Abstract
Fire blight caused by Erwinia amylovora reached Kazakhstan in 2008. Here, the disease poses a threat not only to agricultural production of apples and pears, but also to the forests of wild Malus sieversii, the progenitor of most domesticated apple varieties worldwide. In the period 2019–2021, the spread of fire blight in the growth area of wild apples was limited by the weather conditions. In 2022, late spring and early summer were characterized by increased rainfall and moderate temperatures favorable for the disease. The goal of this study was to monitor the distribution of fire blight in private households and small orchards in the zones adjacent to wild apple distribution areas. A total of 91 samples with fire blight-compatible symptoms were collected from cultural apples (68), wild apple (10), pear (5), hawthorn (7), and quince (1) in south-eastern and eastern Kazakhstan, resulting in 21 isolates (one from pear, one from quince, and 19 from apple) of E. amylovora. All isolates belonged to the archetypal CRISPR genotype A. Considering the relative proximity of the infections to the forests of wild M. sieversii, additional measures for fire blight control and prevention will have to be implemented, including state monitoring of the wild apple forests for disease symptoms and awareness campaigns for specially protected natural territories that safeguard M. sieversii, as well as for local pomaceous-fruit growing communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fire blight caused by the bacterium Erwinia amylovora (Burrill) is a serious disease of apples, pears, and other plants of family Rosaceae, especially of the subfamily Maloideae (Van der Zwet & Keil 1979; Vanneste 2000). Since it was first described in the USA around 1780, fire blight has expanded its distribution area to New Zealand in 1920, reaching Europe in 1957 (Thomson 2000) and continuing its expansion to the east to Central Asia (Djaimurzina et al. 2014; Gaganidze et al. 2021; Kurz et al. 2021), while simultaneously spreading from North America to South-East Asia (Myung et al. 2016), adding new countries in its distribution area in the last decades.
The introduction of fire blight in Kazakhstan, first registered in 2008 (Drenova et al. 2012), was facilitated by massive import of planting material from Europe in the frame of the state program aimed at the increase of cultural apple plantations, which subsidized purchase of imported planting stock of fruit trees for intensive orchards’ development. Along with weak quarantine control of planting material for fire blight, the massive inflow of plants imported from countries with registered fire blight disease (over 10 million seedlings and stocks of apple, pear, and quince in 2008–2015) resulted in a significant spread of the disease in the Almaty fruit zone (Djaimurzina et al. 2014; Umiraliyeva et al. 2021). This, in turn, promoted development of state programs for fire blight monitoring and prevention, including stricter import border control and allocation of funds for chemical treatment (such as phytobacteriomycin) and reimbursements for cut trees. These efforts are ongoing, and in 2021, the state-funded chemical treatment of 728.5 hectares (ha) for fire blight prevention. At the same time, the roadmaps for 2021–2025 agricultural investment projects aim at further expansion of intensive orchards, approving 32 projects for a total of 100 billion tenge (about 2,150,000 US dollars) and creating another 866 ha of apple orchards in 2021 (https://primeminister.kz/ru/news/reviews/itogi-razvitiya-sfery-selskogo-hozyaystva-za-2021-god-i-plany-na-predstoyashchiy-period-22422). The increased demand for apple seedlings to cover such a large area exceeds the capacity of local plant nurseries to deliver the required numbers of plants, and the import of plant material increases the risk of fire blight in the upcoming years.
Whereas most countries have pome fruit trees only in orchards and gardens, in Kazakhstan, wild apple forests are present. The country is home to Malus sieversii, a wild apple species that is the progenitor of the domesticated apple (Cornille et al. 2012; Harris et al. 2002; Velasco et al. 2010). The species is listed as “vulnerable” in the Red List of Threatened Species of the International Union for Conservation of Nature (IUCN) (https://www.iucnredlist.org/species/32363/9693009) and its study and conservation attract both domestic and international attention, given the importance of safeguarding the original gene pool (Dzhangaliev 2007, 2010).
The relative proximity of the wild apple forests to the cultural pome fruit varieties increases the risk of fire blight spread to the wild trees, especially if mediated by birds, insects, and wind (Anonymous 2022), or by human activities in the adjoining areas. The threat of fire blight spreading to the wild apple forests needs to be considered and a regular monitoring of the situation is deemed essential (Djaimurzina et al. 2014; Doolotkeldieva et al. 2021; Feurtey et al. 2020; Umiraliyeva et al. 2021). Trees of M. sieversii may generally be more prone to this disease compared to cultural varieties as, due to the absence of the pathogen in this region, it could not build up a natural resistance. Therefore, some studies have shown increased severity of fire blight once E. amylovora successfully has infected trees of this species (Harshman et al. 2017; Luby et al. 2002).
Materials and methods
Field observations and sampling
The objects of study were culturally grown apple, pear, quince and hawthorn trees, and wild trees of M. sieversii. Geographic coordinates were determined with Garmin ETREX 32x (Vancouver, WA, USA). Maps were created using BatchGeo software (Vancouver, WA, USA).
Observations of trees for fire blight symptoms were carried out on all four sides of the tree crown in two diagonals of the forest/orchard or on separate tree stands. The crowns, trunks, branches, and flowers of the trees were carefully examined for the presence of fire blight-compatible symptoms. In the case of medium- or large-scale cultural plantations, 10–20 trees were inspected. In small plantations and private households with less than 10 trees, all of the trees were inspected.
Symptoms were evaluated on a 6-point scale: 0 healthy tree; 0.1 barely visible signs of illness; 1 the initial stage of disease manifestation (single wilting and blackening of the flowers, twisting, and browning of shoots and leaves are noticeable); 2 flowers, shoots, and leaves are affected more than 10%; 3 damage to the bark of branches, trunks, fruits (bacterial exudate is released on the affected areas); 4 more than 75% of the crown is burnt, as after a fire; 5 tree dead from disease.
The percent of distribution (frequency of occurrence) of the disease was calculated by the following formula:
where P referred to the prevalence or frequency of disease, %; H number of infected trees; N number of studied trees
The degree of disease development is calculated by the following formula:
where R referred to the level of disease development, %; Ʃ the sum of multiplication of a and b; a the number of trees with same disease lesions; b lesion score corresponding to this symptom; N total number of trees; and K the highest score of the lesion scale.
Bacteriology and immunochromatography
Samples for laboratory analyses (flowers, shoots, fruitlets, stem segments) were taken from symptomatic and asymptomatic trees and processed according to standard on diagnostics of the European and Mediterranean Plant Protection Organization (EPPO) (Anonymous 2022). Asymptomatic samples were enriched in liquid King’s B media (King et al. 1954) prior to plating and serological/molecular tests. Enriched cultures were plated on King’s B solid medium and NSA medium in accordance with the same international guidelines.
Immunochromatographic tests were conducted with the Ea AgriStrip (Bioreba, Reinach, Switzerland) (Braun-Kiewnick et al. 2011) according to manufacturer’s instructions. Nucleic acids were extracted with the EasyPure Bacteria Genomic DNA Kit (TransGen Biotech, Beijing, China) according to manufacturer’s instructions.
Immature pear fruit assays were carried out according to the method described by Doolotkeldieva et al. (Doolotkeldieva et al. 2021). In short, immature pear fruits (4–5 cm in diameter) were surface sterilized with 0.5% sodium hypochlorite and thoroughly rinsed in water, then cut in 3–4 mm slices and inoculated with 10 µl of 106 cells ml−1 pure culture suspension. Results were observed 4–5 days after growth in a humid chamber at 25 °C, subcultured on agar and re-checked with PCR.
Molecular test and CRISPR genotyping
Molecular tests were carried out with the protocols of Stöger et al. (2006) for conventional polymerase chain reaction (PCR) on an Eppendorf Mastercycler X50s (Eppendorf, Hamburg, Germany) and the protocol of Pirc et al. (2009) for real-time PCR on QuantStudio 5 (Applied Biosystems, Waltham, MA, USA).
For characterization of the strains, we used the approach that targets spacer 1029 in CRISPR repeat region 1 (CRR1) and allows discrimination between two ancestral populations first colonizing Europe in a single PCR assay (Kurz et al. 2021). The list of primers used for the study is provided in Table 1.
Electrophoresis of the PCR products was conducted on 1.5% agarose gels and visualized using a BioRad Gel-Doc XR + gel-documenting system (Bio-Rad, Hercules, CA, USA).
Results
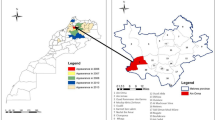
The phytopathological observations were conducted in three regions (oblasts) and one city of Kazakhstan in small and medium size orchards (not larger than 25 ha), private households, public locations, and in stands and forests of M. sieversii in Ile-Alatau and Tarbagatai state national nature parks (Table 2). Samples with symptoms not excluding fire blight (brown and black shoots, flowers and fruitlets, bended shoot tips—“shepherds crook”), or asymptomatic in case of wild apple and hawthorn trees, were collected from each site. A total of 91 samples were collected, including 68 samples from cultural apple, ten from wild apple (M. sieversii), five from pear, seven from hawthorn, and one from quince (Table 3). The collected samples could be clustered into four major sampling areas, being the greater Almaty area, Zharkent area, Zhongar area, and Tarbagatai area (Fig. 1A).
Symptoms such as shoot darkening and bending (“shepherds crook”), wilting, and fruitlets mummification were recorded. These symptoms are compatible with fire blight, so all the samples showing at least one of these symptoms were collected for further laboratory confirmation. The 21 samples yielding colonies with distinct E. amylovora characteristics (milky white or yellowish domed, circular, smooth colonies on levan medium while forming white mucoid colonies on King’s B agar with no fluorescence under UV light) were subcultured to obtain pure cultures (Fig. 2).
Among the isolates obtained in this study, one originated from pear, one from quince, and the remaining 19 isolates were sampled from cultivated apple (Table 4). Both quince and pear were collected in Almaty city. Three isolates from apple host were obtained from Zharkent sampling area and the other sixteen from the greater Almaty area (Fig. 1B). No isolates nor positive molecular and bacteriological results were obtained for samples from Zhongar and Tarbagatai sampling areas.
After the species was positively identified as E. amylovora by classic PCR and immunochromatographic methods (Bioreba Agristrip test; Table 4), the pure cultures were checked for pathogenicity using the immature pear fruitlet assay. All pure cultures with positive results shown by molecular and bacteriologic methods provided positive immature pear fruitlet results, forming milky ooze, which was re-tested with molecular methods and further prepared for long-term storage.
The phylogenetic studies of E. amylovora have shown high genetic homogeneity within isolates of the widely prevalent group (Kurz et al. 2021; Parcey et al. 2020; Zeng et al. 2018). One of the approaches in the characterization of strains is the analysis of hypervariable regions in clustered regularly interspaced short palindromic repeats (CRISPR) (Rezzonico et al. 2011). The analysis of the isolates collected in southeastern Kazakhstan in 2022 revealed that all samples belong to archetypal genotype A like strain CFBP 1430 (Fig. 3), indicating that all strains have the duplication of CRISPR spacer 1029 in CRR1 as observed before in Kazakhstan and in the surrounding Central Asian countries (Doolotkeldieva et al. 2021; Kurz et al. 2021).
Discussion
Fire blight of apples and other pome fruits is present in Kazakhstan since about a decade. The initial unpreparedness and lack of awareness were partially compensated by a set of legal acts that strengthened import control and provided fruit growers with state-funded fire blight treatment preparations and reimbursement opportunities in case a tree had to be eradicated to prevent further spread of the disease. Nevertheless, an awareness-raising campaign is still needed for owners of small orchard and private households, which are sometimes overlooked in more agricultural production-oriented state programs.
Unlike the past several years, the first half of 2022 was favorable for fire blight development due to weather conditions, and the disease was detected in various locations not too far from the growth zone of wild apples. The distance of 20–30 km that separates the infected trees from their wild relatives still leaves some room for action. Although no wild apple and hawthorn trees observed in this study showed any symptoms, urgent measures need to be developed to not let this distance decrease with time. In accordance with the state law, there are 2-km buffer zones surrounding the protected areas, but these may be largely insufficient in case of a pathogen such as E. amylovora that is able to cross these relatively short distances with the help of insects and birds. One underestimated threat is constituted by the local population that can dramatically shorten the distance between infected and healthy zones by carrying the pathogen on themselves (via infected fruits or twigs, clothes, utensils, etc.). This is also the reason a special awareness-raising campaign and training is needed inter alia for the rangers in the specially protected natural territories aimed at the safeguard of wild apple trees. The preparedness of these specialists is key to monitoring and timely prevention of fire blight spread.
The location of the E. amylovora-positive samples around Almaty was quite predictable. Almaty is the largest city of Kazakhstan situated in the apple-growing zone with many people purchasing imported apple trees for personal use in their households and summer houses. These results also correspond to the analysis of large fire blight foci distribution in commercial orchards in 2018 (Umiraliyeva et al. 2021), where the majority of the foci was registered in the commercial orchards in Almaty city area. On the other hand, the Zhongar and Tarbagatai areas are not as densely populated and the local population has less opportunities to purchase imported plant material for private households and small orchards, thus halting the spread of the disease to these areas.
Only 21 out of 91 samples proved that the observed symptoms were due to infection with E. amylovora. This is explained by the strategy of the study, where all the samples are taken with the symptoms that could not exclude fireblight, even if they could be attributed to other diseases and factors, in order to not miss the disease.
Archetypal genotype A, found in all the isolates, can help in speculatively tracking the origin of those isolates to northwestern Europe or the Mediterranean area, where this genotype prevails (Kurz et al. 2021) and from where large batches of planting material were imported. To date, mostly A-derived genotypes were registered in Kazakhstan (Doolotkeldieva et al. 2021; Kurz et al. 2021), congruent to the results of the current study. Nevertheless, evidence is available that archetypal genotype D was present in South Kazakhstan as early as 2014 (Rezzonico, Drenova and Smits, unpublished results).
Although the country promptly reacted to the fire blight introduction, further introduction events and spread of the disease within the orchards of small farmers and household owners have been and will be inevitable due to massive import of improperly tested planting material. It is crucial to protect the wild apple forests against the potential threat that fire blight is posing, and special prevention efforts should be taken timely.
Data availability statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary material; additional data is available from the corresponding author on reasonable request.
References
Anonymous. (2022) PM 7/20 (3) Erwinia amylovora. EPPO Bulletin 52:198–224. https://doi.org/10.1111/epp.12826
Braun-Kiewnick A, Altenbach D, Oberhänsli T, Bitterlin W, Duffy B (2011) A rapid lateral-flow immunoassay for phytosanitary detection of Erwinia amylovora and on-site fire blight diagnosis. J Microbiol Methods 87(1):1–9. https://doi.org/10.1016/j.mimet.2011.06.015
Cornille A, Gladieux P, Smulders MJM, Roldán-Ruiz I, Laurens F, Le Cam B, Nersesyan A, Clavel J, Olonova M, Feugey L, Gabrielyan I, Zhang XG, Tenaillon MI, Giraud T (2012) New insight into the history of domesticated apple: Secondary contribution of the European wild apple to the genome of cultivated varieties. PLoS Genetics 8(5):e1002703. https://doi.org/10.1371/journal.pgen.1002703
Djaimurzina A, Umiralieva Z, Zharmukhamedova G, Born Y, Bühlmann A, Rezzonico F (2014) Detection of the causative agent of fire blight - Erwinia amylovora (Burrill) Winslow et al. - In the Southeast of Kazakhstan. Acta Horticulturae 1056:129–132. https://doi.org/10.17660/ActaHortic.2014.1056.18
Doolotkeldieva T, Bobushova S, Carnal S, Rezzonico F (2021) Genetic characterization of Erwinia amylovora isolates detected in the wild walnut-fruit forest of South Kyrgyzstan. J Plant Pathol 103:109–120. https://doi.org/10.1007/s42161-021-00752-1
Drenova N, Isin M, Dzhaimurzina A, Zharmukhamedova G, Aitkulov A (2012) Bacterial fire blight in the Republic of Kazakhstan. Plant Health Research and Practice 1(3):44–48
Dzhangaliev AD (2007) Unique and global importance of gene fund of apple forests of Kazakhstan. Reports of the National Academy of Sciences of the Republic of Kazakhstan 5:41–27
Dzhangaliev AD (2010) The wild apple tree of Kazakhstan. Hortic Rev (29). https://doi.org/10.1002/9780470650868.ch2
Feurtey A, Guitton E, De Gracia Coquerel M, Duvaux L, Shiller J, Bellanger MN, Expert P, Sannier M, Caffier V, Giraud T, Le Cam B, Lemaire C (2020) Threat to Asian wild apple trees posed by gene flow from domesticated apple trees and their “pestified” pathogens. Mol Ecol 29(24):4925–4941. https://doi.org/10.1111/mec.15677
Gaganidze D, Sadunishvili T, Aznarashvili M, Abashidze E, Gurielidze M, Carnal S, Rezzonico F, Zubadalashvili M (2021) Fire blight distribution in Georgia and characterization of selected Erwinia amylovora isolates. J Plant Pathol 103:121–129. https://doi.org/10.1007/s42161-020-00700-5
Harris SA, Robinson JP, Juniper BE (2002) Genetic clues to the origin of the apple. Trends Genet 18(8):426–430. https://doi.org/10.1016/S0168-9525(02)02689-6
Harshman JM, Evans KM, Allen H, Potts R, Flamenco J, Aldwinckle HS, Wisniewski ME, Norelli JL (2017) Fire blight resistance in wild accessions of Malus sieversii. Plant Dis 101(10):1738–1745. https://doi.org/10.1094/PDIS-01-17-0077-RE
King EO, Ward M, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Kurz M, Carnal S, Dafny-Yelin M, Mairesse O, Gottsberger RA, Ivanović M, Grahovac M, Lagonenko AL, Drenova N, Zharmukhamedova G, Doolotkeldieva T, Smits THM, Rezzonico F (2021) Tracking the dissemination of Erwinia amylovora in the Eurasian continent using a PCR targeted on the duplication of a single CRISPR spacer. Phytopathology Research, 3(1):18. https://doi.org/10.1186/s42483-021-00096-9
Luby JJ, Alspach PA, Bus VGM, Oraguzie NC (2002) Field resistance to fire blight in a diverse apple (Malus sp.) germplasm collection. Journal of the American Society for Horticultural Science, 127(2):245–253. https://doi.org/10.21273/jashs.127.2.245
Myung IS, Lee JY, Yun MJ, Lee YH, Lee YK, Park DH, Oh CS (2016) Fire blight of apple, caused by Erwinia amylovora, a new disease in Korea. Plant Dis 100(8):1774. https://doi.org/10.1094/PDIS-01-16-0024-PDN
Parcey M, Gayder S, Morley-Senkler V, Bakkeren G, Úrbez-Torres JR, Ali S, Castle AJ, Svircev AM (2020) Comparative genomic analysis of Erwinia amylovora reveals novel insights in phylogenetic arrangement, plasmid diversity, and streptomycin resistance. Genomics 112(5):3762–3772. https://doi.org/10.1016/j.ygeno.2020.04.001
Pirc M, Ravnikar M, Tomlinson J, Dreo T (2009) Improved fireblight diagnostics using quantitative real-time PCR detection of Erwinia amylovora chromosomal DNA. Plant Pathol 58(5):872–881. https://doi.org/10.1111/j.1365-3059.2009.02083.x
Rezzonico F, Smits THM, Duffy B (2011) Diversity, evolution, and functionality of clustered regularly interspaced short palindromic repeat (CRISPR) regions in the fire blight pathogen Erwinia amylovora. Appl Environ Microbiol 77(11):3819–3829. https://doi.org/10.1128/AEM.00177-11
Stöger A, Schaffer J, Ruppitsch W (2006) A rapid and sensitive method for direct detection of Erwinia amylovora in symptomatic and asymptomatic plant tissues by polymerase chain reaction. J Phytopathol 154(7–8):469–473. https://doi.org/10.1111/j.1439-0434.2006.01130.x
Thomson S (2000) Epidemiology of fire blight. In Fire blight: the disease and its causative agent, Erwinia amylovora 9–36. CAB International
Umiraliyeva ZZ, Kopzhassarov BK, Jaimurzina AA, Niyazbekov ZB, Issenova GZ, Tursunova AK, Berganayeva GE (2021) Epidemiology of fire blight in fruit crops in Kazakhstan. Agrivita Journal of Agricultural Science 43(2), 273–284. https://doi.org/10.17503/agrivita.v43i2.2674
Van der Zwet T, Keil H (1979) Fire blight. Government Printing Office, A bacterial disease of rosaceous plants. USA
Vanneste J (2000) Fire blight: the disease and its causative agent. CABI, Erwinia amylovora
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, … Viola R (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet, 42(10):833–839. https://doi.org/10.1038/ng.654
Zeng Q, Cui Z, Wang J, Childs KL, Sundin GW, Cooley DR, Yang CH, Garofalo E, Eaton A, Huntley RB, Yuan X, Schultes NP (2018) Comparative genomics of Spiraeoideae-infecting Erwinia amylovora strains provides novel insight to genetic diversity and identifies the genetic basis of a low-virulence strain. Mol Plant Pathol 19(7):1652–1666. https://doi.org/10.1111/mpp.12647
Funding
Open access funding provided by ZHAW Zurich University of Applied Sciences. This work was funded by Ministry of Education and Science of the Republic of Kazakhstan Program OR11465447 “Development of highly sensitive molecular biological and biochemical tests for the detection of pathogens affecting the consumer qualities of the final product, based on monitoring of pathogens of agricultural crops,” the Life Science and Facility Management Department at Zurich University for Applied Sciences (ZHAW), and the Swiss National Science Foundation (SNSF) research project “Preservation of Central Asian fruit tree forest ecosystems, pome fruit varieties and germplasm from the recent epidemics caused by the invasive bacterial pathogen Erwinia amylovora (fire blight)” (Project No. IZ08Z0_177515).
Author information
Authors and Affiliations
Contributions
The list of authors with their background and role in the manuscript preparation is as follows: Elina R. Maltseva, molecular biologist, writing of the manuscript, and overall supervision of the study; Galiya A. Zharmukhamedova, phytopathologist, responsible for phytopathologic assessment, and sample collection; Zhulduzay K. Jumanova, phytopathologist, responsible for phytopathologic assessment, and sample collection; Dinara A. Naizabayeva, molecular biologist, responsible for laboratory analysis (PCR and real-time PCR); Zhanna A. Berdygulova, molecular biologist, responsible for GIS data, and mapping; Karina A. Dmitriyeva, technician, responsible for sample collection and processing in the laboratory; Botakoz Tezekbayeva, technician, responsible for bacteriological and immunochromatographic analysis; Altyn Khassein, technician, responsible for bacteriological analysis and conventional PCR. Yuriy A. Skiba, molecular biologist, responsible for laboratory analyses; Natalya P. Malakhova, molecular biologist, responsible for laboratory analyses; Gulnara Ismagulova, molecular biologist, responsible for manuscript processing and correction; Fabio Rezzonico, molecular biologist, molecular phytopathologist, project manager of the SNF project, supervision of all stages of the study; Theo H.M. Smits, molecular biologist, sequencing specialist, principal investigator of the SNF project, revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maltseva, E.R., Zharmukhamedova, G.A., Jumanova, Z.K. et al. Fire blight cases in Almaty Region of Kazakhstan in the proximity of wild apple distribution area. J Plant Pathol (2023). https://doi.org/10.1007/s42161-023-01416-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-023-01416-y