Abstract
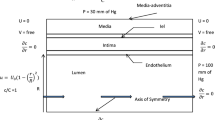
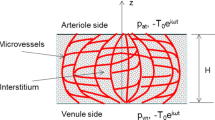
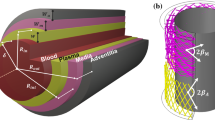
The endothelial dysfunction and the unbalanced secretion of vasoactive substances are the main causes of the macro and micro vascular complications for patients with diabetic mellitus (DM). This paper investigates the blood flow and the nitric oxide (NO) transport in a permeable capillary by using a finite element method. The computational domain consists of a permeable straight or bifurcated capillary and the surrounding tissue with different endothelial hydraulic permeability. The blood flow in the lumen of a capillary is assumed to be governed by the Stokes equation. The fluid flow in the surrounding tissue is simplified as the Darcy flow. The advection–diffusion reaction equation is employed to investigate the NO distribution in a lumen-tissue model, which is originated from the endothelial cells inside the capillary wall. The characteristic Galerkin method is employed for the discretization of the advection-diffusion reaction equation. The simulated results show that the NO transport is effectively affected by different hydraulic permeabilities due to the changes of the blood velocity. When the hydraulic permeability increases considerably, the NO concentration ([NO]) in the whole domain decreases accordingly. Moreover, the NO concentration increases in the area after the bifurcation of the capillary owing to the convective effect. It is also shown that even if the NO production by the endothelial cells is enhanced, the increase of the convection inside the vessel may reduce the endothelial NO concentration. As a signal transduction molecule, the spatial location with discriminating NO distribution may be useful for determining the position of the microangiopathy in the DM.
Similar content being viewed by others
References
Perrin R. M., Harper S. J., Bates D. O. A. Role for the endothelial glycocalyx in regulating microvascular permeability in mellitus [J]. Cell Biochemistry and Biophysics, 2007, 49(2): 65–72.
Cho Y. I., Mooney M. P., Cho D. J. Hemorheological disorders in diabetes mellitus [J]. Journal of Diabetes Science and Technology, 2008, 2(6): 1130–1138.
Yu Y., Xu J., Wang X. L. et al. Numerical study of the effect of erythrocytes mechanical properties on the aggregation of platelets [J]. Chinese Journal of Hydrodynamics, 2015, 30(6): 624–631(in Chinese).
Mónica F. Z., Bian K., Murad F. The endothelium-dependent nitric oxide-cGMP pathway [J]. Advances in Pharmacology, 2016, 77: 1–27.
Sylman J. L., Lantvit S. M., Vedepo M. C. et al. Transport limitations of nitric oxide inhibition of platelet aggregation under flow [J]. Annals of Biomedical Engineering, 2013, 41(10): 2193–2205.
Helisch A., Schaper W. Arteriogenesis: The development and growth of collateral arteries [J]. Microcirculation, 2015, 10(1): 83–97.
Snyder S. H., Ferris C. D. Novel neurotransmitters and their neuropsychiatric relevance [J]. American Journal of Psychiatry, 2000, 157(11): 1738–1751.
Giuffrè A., Forte E., Brunori M. et al. Nitric oxide, cytochrome c oxidase and myoglobin: competition and reaction pathways [J]. Febs Letters, 2005, 579(11): 2528–2532.
Pearce L. L., Kanai A. J., Birder L. A. et al. The catabolic fate of nitric oxide: the nitric oxide oxidase and peroxynitrite reductase activities of cytochrome oxidase [J]. Journal of Biological Chemistry, 2002, 277(16): 13556–13562.
Vaughn M. W., Kuo L., Liao J. C. Effective diffusion distance of nitric oxide in the microcirculation [J]. American Journal of Physiology, 1998, 274(2): 1705–1714.
Chen X., Buerk D. G., Barbee K. A. et al. 3D network model of NOtransport in tissue [J]. Medical and Biological Engineering Computing, 2011, 49(6): 633–647.
Liu X., Wang Z., Zhao P. et al. Nitric oxide transport in normal human thoracic aorta: effects of hemodynamics and nitric oxide scavengers [J]. Plos One, 2013, 9(11): e112395.
Mitchell D., Tyml K. Nitric oxide release in rat skeletal muscle capillary [J]. American Journal of Physiology, 1996, 270(5): H1696–1703.
Tsoukias N. M., Popel A. S. A model of nitric oxide capillary exchange [J]. Microcirculation, 2003, 10(6): 479–495.
Liu X., Fan Y., Xu X. Y. et al. Nitric oxide transport in an axisymmetric stenosis [J]. Journal of Royal Society Interface, 2012, 9(75): 2468–2478.
Fadel A. A., Barbee K. A., Jaron D. A computational model of nitric oxide production and transport in a parallel plate flow chamber [J]. Annals of Biomedical Engineering, 2009, 37(5): 943–954.
Plata A. M., Sherwin S. J., Krams R. Endothelial nitric oxide production and transport in flow chambers: The importance of convection [J]. Annals of Biomedical Engineering, 2010, 38(9): 2805–2816.
He Y., Liu H., Himeno R. A One-dimensional thermo-fluid model of blood circulation in the human upper limb [J]. International Journal of Heat and Mass Tranfer, 2004, 47(12): 2735–2745.
Nield D. A., Bejan A. Convection in porous media [M]. Berlin Heidelberg, Germany: Springer, 2013.
Azarov I., Huang K., Basu S. et al. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation [J]. Journal of Biological Chemistry, 2005, 280(47): 39024–39032.
Andrews A. M., Jaron D., Buerk D. G. et al. Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro [J]. Nitric Oxide, 2010, 23(4): 335–342.
He Y., Himeno R. Finite element analysis on fluid filtration in system of permeable curved capillary and tissue [J]. Journal of Mechanics in Medicine and Biology, 2012, 12(4): 271–276.
Pozrikidis C., Farrow D. A. A model of fluid flow in solid tumors [J]. Annals of Biomedical Engineering, 2003, 31(2): 181–194.
Hanspal N. S., Waghode A. N., Nassehi V. et al. Numerical Analysis of Coupled Stokes/Darcy Flows in Industrial Filtrations [J]. Transport in Porous Media, 2006, 64(1): 73–101.
Pozrikidis C. Stokes flow through a permeable tube [J]. Archive of Applied Mechanics, 2010, 80(4): 323–333.
Kanai A. J., Strauss H. C., Truskey G. A. et al. Shear stress induces ATP-independent transient nitric oxide release from vascular endothelial cells, measured directly with a porphyrinic microsensor [J]. Circulation Research, 1995, 77(2): 284–293.
Lu X., Kassab G. S. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production [J]. Journal of Biomechanics, 2004, 561(2): 575–582.
Lai S. S. M., Tang A. Y. S., Tsang A. C. O. et al. A joint computational-experimental study of intracranial aneurysms: Importance of the aspect ratio [J]. Journal of Hydrodynamics, 2016, 28(3): 462–472.
Shi X., Zhang S., Wang S. L. Numerical simulation of hemodynamic interactions of red blood cells in micro-capillary flow [J]. Journal of Hydrodynamics, 2014, 26(2): 178–186.
Liu X. G., Yu X. L., Li C. Y. et al. A microfluidic chip for co-culture of human vascular endothelial cells and smooth muscle cells [J]. Chinese Journal of Hydrodynamics, 2015, 30(6): 638–642(in Chinese).
Wang Y. X., Gao Z. M., Bo L. et al. A flow chamber device for simulating oscillatory shear stress in the common carotid artery after exercise trainingai][J]. Chinese Journal of Hydrodynamics, 2015, 30(6): 650–656(in Chinese).
Acknowledgements
This work was supported by the Dalian Science and Technology R & D Program (Grant No. 2015F11GH092), the Scientific Research Foundation of Liaoning Education Department (Grant No. L2015113).
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (Grant No. 51576033).
Rights and permissions
About this article
Cite this article
Wei, Yj., He, Y., Tang, Yl. et al. Finite element analysis of nitric oxide (NO) transport in system of permeable capillary and tissue. J Hydrodyn 30, 722–737 (2018). https://doi.org/10.1007/s42241-018-0076-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42241-018-0076-8