pulse electrochemical polymerization. Biomater Sci 6(3):575–585. https://doi.org/10.1039/C7BM01104K
Tian G, Zhu G, Xu S et al (2019) A novel shape memory poly (ɛ-caprolactone)/hydroxyapatite nanoparticle networks for potential biomedical applications. J Solid State Chem 272:78–86. https://doi.org/10.1016/j.jssc.2019.01.029
Hong W, Guo F, Chen J et al (2018) Bioactive glass–chitosan composite coatings on PEEK: effects of surface wettability and roughness on the interfacial fracture resistance and in vitro cell response. Appl Surf Sci 440:514–523. https://doi.org/10.1016/j.apsusc.2018.01.183
Mohamed MS, Kumar DS (2016) Methods of using nanoparticles, plant nanotechnology, Springer, Cham, pp 65–93. https://doi.org/10.1007/978-3-319-42154-4
Mallakpour S, Nouruzi N (2016) Effect of modified ZnO nanoparticles with biosafe molecule on the morphology and physiochemical properties of novel polycaprolactone nanocomposites. Polymer 89:94–101. https://doi.org/10.1016/j.polymer.2016.02.038
Nayak GC, Das CK (2016) LCP based polymer blend nanocomposites. Liq Cryst Polym 2016:251–272. https://doi.org/10.1007/978-3-319-22894-5
Zhu Y, Shen J, Zhou K et al (2010) Multifunctional magnetic composite microspheres with in situ growth Au nanoparticles: a highly efficient catalyst system. J Phys Chem C 115(5):1614–1619. https://doi.org/10.1021/jp109276q
Zhang X, Liu Z, Zhang X et al (2018) High-adhesive superhydrophobic litchi-like coatings fabricated by in-situ growth of nano-silica on polyethersulfone surface. Chem Eng J 343:699–707. https://doi.org/10.1016/j.cej.2018.03.012
Lee YH, Hsu YT, Yeh PS et al (2019) Embossed hybrid synthesis of ZnO nanocrystals on self-organized one-dimensional poly (3-butylthiophene) nanowires. Physica Status Solidi(a) 216(9):1801001. https://doi.org/10.1002/pssa.201801001
Yang Y, Huang Q, Payne GF et al (2019) A highly conductive, pliable and foldable Cu/cellulose paper electrode enabled by controlled deposition of copper nanoparticles. Nanoscale 11(2):725–732. https://doi.org/10.1039/C8NR07123C
Tang W, Ma T, Zhou L et al (2019) Polyamine-induced tannic acid co-deposition on magnetic nanoparticles for enzyme immobilization and efficient biodiesel production catalysed by an immobilized enzyme under an alternating magnetic field. Catal Sci Technol 9(21):6015–6026. https://doi.org/10.1039/C9CY01350D
Xie Y, Yue L, Zheng Y et al (2019) The antibacterial stability of poly (dopamine) in-situ reduction and chelation nano-Ag based on bacterial cellulose network template. Appl Surf Sci 491:383–394. https://doi.org/10.1016/j.apsusc.2019.06.096
JináLee S, MináSeok J, HeeáLee J et al (2018) In situ gold nanoparticle growth on polydopamine-coated 3D-printed scaffolds improves osteogenic differentiation for bone tissue engineering applications: in vitro and in vivo studies. Nanoscale 10(33):15447–15453. https://doi.org/10.1039/C8NR04037K
Bian SW, Liu S, Chang L (2016) Synthesis of magnetically recyclable Fe3O4@ polydopamine–Pt composites and their application in hydrogenation reactions. J Mater Sci 51(7):3643–3649. https://doi.org/10.1007/s10853-015-9688-3
Yang Y, Cheng Y, Peng S et al (2021) Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact Mater 6(5):1230–1241. https://doi.org/10.1016/j.bioactmat.2020.10.017
Shuai C, Li S, Yang W et al (2020) MnO2 catalysis of oxygen reduction to accelerate the degradation of Fe–C composites for biomedical applications. Corros Sci 170:108679. https://doi.org/10.1016/j.corsci.2020.108679
Gao C, Li S, Liu L et al (2020) Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting. J Magn Alloys 9(1):305–316. https://doi.org/10.1016/j.jma.2020.03.016
Li M, Chen AN, Lin X et al (2019) Lightweight mullite ceramics with controlled porosity and enhanced properties prepared by SLS using mechanical mixed FAHSs/polyamide12 composites. Ceram Int 45(16):20803–20809. https://doi.org/10.1016/j.ceramint.2019.07.067
Wang G, Qian G, Zan J et al (2020) A co-dispersion nanosystem of graphene oxide @silicon-doped hydroxyapatite to improve scaffold properties. Mater Des 199:109399. https://doi.org/10.1016/j.matdes.2020.109399
Qi F, Wang C, Peng S et al (2021) A co-dispersed nanosystem from strontium-anchored reduced graphene oxide to enhance bioactivity and mechanical property in polymer scaffolds. Mater Chem Front. https://doi.org/10.1039/D0QM00958J
Wei D, Zhou Y, Jia D et al (2008) Chemical treatment of TiO2-based coatings formed by plasma electrolytic oxidation in electrolyte containing nano-HA, calcium salts and phosphates for biomedical applications. Appl Surf Sci 254(6):1775–1782. https://doi.org/10.1016/j.apsusc.2007.07.144
Xu YC, Wang ZX, Cheng XQ et al (2016) Positively charged nanofiltration membranes via economically mussel-substance-simulated co-deposition for textile wastewater treatment. Chem Eng J 303:555–564. https://doi.org/10.1016/j.cej.2016.06.024
Ghorbani F, Zamanian A, Behnamghader A et al (2019) Bone-like hydroxyapatite mineralization on the bio-inspired PDA nanoparticles using microwave irradiation. Surf Interf 15:38–42. https://doi.org/10.1016/j.surfin.2019.01.007
Huan Y, Zhang X, Song J et al (2018) High-performance piezoelectric composite nanogenerator based on Ag/(K, Na) NbO3 heterostructure. Nano Energy 50:62–69. https://doi.org/10.1016/j.nanoen.2018.05.012
Chen K, Xie K, Long Q et al (2017) Fabrication of core–shell Ag@ pDA@ HAp nanoparticles with the ability for controlled release of Ag+ and superior hemocompatibility. RSC Adv 7(47):29368–29377. https://doi.org/10.1039/C7RA03494F
Zhang J, Xiao D, He X et al (2018) A novel porous bioceramic scaffold by accumulating hydroxyapatite spheres for large bone tissue engineering. III: characterization of porous structure. Mater Sci Eng C 89:223–229. https://doi.org/10.1016/j.msec.2018.04.013
Shuai C, He C, Qian G et al (2021) Mechanically driving supersaturated Fe–Mg solid solution for bone implant: Preparation, solubility and degradation. Compos B Eng 207:108564. https://doi.org/10.1016/j.compositesb.2020.108564
Liang H, Yang Y, Xie D et al (2019) Trabecular-like Ti-6Al-4V scaffolds for orthopedic: fabrication by selective laser melting and in vitro biocompatibility. J Mater Sci Technol 35(7):1284–1297. https://doi.org/10.1016/j.jmst.2019.01.012
Fernández-Arias M, Boutinguiza M, del Val J et al (2019) RE-irradiation of silver nanoparticles obtained by laser ablation in water and assessment of their antibacterial effect. Appl Surf Sci 473:548–554. https://doi.org/10.1016/j.apsusc.2018.12.182
Zeng J, Xu L, Luo X et al (2021) A novel design of SiH/CeO2(111) van der Waals type-II heterojunction for water splitting. Phys Chem Chem Phys 23:2812–2818. https://doi.org/10.1039/D0CP05238H
Shuai C, Zan J, Deng F et al (2021) Core–shell-structured ZIF-8@PDA-HA with controllable Zinc ion release and superior bioactivity for improving a poly-l-lactic acid scaffold. ACS Sustain Chem Eng 9(4):1814–1825. https://doi.org/10.1021/acssuschemeng.0c08009
Chen L, Deng C, Li J et al (2019) 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials 196:138–150. https://doi.org/10.1016/j.biomaterials.2018.04.005
Mao D, Li Q, Bai N et al (2018) Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohyd Polym 180:104–111. https://doi.org/10.1016/j.carbpol.2017.10.031
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug Anal 22(1):116–127. https://doi.org/10.1016/j.jfda.2014.01.010
Shuai C, Liu G, Yang Y et al (2020) A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 74:104825. https://doi.org/10.1016/j.nanoen.2020.104825
Kim JS, Kuk E, Yu KN et al (2007) Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med 3(1):95–101. https://doi.org/10.1016/j.nano.2006.12.001
Zhu X, Wu D, Wang W et al (2016) Highly effective antibacterial activity and synergistic effect of Ag–MgO nanocomposite against Escherichia coli. J Alloys Compd 684:282–290. https://doi.org/10.1016/j.jallcom.2016.05.179
Wang Z, Liu S, Ma J et al (2013) Silver nanoparticles induced RNA polymerase-silver binding and RNA transcription inhibition in erythroid progenitor cells. ACS Nano 7(5):4171–4186. https://doi.org/10.1021/nn400594s
Zou Z, Liu W, Cao L et al (2020) Advances in the occurrence and biotherapy of osteoporosis. Biochem Soc Trans 48(4):1623–1636. https://doi.org/10.1042/BST20200005
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4(7):518. https://doi.org/10.1038/nmat1421
Zou A, Liang H, Jiao C et al (2021) Fabrication and properties of CaSiO3/ Sr3(PO4)2 composite scaffold based on extrusion deposition. Ceram Int 47(4):4783–4792. https://doi.org/10.1016/j.ceramint.2020.10.048
Yang W, Han W, He W et al (2016) Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C 60:45–53. https://doi.org/10.1016/j.msec.2015.11.012
Tang W, Lin D, Yu Y et al (2016) Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomater 32:309–323. https://doi.org/10.1016/j.actbio.2015.12.006
Shuai C, Yu L, Feng P et al (2020) Organic montmorillonite produced an interlayer locking effect in a polymer scaffold to enhance interfacial bonding. Mater Chem Front 4(8):2398–2408. https://doi.org/10.1039/D0QM00254B
Xia L, Lin K, Jiang X et al (2013) Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J Mater Chem B 1(40):5403–5416. https://doi.org/10.1039/C3TB20945H
Wang C, Liu D, Zhang C et al (2016) Defect-related luminescent hydroxyapatite-enhanced osteogenic differentiation of bone mesenchymal stem cells via an ATP-induced cAMP/PKA pathway. ACS Appl Mater Interface 8(18):11262–11271. https://doi.org/10.1021/acsami.6b01103
Acknowledgements
This study was supported by the following funds: (1) National Natural Science Foundation of China (Nos. 51935014, 82072084, and 81871498); (2) Jiangxi Provincial Natural Science Foundation of China (Nos. 20192ACB20005 and 2020ACB214004); (3) The Provincial Key R & D Projects of Jiangxi (No. 20201BBE51012); (4) Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2018); (5) Shenzhen Science and Technology Plan Project (No. JCYJ20170817112445033); (6) Innovation Team Project on University of Guangdong Province (No. 2018GKCXTD001); (7) Technology Innovation Platform Project of Shenzhen Institute of Information Technology 2020 (No. PT2020E002); (8) Open Research Fund of Jiangsu Key Laboratory of Precision and Micro-Manufacturing Technology; (9) China Postdoctoral Science Foundation (No. 2020M682114).
Author information
Authors and Affiliations
Contributions
YWY and YC were involved in conceptualization, investigation, writing—original draft; FD and LDS were involved in visualization and resources; ZYZ and SPP helped in writing—review & editing; and CJS contributed to supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The animal experiments in this work have obtained the permission from Xiangya Hospital Animal Experimental Ethics Committee.
Rights and permissions
About this article
Cite this article
Yang, Y., Cheng, Y., Deng, F. et al. A bifunctional bone scaffold combines osteogenesis and antibacterial activity via in situ grown hydroxyapatite and silver nanoparticles. Bio-des. Manuf. 4, 452–468 (2021). https://doi.org/10.1007/s42242-021-00130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-021-00130-x