Abstract
Many models of decision-making assume accumulation of evidence to threshold as a core mechanism to predict response probabilities and response times. A spiking neural network model (Wang, 2002) instantiates these mechanisms at the level of biophysically-plausible pools of neurons with excitatory and inhibitory connections and has numerous model parameters tuned by physiological measures. The diffusion model (Ratcliff, 1978) is a cognitive model that can be fitted to a range of behaviors and conditions. We investigated how parameters of the cognitive-level diffusion model relate to the parameters of a neural-level spiking model. In each simulated “experiment,” we generated “data” from the spiking neural network by factorially combining a manipulation of choice difficulty (via the input to the spiking model) and a manipulation of one of the core parameters of the spiking model. We then fitted the diffusion model to these simulated data to observe how manipulation of each core spiking model parameter mapped on to fitted drift rate, response threshold, and non-decision time. Manipulations of parameters in the spiking model related to input sensitivity, threshold, and stimulus processing time mapped on to their conceptual analogues in the diffusion model, namely drift rate, threshold, and non-decision time. Manipulations of parameters in the spiking model with no direct analogue to the diffusion model, non-stimulus-specific background input, strength of recurrent excitation, and receptor conductances mapped on to threshold in the diffusion model. We discuss implications of these results for interpretations of fits of the diffusion model to behavioral data.






Similar content being viewed by others
Data Availability
Simulation code is available from the authors upon request.
Notes
Variously called the diffusion decision modeling or drift diffusion model (DDM), the Ratcliff diffusion model, or most simply just the diffusion model (of decision-making).
With noise (variability) originating from sources that are internal, external, or both, with variability arising within a single decision (within-trial variability), across decisions (between-trial variability), or both.
While the spiking neural network model was applied to data from a motion discrimination task, the model does not instantiate motion-detection circuits in extrastriate visual cortex, nor does it take a motion signal as input. Rather, different levels of motion coherence simply map onto different numeric levels of input to the spiking neural network model. We can think of the model as generically simulating choice decision-making across various levels of difficulty rather than specifically simulating performance in a motion discrimination task per se.
Quasi-independent in the sense that they could simulate difference between different subjects (for example, caused by differences in genetics, development, IQ, sex, age, experience, disease, damage, and whole host of other factors not manipulated by the experimenter).
Stimulus processing time (Ts) reflects both afferent conductance delays from the retinal stimulation and time needed to process the stimulus input to drive the appropriate choice-selective pools.
Using a slightly larger or smaller window size did not qualitatively change model simulations.
So-called “degenerate” distributions asymptote at the rate of correct or error proportion in each condition, rather than asymptote at 1.
For drift rate, we compared the linear assumption described in the text with a more complex model assuming a separate free drift rate parameter for each motion coherence condition; for threshold, we compared the constant value assumption described in the text with a more complex model assuming a separate free threshold parameter for each motion coherence condition. Nested model comparisons were conducted using the same BIC-based methods described later in this article.
This is a common approach to maximum likelihood parameter estimation for models that predict response time (see Heathcote et al., 2002; Farrell & Lewandowsky, 2018 for details). Observed RT distributions are defined in terms of quantile bins, and model predictions are evaluated based on how well the predicted probabilities account for the observed frequencies with which responses fall into each RT bin. This essentially turns a continuous data space into a discrete data space using a multinomial distribution to link model predicted probabilities to observed frequencies and amounts to evaluating a model based on its fit to the (defective) cumulative RT distribution functions (see Van Zandt, 2000). While the diffusion model does predict probability density functions for RT, fitting the cumulative distribution function helps to mitigate against the overinfluence that outliers (overly fast or overly slow individual RTs) can have on likelihood using a pdf approach; for example, just a single observed RT from one trial out of thousands that falls below the minimum possible RT predicted by a set of diffusion model parameters can send the log likelihood to negative infinity using a pdf approach.
We chose to compare the fit of the unrestricted (all-free) model with the fit of each restricted (one-fixed) model to mirror a common model comparison approach whereby adding a parameter constraint (in this case fixing the value of one free parameter across conditions) allows the modeler to ask whether flexibility in that parameter is necessary to maintain an adequate fit to the observed data (in other words, does the added parameter constraint “break” the model, producing a significantly worse fit; for example, see Farrell and Lewandowsky, 2018).
References
Abbott, L. F. (1999). Lapicque’s introduction of the integrate-and-fire model neuron (1907). Brain Research Bulletin, 50(5–6), 303–304.
Boehm, U., Annis, J., Frank, M.J., Hawkins, G.E., Heathcote, A., Kellen, D., ... & Wagenmakers, E.J. (2018). Estimating across-trial variability parameters of the diffusion decision model: Expert advice and recommendations. Journal of Mathematical Psychology, 87, 46-75
Bogacz, R., Wagenmakers, E.-J., Forstmann, B. U., & Nieuwenhuis, S. (2009). The neural basis of the speed–accuracy tradeoff. Trends in Neuroscience, 33, 10–16.
Bogacz, R., Brown, E., Moehlis, J., Holmes, P., & Cohen, J. D. (2006). The physics of optimal decision making: A formal analysis of models of performance in two-alternative forced-choice tasks. Psychological Review, 113, 700–765.
Boucher, L., Palmeri, T. J., Logan, G. D., & Schall, J. D. (2007). Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychological Review, 114, 376–397.
Britten, K. H., Shadlen, M. N., Newsome, W. T., & Movshon, J. A. (1992). The analysis of visual motion: A comparison of neuronal and psychophysical performance. Journal of Neuroscience, 12, 4745–4765.
Brown, S. D., & Heathcote, A. (2008). The simplest complete model of choice response time: Linear ballistic accumulation. Cognitive Psychology, 57, 153–178.
Brunel, N., & Wang, X.-J. (2001). Effects of neuromodulation in a cortical network model. Journal of Computational Neuroscience, 11, 63–85.
Busemeyer, J.R., Townsend, J.T., Wang, Z.J. , & Eidels, A. (Eds.) (2015). Mathematical and computational models of cognition. Oxford University Press.
Carandini, M. (2012). From circuits to behavior: A bridge too far? Nature Neuroscience, 15, 507–509.
Cepeda, C., Buchwald, N. A., & Levine, M. S. (1993). Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proceedings of the National Academy of Sciences, 90(20), 9576–9580.
Cisek, P., Puskas, G. A., & El-Murr, S. (2009). Decisions in changing conditions: The urgency-gating model. Journal of Neuroscience, 29(37), 11560–11571.
Churchland, A. K., Kiani, R., & Shadlen, M. N. (2008). Decision-making with multiple alternatives. Nature Neuroscience, 11, 693–702.
Cox, G.E., Palmeri, T.J. Logan, G.D., Smith, P.L., Schall, J.D. (in press). Salience by competitive and recurrent interactions: Bridging neural spiking and computation in visual attention. Psychological Review. PsyArXiv: https://psyarxiv.com/rkh8g/
Cox, G.E., Lilburn, S., Logan, G.D., Schall, J.D., Zandbelt, B., & Palmeri, T.J. (under revision). Decision making by ensembles of accumulators. Manuscript under revision. PsyArXiv: https://psyarxiv.com/qdk7b/
Dayan, P., & Abbott, L. F. (2005). Theoretical neuroscience: Computational and mathematical modeling of neural systems. MIT Press.
de Lafuente, V., Jazayeri, M., & Shadlen, M. N. (2015). Representation of accumulating evidence for a decision in two parietal areas. Journal of Neuroscience, 35(10), 4306–4318.
Donkin, C., Brown, S., Heathcote, A., & Wagenmakers, E.-J. (2011). Diffusion versus linear ballistic accumulation: Different models but the same conclusions about psychological processes? Psychonomic Bulletin & Review, 18, 61–69.
Farrell, S., & Lewandowsky, S. (2018). Computational modeling of cognition and behavior. Cambridge University Press.
Forstmann, B. U., Dutilh, G., Brown, S., Neumann, J., Von Cramon, D. Y., Ridderinkhof, K. R., & Wagenmakers, E. J. (2008). Striatum and pre-SMA facilitate decision-making under time pressure. Proceedings of the National Academy of Sciences, 105, 17538–17542.
Forstmann, B. U., Ratcliff, R., & Wagenmakers, E. J. (2016). Sequential sampling models in cognitive neuroscience: Advantages, applications, and extensions. Annual Review of Psychology, 67, 641–666.
Forstmann, B. U., Wagenmakers, E.-J., Eichele, T., Brown, S., & Serences, J. T. (2011). Reciprocal relations between cognitive neuroscience and formal cognitive models: Opposites attract? Trends in Cognitive Sciences, 15(6), 272–279.
Frank, M. J. (2015). Linking across levels of computation in model-based cognitive neuroscience. In B. Forstmann & E. J. Wagenmakers (Eds.), An Introduction to Model-Based Cognitive Neuroscience (pp. 159–177). Springer Neuroscience.
Frank, M. J., & Claus, E. D. (2006). Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review, 113(2), 300–326.
Gold, J. I., & Shadlen, M. N. (2007). The neural basis of decision making. Annual Review of Neuroscience, 30, 535–560.
Hanes, D. P., & Schall, J. D. (1996). Neural control of voluntary movement initiation. Science, 274, 427–430.
Hawkins, G. E., Forstmann, B. U., Wagenmakers, E. J., Ratcliff, R., & Brown, S. D. (2015). Revisiting the evidence for collapsing boundaries and urgency signals in perceptual decision-making. Journal of Neuroscience, 35(6), 2476–2484.
Heathcote, A., Brown, S., & Mewhort, D. J. K. (2002). Quantile maximum likelihood estimation of response time distributions. Psychonomic Bulletin & Review, 9(2), 394–401.
Heitz, R. P. (2014). The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Frontiers in Neuroscience, 8, 150.
Heitz, R. P., & Schall, J. D. (2012). Neural mechanisms of speed-accuracy tradeoff. Neuron, 76, 616–628.
Jones, M., & Love, B. C. (2011). Bayesian fundamentalism or enlightenment? On the explanatory status and theoretical contributions of Bayesian models of cognition. Behavioral and Brain Sciences, 34, 169–231.
Link, S. W. (1975). The relative judgment theory of two choice response time. Journal of Mathematical Psychology, 12(1), 114–135.
Lo, C.-C., Wang, C. T., & Wang, X. J. (2015). Speed-accuracy tradeoff by a control signal with balanced excitation and inhibition. Journal of Neurophysiology, 114(1), 650–661.
Logan, G.D., Schall, J.D., & Palmeri, T.J. (2015). Inhibitory control in mind and brain: The mathematics and neurophysiology of the underlying computation. In B. Forstmann & E.J. Wagenmakers (Eds.), An introduction to model-based cognitive neuroscience, Springer Neuroscience.
Love, B. C. (2015). The algorithmic level is the bridge between computation and brain. Topics in Cognitive Science, 7(2), 230–242.
Marr, D. (1982). Vision: A computational investigation into the human representation and processing of visual information. W.H. Freeman and Company, New York.
Mazurek, M. E., Roitman, J. D., Ditterich, J., & Shadlen, M. N. (2003). A role for neural integrators in perceptual decision making. Cerebral Cortex, 13, 1257–1269.
Montague, P. R., Dolan, R. J., Friston, K. J., & Dayan, P. (2012). Computational psychiatry. Trends in Cognitive Sciences, 16, 72–80.
Nelder, J. A., & Mead, R. (1965). A simplex method for function optimization. The Computer Journal, 7(4), 308–313.
Nosofsky, R. M., & Palmeri, T. J. (1997). An exemplar-based random walk model of speeded classification. Psychological Review, 104, 266–300.
Nosofsky, R.M., & Palmeri, T.J. (2015). Exemplar-based random walk model. To appear in J.R. Busemeyer, J. Townsend, Z.J. Wang, & A. Eidels (Eds.), Mathematical and computational models of cognition, Oxford University Press.
Oaksford, M., & Chater, N. (2007). Bayesian rationality: The probabilistic approach to human reasoning. Oxford University Press.
O’Connell, R. G., Dockree, P. M., & Kelly, S. P. (2012). A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nature Neuroscience, 15, 1729–1735.
O’Connell, R. G., Shadlen, M. N., Wong-Lin, K., & Kelly, S. P. (2018). Bridging neural and computatio nal viewpoints on perceptual decision-making. Trends in Neurosciences, 41(11), 838–852. Chicago.
Palmer, J., Huk, A. C., & Shadlen, M. N. (2005). The effect of stimulus strength on the speed and accuracy of a perceptual decision. Journal of Vision, 5(5), 376–404.
Palmeri, T. J. (1997). Exemplar similarity and the development of automaticity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 23, 324–354.
Palmeri, T. J., Love, B. C., & Turner, B. M. (2017). Model-based cognitive neuroscience. Journal of Mathematical Psychology, 76, 59–64.
Palmeri, T.J., Schall, J.D. & Logan, G.D. (2015). Neurocognitive modelling of perceptual decisions. To appear in J.R. Busemeyer, J. Townsend, Z.J. Wang, & A. Eidels (Eds.), Oxford handbook of computational and mathematical psychology, Oxford University Press.
Philiastides, M. G., Ratcliff, R., & Sajda, P. (2006). Neural representation of task difficulty and decision making during perceptual categorization: A timing diagram. Journal of Neuroscience, 26(35), 8965–8975.
Purcell, B. A., Heitz, R. P., Cohen, J. Y., Schall, J. D., Logan, G. D., & Palmeri, T. J. (2010). Neurally-constrained modeling of perceptual decision making. Psychological Review, 117, 1113–1143.
Purcell, B. A., & Kiani, R. (2016). Neural mechanisms of post-error adjustments of decision policy in parietal cortex. Neuron, 89, 658–671.
Purcell, B. A., & Palmeri, T. J. (2017). Relating accumulator model parameters and neural dynamics. Journal of Mathematical Psychology, 76, 156–171.
Purcell, B. A., Schall, J. D., Logan, G. D., & Palmeri, T. J. (2012). From salience to saccades: Multiple-alternative gated stochastic accumulator model of visual search. Journal of Neuroscience, 32(10), 3433–3446.
Ratcliff, R. (1978). A theory of memory retrieval. Psychological Review, 85, 59–108.
Ratcliff, R. (1979). Group reaction time distributions and an analysis of distribution statistics. Psychological Bulletin, 86, 446–461.
Ratcliff, R. (2006). Modeling response signal and response time data. Cognitive Psychology, 53, 195–237.
Ratcliff, R., Cherian, A., & Segraves, M. (2003). A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. Journal of Neurophysiology, 90, 1392–1407.
Ratcliff, R., & Frank, M. J. (2012). Reinforcement-based decision making in corticostriatal circuits: Mutual constraints by neurocomputational and diffusion models. Neural Computation, 24, 1186–1229.
Ratcliff, R., Hasegawa, Y. T., Hasegawa, R. P., Smith, P. L., & Segraves, M. A. (2007). Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. Journal of Neurophysiology, 97, 1756–1774.
Ratcliff, R., & McKoon, G. (2008). The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation, 20(4), 873–922.
Ratcliff, R., & Rouder, J. N. (1998). Modeling response times for two-choice decisions. Psychological Science, 9, 347–356.
Ratcliff, R., & Smith, P. L. (2004). A comparison of sequential sampling models for two-choice reaction time. Psychological Review, 111, 333–367.
Ratcliff, R., Thapar, A., & McKoon, G. (2006). Aging and individual differences in rapid two-choice decisions. Psychonomic Bulletin and Review, 13, 626–635.
Ratcliff, R., Thapar, A., & McKoon, G. (2010). Individual differences, aging, and IQ in two-choice tasks. Cognitive Psychology, 60, 127–157.
Ratcliff, R., & Tuerlinckx, F. (2002). Estimating parameters of the diffusion model: Approaches to dealing with contaminant reaction times and parameter variability. Psychonomic Bulletin & Review, 9, 438–481.
Roitman, J. D., & Shadlen, M. N. (2002). Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. Journal of Neuroscience, 22, 9475–9489.
Rolls, E. T., & Deco, G. (2011). A computational neuroscience approach to schizophrenia and its onset. Neuroscience & Biobehavioral Reviews, 35, 1644–1653.
Schall, J. D. (2001). Neural basis of deciding, choosing and acting. Nature Reviews Neuroscience, 2, 33–42.
Schall, J. D. (2004). On building a bridge between brain and behavior. Annual Review of Psychology, 55, 23–50.
Schall, J. D., Purcell, B. A., Heitz, R. P., Logan, G. D., & Palmeri, T. J. (2011). Neural mechanisms of saccade target selection: Gated accumulator model of visual-motor cascade. European Journal of Neuroscience, 33, 1991–2002.
Schurger, A., Sitt, J. D., & Dehaene, S. (2012). An accumulator model for spontaneous neural activity prior to self-initiated movement. Proceedings of the National Academy of Sciences, 109, 2904–2913.
Schwarz, G. (1978). Estimating the dimension of a model. The Annals of Statistics, 6(2), 461–464.
Servant, M., Tillman, G., Schall, J. D., Logan, G. D., & Palmeri, T. J. (2019). Neurally constrained modeling of speed-accuracy tradeoff during visual search: Gated accumulation of modulated evidence. Journal of Neurophysiology, 121, 1300–1314.
Shadlen, M. N., & Newsome, W. T. (2001). Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. Journal of Neurophysiology, 86(4), 1916–1936.
Shen, J., & Palmeri, T. J. (2016). Modeling individual differences in visual categorization. Visual Cognition, 24, 260–283.
Smith, P. L. (2010). From Poisson shot noise to the integrated Ornstein-Uhlenbeck process: Neurally principled models of information accumulation in decision-making and response time. Journal of Mathematical Psychology, 54, 266–283.
Smith, P. L., & Ratcliff, R. (2004). Psychology and neurobiology of simple decisions. Trends in Neuroscience, 27, 161–168.
Sternberg, S. (1998). Discovering mental processing stages: The method of additive factors. Methods, models, and conceptual issuesIn D. Scarborough & S. Sternberg (Eds.), Invitation to cognitive science (Vol. 4, pp. 703–863). MIT Press.
Sun, R. (Ed.). (2008). The Cambridge handbook of computational psychology. Cambridge University Press.
Tenenbaum, J. B., Kemp, C., Griffiths, T. L., & Goodman, N. D. (2011). How to grow a mind: Statistics, structure, and abstraction. Science, 331(6022), 1279–1285.
Treue, S., & Maunsell, J. H. R. (1999). Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. Journal of Neuroscience, 19, 7591–7602.
Tuerlinckx, F. (2004). The efficient computation of the cumulative distribution and probability density functions in the diffusion model. Behavior Research Methods, Instruments & Computers, 36(4), 702–716.
Turner, B. M., Forstmann, B. U., Love, B. C., Palmeri, T. J., & Van Maanen, L. (2017). Approaches to analysis in model-based cognitive neuroscience. Journal of Mathematical Psychology, 76, 65–79.
Turner, B. M., Forstmann, B. U., Wagenmakers, E. J., Brown, S. D., Sederberg, P. B., & Steyvers, M. (2013). A Bayesian framework for simultaneously modeling neural and behavioral data. NeuroImage, 72, 193–206.
Turner, B. M., van Maanen, L., & Forstmann, B. U. (2015). Informing cognitive abstractions through neuroimaging: The neural drift diffusion model. Psychological Review, 122, 312–336.
Usher, M., & McClelland, J. L. (2001). The time course of perceptual choice: The leaky, competing accumulator model. Psychological Review, 108, 550–592.
Wagenmakers, E. J., Ratcliff, R., Gomez, P., & Iverson, G. J. (2004). Assessing model mimicry using the parametric bootstrap. Journal of Mathematical Psychology, 48(1), 28–50.
Van Zandt, T. (2000). How to fit a response time distribution. Psychonomic Bulletin & Review, 7(3), 424–465.
Wagenmakers, E.-J., Ratcliff, R., Gomez, P., & McKoon, G. (2008). A diffusion model account of criterion shifts in the lexical decision task. Journal of Memory and Language, 58, 140–159.
Wang, X.-J. (1999). Synaptic basis of cortical persistent activity: The importance of NMDA receptors to working Mmmory. Journal of Neuroscience, 19(21), 9587–9603.
Wang, X.-J. (2002). Probabilistic decision making by slow reverberation in cortical circuits. Neuron, 36, 955–968.
Wang, X.-J. (2008). Decision making in recurrent neuronal circuits. Neuron, 60, 215–234.
Wiecki, T. V., Poland, J., & Frank, M. J. (2015). Model-based cognitive neuroscience approaches to computational psychiatry: Clustering and classification. Clinical Psychological Science, 3(3), 378–379.
White, C. N., Mumford, J. A., & Poldrack, R. A. (2012). Perceptual criteria in the human brain. Journal of Neuroscience, 32(47), 16716–16724.
Wong, K. F., Huk, A. C., Shadlen, M. N., & Wang, X.-J. (2007). Neural circuit dynamics underlying accumulation of time-varying evidence during perceptual decision making. Frontiers in Computational Neuroscience, 1, 1–11.
Wong, K. F., & Wang, X.-J. (2006). A recurrent network mechanism of time integration in perceptual decisions. Journal of Neuroscience, 26, 1314–1328.
Zandbelt, B. B., Purcell, B. A., Palmeri, T. J., Logan, G. D., & Schall, J. D. (2014). Response times from ensembles of accumulators. Proceedings of the National Academy of Sciences, 111(7), 2848–2853.
Acknowledgements
We thank Xiao-Jing Wang for providing a Python-based implementation of his spiking neural network model that was adapted for use in our simulations. Portions of this article represented an undergraduate honors thesis by the first author (AU) when he was at Vanderbilt University. Much of the original modeling work and writing was done when the second author (BAP) was a graduate student at Vanderbilt University and then a postdoctoral fellow at New York University.
Funding
This work was supported by NEI grant R01 EY021833, the Temporal Dynamics of Learning Center (NSF grant SMA 1041755), and the Vanderbilt Vision Research Center (P30 EY008126).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Simulation and model fits were performed by Akash Umakantha, with significant input from Braden Purcell and Thomas Palmeri. The first draft of the manuscript was written by Akash Umakantha and Braden Purcell, the final version edited by Thomas Palmeri. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix Spiking Neural Network Model Dynamics
Appendix Spiking Neural Network Model Dynamics
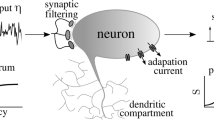
Neuron and synapse models. Each neuron in the spiking network is modeled as a leaky integrate-and-fire neuron (e.g., Abbott, 1999) with a membrane potential V(t) defined by a differential equation
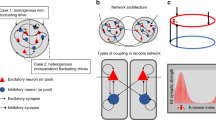
where Cm is the membrane capacitance, gL is the leakage conductance, VL is the resting potential, and Isyn(t) is the synaptic current. When the membrane potential V(t) of a neuron reaches a threshold potential Vth = -50 mV, the neuron generates a spike and is reset to Vr = − 55 mV for a refractory period of Tref. For excitatory neurons, Cm = 0.5 nF, gL = 25 nS, VL = -70 mV, and Tref = 2 ms; for inhibitory neurons, Cm = 0.2 nF, gL = 20 nS, VL = -70 mV, and Tref = 1 ms. We fixed these parameters at values that replicate known biophysical properties of cortical neurons (as per Wang, 2002).
The total synaptic current Isyn(t) is the sum of external input currents and excitatory and inhibitory currents from recurrent network connections. There are four types of currents at the synaptic connections: external AMPA, recurrent AMPA, recurrent NMDA, and recurrent GABA. Therefore, the equation for total synaptic current Isyn(t) is:
The individual receptor currents are given by:
where VE = 0 and VI = − 70 mV are the reversal potentials and [Mg2+] = 1 mM is the extracellular magnesium concentration. The sums are over all excitatory connections Ce or all inhibitory connections Ci. We fixed these parameters at values that replicate known biophysical properties of cortical neurons (as per Wang, 2002).
The g variables are conductance values of the receptor channels. All default conductance values were chosen to match known biophysical measurements (Wang, 2002). For excitatory neurons gext,AMPA = 2.100 nS, grec,AMPA = 0.050 nS, gNMDA = 0.165 nS, and gGABA = 1.300 nS. For inhibitory neurons gext,AMPA = 1.620 nS, grec,AMPA = 0.040 nS, gNMDA = 0.130, and gGABA = 1.000 nS. To explore the relationship between diffusion model parameters and conductance values, in simulated experiments, we used the following ranges: grec,AMPA = 0.045, 0.0475, 0.050 (default), 0.0525, and 0.055 nS; gNMDA = 0.163, 0.164, 0.165 (default), 0.166, and 0.167 nS; and gGABA = 1.290, 1.295, 1.300 (default), 1.305, and 1.310 nS. As with other choices of manipulated parameter values, these values were chosen because they allowed the model to exhibit appropriate competitive dynamics while also producing reasonable differences in predicted behavior.
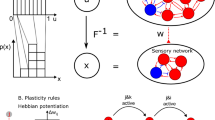
The w variables are synaptic weights that control the strength of connections between neural pools. The network is connected all-to-all, meaning that a single neuron, j, is connected to every other neuron in the network with weight wj. The synaptic weights were structured with a “Hebbian rule” with stronger connections between neurons representing the same choice (wj = w+) and weaker connections between neurons representing opposite choices (wj = w–). All other connections assume wj = 1. Following Wang (2002), we used the default value w+ = 1.7 and w– was determined by the Eq. 1 – f(w+ – 1)/(1 – f). f is the proportion of excitatory neurons in the spiking neural network that belong to a choice-selective subpool (in our case, f = 240/1600 = 0.15). To identify the relationship between diffusion model parameters and synaptic weights, we explored the following ranges: w+ = 1.650, 1.675, 1.700 (default), 1.725, and 1.750. Beyond these ranges, the model failed to exhibit competitive dynamics (Wong & Wang, 2006).
The s variables are gating variables and represent the fraction of receptor channels that are open at a given time. These parameters control the receptor dynamics and are presumed to be a fixed biophysical property of the cells. They are governed by the following equations:
where τAMPA = 2 ms is the decay time and the sum is over the presynaptic spikes from neuron j. For external AMPA currents, the sum is over Poisson spike trains generated with means specified by the parameters μext, μ1, and μ2 that are independent for each cell. For NMDA receptors:
where τNMDA,decay = 100 ms and α = 0.5/ms. The rise time dynamics of NMDA receptors is modeled by xj, where τNMDA,rise = 2 ms. Finally, for GABA:
where τGABA = 100 ms.
Rights and permissions
About this article
Cite this article
Umakantha, A., Purcell, B.A. & Palmeri, T.J. Relating a Spiking Neural Network Model and the Diffusion Model of Decision-Making. Comput Brain Behav 5, 279–301 (2022). https://doi.org/10.1007/s42113-022-00143-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42113-022-00143-4