Abstract
Purpose
To explore the feasibility of machine learning-based cascade classification models for screening high-risk COPD populations.
Materials and methods
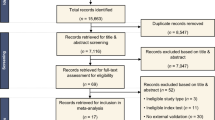
A total of 1637 community residents with available demographic data, smoking history, and pulmonary function tests (PFT) who underwent low-dose chest computed tomography (CT) from 2018 to 2020 were included. All subjects were divided into COPD and non-COPD groups according to their FEV1/FVC threshold of 0.7. Furthermore, the non-COPD groups were further subdivided into normal and high-risk COPD groups subgroups according to FEV1% predicted value (FEV1% pre) thresholds of 72%, 80%, and 95%, respectively. Based on the basic information and CT quantitative parameters of subjects, random forest model 1 (RF_1) was established to distinguish COPD from non-COPD groups, and RF_2 was established to distinguish high-risk COPD from normal groups. Then, we combined RF_1 and RF_2 to form triple classification model using cascade classification method. Subjects were randomly divided into training and test sets in the ratio of 8:2. Model performances were evaluated using AUC, accuracy, sensitivity, and specificity.
Results
The accuracy of the triple classification model was 0.63 for FEV1/FVC threshold of 0.7 and FEV1% threshold of 72%. For FEV1/FVC threshold of 0.7 and FEV1% threshold of 80%, accuracy of the model was 0.51. For FEV1/FVC threshold of 0.7 and FEV1% threshold of 95%, accuracy of the model was 0.58.
Conclusions
Machine learning-based cascade classification models is a potential method to screen high-risk COPD populations from general population. This method lays a foundation for a uniform method to screen high-risk COPD populations.





Similar content being viewed by others
Data availability
The data sets supporting the conclusion of this article are included within the article. All data are available from the corresponding author upon reasonable request.
Abbreviations
- AI:
-
Artificial intelligence
- AUC:
-
Area under curve
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1% pre:
-
FEV1% predicted values
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- FEV1/FVC:
-
The ratio of forced expiratory volume in 1 s to forced vital capacity
- FOV:
-
Field of view
- fSAD:
-
Functional small airway disease
- PFT:
-
Pulmonary function test
- PRM:
-
Parameter response mapping
- TBV:
-
Total blood volume
- RF:
-
Random forest
- No. vessels:
-
Number of small pulmonary vessels
- No. vessels CSA < 5 mm2 :
-
Numbers of pulmonary vessels with a cross-sectional area less than 5 mm2
- Vessel area:
-
Area of small pulmonary vessels
- Total vessel surface area:
-
Total surface area of small pulmonary vessels
- LV:
-
Lung volume
- TBV:
-
Total blood volume
- BV5:
-
Vessel volume for vessels less than 5 mm2
References
Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–17. https://doi.org/10.1016/S0140-6736(18)30841-9.
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. https://doi.org/10.1016/S0140-6736(18)32203-7.
Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–40. https://doi.org/10.1016/S0140-6736(17)31222-9.
Santos S, Peinado VI, Ramírez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–8. https://doi.org/10.1183/09031936.02.00245902.
Takayanagi S, Kawata N, Tada Y, et al. Longitudinal changes in structural abnormalities using MDCT in COPD: do the CT measurements of airway wall thickness and small pulmonary vessels change in parallel with emphysematous progression? Int J Chron Obstruct Pulmon Dis. 2017;12:551–60. https://doi.org/10.2147/COPD.S121405.
Lowe KE, Regan EA, Anzueto A, et al. COPDGene®2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019;6(5):384–99. https://doi.org/10.15326/jcopdf.6.5.2019.0149.
Chen S, Wang C, Li B, et al. Risk factors for FEV1 decline in mild COPD and high-risk populations. Int J Chron Obstruct Pulmon Dis. 2017;12:435–42. https://doi.org/10.2147/COPD.S118106.
Pu Y, Zhou X, Zhang D, et al. Re-defining high risk COPD with parameter response mapping based on machine learning models. Int J Chron Obstruct Pulmon Dis. 2022;17:2471–83. https://doi.org/10.2147/COPD.S369904.
Hueper K, Vogel-Claussen J, Parikh MA, et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am J Respir Crit Care Med. 2015;192:570–80. https://doi.org/10.1164/rccm.201411-2120OC.
Saruya S, Yamashiro T, Matsuoka S, et al. Decrease in small pulmonary vessels on chest computed tomography in light smokers without COPD: an early change, but correlated with smoking index. Lung. 2017;195:179–84. https://doi.org/10.1007/s00408-017-9985-5.
Xia Y, Guan Y, Fan L, et al. Dynamic contrast enhanced magnetic resonance perfusion imaging in high-risk smokers and smoking-related COPD: correlations with pulmonary function tests and quantitative computed tomography. COPD. 2014;11:510–20. https://doi.org/10.3109/15412555.2014.948990.
Balkissoon R. Journal Club-COPD2020 update. Global initiative for chronic obstructive lung disease 2020 report and the journal of the COPD foundation special edition, moving to a new definition for COPD: “COPDGene® 2019.” Chronic Obstr Pulm Dis. 2019;6:64–72. https://doi.org/10.15326/jcopdf.7.1.2020.0133.
Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med. 2023;11:18. https://doi.org/10.1016/S2213-2600(22)00494-5.
González G, Ash SY, Vegas-Sánchez-Ferrero G, et al. Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med. 2018;197:193–203. https://doi.org/10.1164/rccm.201705-0860OC.
Chen W, Sin DD, FitzGerald JM, et al. An individualized prediction model for long-term lung function trajectory and risk of COPD in the general population. Chest. 2020;157:547–57. https://doi.org/10.1016/j.chest.2019.09.003.
Liaw A, Wiener M. Classification and regression by random forest. R News. 2002;2(3):18–22.
Gosain A, Sardana S. Handling class imbalance problem using oversampling techniques: a review. In 2017 International Conference on Advances in Computing, Communications and Informatics. IEEE. 2017;79–85.
Sharma S, Gosain A, Jain S (2022) A review of the oversampling techniques in class imbalance problem. In: Khanna A, Gupta D, Bhattacharyya S, Hassanien AE, Anand S, Jaiswal A (eds) International conference on innovative computing and communications. Advances in Intelligent Systems and Computing, vol 1387. Springer, Singapore, pp 459–472. https://doi.org/10.1007/978-981-16-2594-7_38
Mohammed R, Rawashdeh J, Abdullah M (2020) Machine learning with oversampling and undersampling techniques: overview study and experimental results. In: 2020 11th international conference on information and communication systems (ICICS). IEEE, pp 243–248
Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: machine learning in python. J Mach Learn Res. 2011;12:122825–30.
McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–75. https://doi.org/10.1056/NEJMoa1106955.
Mohamed Hoesein FA, de Jong PA, Lammers JW, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45:644–51. https://doi.org/10.1183/09031936.00020714.
Koo HK, Vasilescu DM, Booth S, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med. 2018;6:591–602. https://doi.org/10.1016/S2213-2600(18)30196-6.
Berg K, Wright JL. The pathology of chronic obstructive pulmonary disease: progress in the 20th and 21st centuries. Arch Pathol Lab Med. 2016;140:1423–8. https://doi.org/10.5858/arpa.2015-0455-RS.
Tuder RM, Cool CD. Pulmonary arteries and microcirculation in COPD with pulmonary hypertension: bystander or culprit? Chest. 2019;156:4–6. https://doi.org/10.1016/j.chest.2019.04.100.
Scarrow GD. The pulmonary angiogram in chronic bronchitis and emphysema. Clin Radiol. 1966;17:54–67. https://doi.org/10.1016/s0009-9260(66)80123-x.
Jacobson G, Turner AF, Balchum OJ, et al. Vascular changes in pulmonary emphysema. The radiologic evaluation by selective and peripheral pulmonary wedge angiography. Am J Roentgenol Radium Ther Nucl Med. 1967;100:374–96.
Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–8. https://doi.org/10.2147/COPD.S176122.
Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–59. https://doi.org/10.1146/annurev.pathol.4.110807.092145.
Eom JS, Lee G, Lee HY, et al. The relationships between tracheal index and lung volume parameters in mild-to-moderate COPD. Eur J Radiol. 2013;82:e867–72. https://doi.org/10.1016/j.ejrad.2013.08.028.
Wright JL, Levy RD, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax. 2005;60:605–9. https://doi.org/10.1136/thx.2005.042994.
Acknowledgements
We greatly appreciate Ms. Qian He (Department of Statistics, Naval Medical University, Shanghai, China) for her assistance in statistical analysis.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 82171926, 81930049); National Key R&D Program of China (grant number: 2022YFC2010000, 2022YFC2010002, 2022YFC2010005); the program of Science and Technology Commission of Shanghai Municipality (grant number 21DZ2202600); the construction of CT standardized database for chronic obstructive pulmonary disease (grant number: YXFSC2022JJSJ002); Clinical Innovation Project of Shanghai Changzheng Hospital (grant number: 2020YLCYJ-Y24).
Author information
Authors and Affiliations
Contributions
YP: conceptualization, formal analysis, data curation, writing—original draft, writing—review and editing, visualization. XZ: conceptualization, formal analysis, data curation, writing—original draft, writing—review and editing, visualization. DZ: methodology, formal analysis, investigation, writing—original draft, visualization. YG: investigation, resources, visualization, supervision. YX: validation, formal analysis. YL: methodology, software, validation, formal analysis. XZ: software, validation, formal analysis. CH: supervision, project administration. SL: resources, supervision, project administration, funding acquisition. LF: conceptualization, methodology, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interest.
Ethical approval
The study was approved by the institutional review board of Changzheng Hospital, Naval Medical University, Shanghai, China, and the study was registered in the Chinese Clinical Trials Registry (http://www.chictr.org.cn/index.aspx; ChiCTR2000035283). The study was conducted in accordance with the Declaration of Helsinki. All the subjects signed written informed consent for participating in this study.
Consent for publication
The authors confirm that all the contents in this review can be published. Our study has not been published in another journal or presented at a congress.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pu, Y., Zhou, X., Zhang, D. et al. A new method to screen high-risk COPD populations: machine learning-based cascade classification models based on low-dose CT scan. Chin J Acad Radiol 7, 28–39 (2024). https://doi.org/10.1007/s42058-023-00134-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42058-023-00134-9