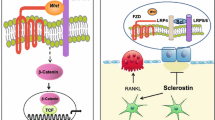
Abstract
Sclerostin inhibits osteoblast activity by hampering activation of the canonical Wnt signaling pathway and simultaneously stimulates osteoclastogenesis through upregulation of the receptor activator of NFκB ligand (RANKL). Thus, antibodies against sclerostin (Scl-Abs), besides promoting bone formation, suppress bone resorption and dissociate bone formation from resorption. This dual action results in remarkable increases of bone mineral density which are of a greater magnitude compared to the other antiosteoporotic treatments and are accompanied by decreases of fracture risk at all skeletal sites. The anabolic effect subsides after the first few months of treatment and a predominantly antiresorptive effect remains after this period, limiting its use to 12 months. Furthermore, these effects are largely reversible upon discontinuation; therefore, subsequent treatment with antiresorptives is indicated to maintain or further increase the bone gains achieved. Romosozumab is currently the only Scl-Ab approved for the treatment of severe postmenopausal osteoporosis. Indications for use in other populations, such as males, premenopausal women, and patients with glucocorticoid-induced osteoporosis, are pending. Additionally, the efficacy of Scl-Abs in other bone diseases, such as osteogenesis imperfecta, hypophosphatasia, X-linked hypophosphatemia, and bone loss associated with malignancies, is under thorough investigation. Cardiovascular safety concerns currently exclude patients at high cardiovascular risk from this treatment.
Similar content being viewed by others
Data availability
Not applicable.
References
Anastasilakis AD, Polyzos SA, Toulis KA (2011) Role of wingless tail signaling pathway in osteoporosis: an update of current knowledge. Curr Opin Endocrinol Diabetes Obes 18(6):383–388. https://doi.org/10.1097/MED.0b013e32834afff2
Toulis KA, Anastasilakis AD, Polyzos SA, Makras P (2011) Targeting the osteoblast: approved and experimental anabolic agents for the treatment of osteoporosis. Hormones 10(3):174–195. https://doi.org/10.14310/horm.2002.1308
Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R (2006) Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21(11):1738–1749. https://doi.org/10.1359/jbmr.060810
Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, Latham JA (2004) Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 35(4):828–835. https://doi.org/10.1016/j.bone.2004.05.023
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 6(10):e25900. https://doi.org/10.1371/journal.pone.0025900
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39(2):91–97. https://doi.org/10.1136/jmg.39.2.91
van Lierop AH, Appelman-Dijkstra NM, Papapoulos SE (2017) Sclerostin deficiency in humans. Bone 96:51–62. https://doi.org/10.1016/j.bone.2016.10.010
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23(6):860–869. https://doi.org/10.1359/jbmr.080216
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276. https://doi.org/10.1093/emboj/cdg599
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588. https://doi.org/10.1359/jbmr.081206
Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, Dwyer D, Stouch B, Thway TM, Stolina M, Ominsky MS, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ (2010) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25(12):2647–2656. https://doi.org/10.1002/jbmr.182
Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25(5):948–959. https://doi.org/10.1002/jbmr.14
Ominsky MS, Boyd SK, Varela A, Jolette J, Felx M, Doyle N, Mellal N, Smith SY, Locher K, Buntich S, Pyrah I, Boyce RW (2017) Romosozumab improves bone mass and strength while maintaining bone quality in ovariectomized cynomolgus monkeys. J Bone Miner Res 32(4):788–801. https://doi.org/10.1002/jbmr.3036
Padhi D, Jang G, Stouch B, Fang L, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26(1):19–26. https://doi.org/10.1002/jbmr.173
Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, Jang G (2014) Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol 54(2):168–178. https://doi.org/10.1002/jcph.239
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370(5):412–420. https://doi.org/10.1056/NEJMoa1305224
Holdsworth G, Greenslade K, Jose J, Stencel Z, Kirby H, Moore A, Ke HZ, Robinson MK (2018) Dampening of the bone formation response following repeat dosing with sclerostin antibody in mice is associated with up-regulation of Wnt antagonists. Bone 107:93–103. https://doi.org/10.1016/j.bone.2017.11.003
Boyce RW, Niu QT, Ominsky MS (2017) Kinetic reconstruction reveals time-dependent effects of romosozumab on bone formation and osteoblast function in vertebral cancellous and cortical bone in cynomolgus monkeys. Bone 101:77–87. https://doi.org/10.1016/j.bone.2017.04.005
Taylor S, Ominsky MS, Hu R, Pacheco E, He YD, Brown DL, Aguirre JI, Wronski TJ, Buntich S, Afshari CA, Pyrah I, Nioi P, Boyce RW (2016) Time-dependent cellular and transcriptional changes in the osteoblast lineage associated with sclerostin antibody treatment in ovariectomized rats. Bone 84:148–159. https://doi.org/10.1016/j.bone.2015.12.013
Boyce RW, Brown D, Felx M, Mellal N, Locher K, Pyrah I, Ominsky MS, Taylor S (2018) Decreased osteoprogenitor proliferation precedes attenuation of cancellous bone formation in ovariectomized rats treated with sclerostin antibody. Bone Rep 8:90–94. https://doi.org/10.1016/j.bonr.2018.03.001
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375(16):1532–1543. https://doi.org/10.1056/NEJMoa1607948
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377(15):1417–1427. https://doi.org/10.1056/NEJMoa1708322
Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, Miyauchi A, Maddox J, Chen L, Horlait S (2018) A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 103(9):3183–3193. https://doi.org/10.1210/jc.2017-02163
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, Dokoupilova E, Engelke K, Finkelstein JS, Genant HK, Goemaere S, Hyldstrup L, Jodar-Gimeno E, Keaveny TM, Kendler D, Lakatos P, Maddox J, Malouf J, Massari FE, Molina JF, Ulla MR, Grauer A (2017) Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390(10102):1585–1594. https://doi.org/10.1016/S0140-6736(17)31613-6
Graeff C, Campbell GM, Peña J, Borggrefe J, Padhi D, Kaufman A, Chang S, Libanati C, Glüer CC (2015) Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone 81:364–369. https://doi.org/10.1016/j.bone.2015.07.036
Genant HK, Engelke K, Bolognese MA, Mautalen C, Brown JP, Recknor C, Goemaere S, Fuerst T, Yang YC, Grauer A, Libanati C (2017) Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res 32(1):181–187. https://doi.org/10.1002/jbmr.2932
Keaveny TM, Crittenden DB, Bolognese MA, Genant HK, Engelke K, Oliveri B, Brown JP, Langdahl BL, Yan C, Grauer A, Libanati C (2017) Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Res 32(9):1956–1962. https://doi.org/10.1002/jbmr.3176
Poole KE, Treece GM, Pearson RA, Gee AH, Bolognese MA, Brown JP, Goemaere S, Grauer A, Hanley DA, Mautalen C, Recknor C, Yang YC, Rojeski M, Libanati C, Whitmarsh T (2022) Romosozumab enhances vertebral bone structure in women with low bone density. J Bone Miner Res 37(2):256–264. https://doi.org/10.1002/jbmr.4465
Brown JP, Engelke K, Keaveny TM, Chines A, Chapurlat R, Foldes AJ, Nogues X, Civitelli R, De Villiers T, Massari F, Zerbini CAF, Wang Z, Oates MK, Recknor C, Libanati C (2021) Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: results from the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial. J Bone Miner Res 36(11):2139–2152. https://doi.org/10.1002/jbmr.4409
Chavassieux P, Chapurlat R, Portero-Muzy N, Roux JP, Garcia P, Brown JP, Libanati C, Boyce RW, Wang A, Grauer A (2019) Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: bone histomorphometry and microcomputed tomography analysis after 2 and 12 months of treatment. J Bone Miner Res 34(9):1597–1608. https://doi.org/10.1002/jbmr.3735
Eriksen EF, Chapurlat R, Boyce RW, Shi Y, Brown JP, Horlait S, Betah D, Libanati C, Chavassieux P (2022) Modeling-based bone formation after 2 months of romosozumab treatment: results from the FRAME Clinical Trial. J Bone Miner Res 37(1):36–40. https://doi.org/10.1002/jbmr.4457
Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, Matsumoto T, Milmont CE, Libanati C, Grauer A (2018) FRAME Study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res 33(7):1219–1226. https://doi.org/10.1002/jbmr.3427
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C, Investigators AS (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316(7):722–733. https://doi.org/10.1001/jama.2016.11136
McClung MR, Bolognese MA, Brown JP, Reginster JY, Langdahl BL, Shi Y, Timoshanko J, Libanati C, Chines A, Oates MK (2021) Skeletal responses to romosozumab after 12 months of denosumab. JBMR Plus 5(7):e10512. https://doi.org/10.1002/jbm4.10512
Cosman F, Kendler DL, Langdahl BL, Leder BZ, Lewiecki EM, Miyauchi A, Rojeski M, McDermott M, Oates MK, Milmont CE, Libanati C, Ferrari S (2022) Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int 33(6):1243–1256. https://doi.org/10.1007/s00198-021-06174-0
Ebina K, Tsuboi H, Nagayama Y, Kashii M, Kaneshiro S, Miyama A, Nakaya H, Kunugiza Y, Hirao M, Okamura G, Etani Y, Takami K, Goshima A, Miura T, Nakata K, Okada S (2021) Effects of prior osteoporosis treatment on 12-month treatment response of romosozumab in patients with postmenopausal osteoporosis. Joint Bone Spine 88(5):105219. https://doi.org/10.1016/j.jbspin.2021.105219
Ebina K, Etani Y, Tsuboi H, Nagayama Y, Kashii M, Miyama A, Kunugiza Y, Hirao M, Okamura G, Noguchi T, Takami K, Goshima A, Miura T, Fukuda Y, Kurihara T, Okada S, Nakata K (2022) Impact of the duration of previous osteoporosis treatment on the effect of romosozumab in patients with postmenopausal osteoporosis. Osteoporos Int 33(11):2441–2443. https://doi.org/10.1007/s00198-022-06545-1
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386(9999):1147–1155. https://doi.org/10.1016/S0140-6736(15)61120-5
McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, Bolognese MA, Goemaere S, Bone HG, Zanchetta JR, Maddox J, Bray S, Grauer A (2018) Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res 33(8):1397–1406. https://doi.org/10.1002/jbmr.3452
Kendler DL, Bone HG, Massari F, Gielen E, Palacios S, Maddox J, Yan C, Yue S, Dinavahi RV, Libanati C, Grauer A (2019) Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos Int 30(12):2437–2448. https://doi.org/10.1007/s00198-019-05146-9
McClung MR, Bolognese MA, Brown JP, Reginster JY, Langdahl BL, Maddox J, Shi Y, Rojeski M, Meisner PD, Grauer A (2020) A single dose of zoledronate preserves bone mineral density for up to 2 years after a second course of romosozumab. Osteoporos Int 31(11):2231–2241. https://doi.org/10.1007/s00198-020-05502-0
Kaveh S, Hosseinifard H, Ghadimi N, Vojdanian M, Aryankhesal A (2020) Efficacy and safety of romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis. Clin Rheumatol 39(11):3261–3276. https://doi.org/10.1007/s10067-020-04948-1
Lv F, Cai X, Yang W, Gao L, Chen L, Wu J, Ji L (2020) Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: systematic review and meta- analysis. Bone 130:115121. https://doi.org/10.1016/j.bone.2019.115121
Cummings SR, McCulloch C (2020) Explanations for the difference in rates of cardiovascular events in a trial of alendronate and romosozumab. Osteoporos Int 31(6):1019–1021. https://doi.org/10.1007/s00198-020-05379-z
Zheng J, Wheeler E, Pietzner M, Andlauer TFM, Yau MS, Hartley AE, Brumpton BM, Rasheed H, Kemp JP, Frysz M, Robinson J, Reppe S, Prijatelj V, Gautvik KM, Falk L, Maerz W, Gergei I, Peyser PA, Kavousi M, de Vries PS, Miller CL, Bos M, van der Laan SW, Malhotra R, Herrmann M, Scharnagl H, Kleber M, Dedoussis G, Zeggini E, Nethander M, Ohlsson C, Lorentzon M, Wareham N, Langenberg C, Holmes MV, Davey Smith G, Tobias JH (2023) Lowering of circulating sclerostin may increase risk of atherosclerosis and its risk factors: evidence from a genome-wide association meta-analysis followed by mendelian randomization. Arthritis Rheumatol 75(10):1781–1792. https://doi.org/10.1002/art.42538
Miller PD, Adachi JD, Albergaria BH, Cheung AM, Chines AA, Gielen E, Langdahl BL, Miyauchi A, Oates M, Reid IR, Santiago NR, Vanderkelen M, Wang Z, Yu Z (2022) Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J Bone Miner Res 37(8):1437–1445. https://doi.org/10.1002/jbmr.4563
Miyauchi A, Hamaya E, Nishi K, Tolman C, Shimauchi J (2022) Efficacy and safety of romosozumab among Japanese postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J Bone Miner Metab 40(4):677–687. https://doi.org/10.1007/s00774-022-01332-8
Hsu CP, Maddox J, Block G, Bartley Y, Yu Z (2022) Influence of renal function on pharmacokinetics, pharmacodynamics, and safety of a single dose of romosozumab. J Clin Pharmacol 62(9):1132–1141. https://doi.org/10.1002/jcph.2050
Jankowski J, Floege J, Fliser D, Böhm M, Marx N (2021) Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 143(11):1157–1172. https://doi.org/10.1161/circulationaha.120.050686
Romosozumab (2012) In: LiverTox: Clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
Hamann C, Rauner M, Hohna Y, Bernhardt R, Mettelsiefen J, Goettsch C, Gunther KP, Stolina M, Han CY, Asuncion FJ, Ominsky MS, Hofbauer LC (2013) Sclerostin antibody treatment improves bone mass, bone strength, and bone defect regeneration in rats with type 2 diabetes mellitus. J Bone Miner Res 28(3):627–638. https://doi.org/10.1002/jbmr.1803
Marino S, Akel N, Li S, Cregor M, Jones M, Perez B, Troncoso G, Meeks J, Stewart S, Sato AY, Nookaew I, Bellido T (2023) Reversal of the diabetic bone signature with anabolic therapies in mice. Bone Res 11(1):19. https://doi.org/10.1038/s41413-023-00261-0
Anastasilakis AD, Pepe J, Napoli N, Palermo A, Magopoulos C, Khan AA, Zillikens MC, Body JJ (2022) Osteonecrosis of the jaw and antiresorptive agents in benign and malignant diseases: a critical review organized by the ECTS. J Clin Endocrinol Metab 107(5):1441–1460. https://doi.org/10.1210/clinem/dgab888
Hadaya D, Gkouveris I, Soundia A, Bezouglaia O, Boyce RW, Stolina M, Dwyer D, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S (2019) Clinically relevant doses of sclerostin antibody do not induce osteonecrosis of the jaw (ONJ) in rats with experimental periodontitis. J Bone Miner Res 34(1):171–181. https://doi.org/10.1002/jbmr.3581
Peng J, Wang H, Liu Z, Xu ZL, Wang MX, Chen QM, Wu ML, Ren XL, Liang QH, Liu FP, Ban B (2022) Real-world study of antiresorptive-related osteonecrosis of jaw based on the US food and drug administration adverse event reporting system database. Front Pharmacol 13:1017391. https://doi.org/10.3389/fphar.2022.1017391
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29(1):1–23. https://doi.org/10.1002/jbmr.1998
Makras P, Delaroudis S, Anastasilakis AD (2015) Novel therapies for osteoporosis. Metabolism 64(10):1199–1214. https://doi.org/10.1016/j.metabol.2015.07.011
McColm J, Hu L, Womack T, Tang CC, Chiang AY (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 29(4):935–943. https://doi.org/10.1002/jbmr.2092
Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL (2015) A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 30(2):216–224. https://doi.org/10.1002/jbmr.2351
Recknor CP, Recker RR, Benson CT, Robins DA, Chiang AY, Alam J, Hu L, Matsumoto T, Sowa H, Sloan JH, Konrad RJ, Mitlak BH, Sipos AA (2015) The effect of discontinuing treatment with blosozumab: follow-up results of a phase 2 randomized clinical trial in postmenopausal women with low bone mineral density. J Bone Miner Res 30(9):1717–1725. https://doi.org/10.1002/jbmr.2489
Seefried L, Baumann J, Hemsley S, Hofmann C, Kunstmann E, Kiese B, Huang Y, Chivers S, Valentin MA, Borah B, Roubenoff R, Junker U, Jakob F (2017) Efficacy of anti-sclerostin monoclonal antibody BPS804 in adult patients with hypophosphatasia. J Clin Invest 127(6):2148–2158. https://doi.org/10.1172/JCI83731
Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, Junker U, Ruckle J, Seefried L, Winkle PJ (2017) BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial. J Bone Miner Res 32(7):1496–1504. https://doi.org/10.1002/jbmr.3143
Glorieux F, Javaid MK, Langdahl B, Chapurlat R, De Beur SJ, Sutton VR, Poole K, Orwoll ES, Willie B, Mikolajewicz N, Clancy J, MacKinnon A, Mistry A, Ominsky MS, Saville C (2021) It was an oral presentation with presentation number: 1016 Session: Oral Presentations: Rare Bone Disease (Clinical), San Diego Convention Center, Ballroom 6AB
Dai Z, Fang P, Yan X, Zhu R, Feng Q, Yan Q, Yang L, Fan X, Xie Y, Zhuang L, Feng S, Liu Y, Zhong S, Yang Z, Sheng Z, Zhou Z (2021) Single dose of SHR-1222, a sclerostin monoclonal antibody, in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled, dose-escalation, phase I study. Front Pharmacol 12:770073. https://doi.org/10.3389/fphar.2021.770073
Dai Z, Zhu R, Sheng Z, Qin G, Luo X, Qin Q, Song C, Li L, Jin P, Yang G, Cheng Y, Peng D, Zou C, Wang L, Shentu J, Zhang Q, Zhang Z, Yan X, Fang P, Yan Q, Yang L, Fan X, Liu W, Wu B, Cui R, Wu X, Xie Y, Shu C, Shen K, Wei W, Lu W, Chen H, Zhou Z (2023) Multiple doses of SHR-1222, a sclerostin monoclonal antibody, in postmenopausal women with osteoporosis: a randomized, double-blind, placebo-controlled, dose-escalation phase 1 trial. Front Endocrinol 14:1168757. https://doi.org/10.3389/fendo.2023.1168757
Boschert V, Frisch C, Back JW, van Pee K, Weidauer SE, Muth EM, Schmieder P, Beerbaum M, Knappik A, Timmerman P, Mueller TD (2016) The sclerostin-neutralizing antibody AbD09097 recognizes an epitope adjacent to sclerostin's binding site for the Wnt co-receptor LRP6. Open Biol 6(8). https://doi.org/10.1098/rsob.160120
Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM (2015) Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone 71:115–123. https://doi.org/10.1016/j.bone.2014.10.012
Cardinal M, Tys J, Roels T, Lafont S, Ominsky MS, Devogelaer JP, Chappard D, Mabilleau G, Ammann P, Nyssen-Behets C, Manicourt DH (2019) Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 124:137–147. https://doi.org/10.1016/j.bone.2019.04.011
Cardinal M, Dessain A, Roels T, Lafont S, Ominsky MS, Devogelaer JP, Chappard D, Mabilleau G, Ammann P, Nyssen-Behets C, Manicourt DH (2020) Sclerostin-antibody treatment decreases fracture rates in axial skeleton and improves the skeletal phenotype in growing oim/oim mice. Calcif Tissue Int 106(5):494–508. https://doi.org/10.1007/s00223-019-00655-5
Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, Rauch F (2014) Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 66:182–188. https://doi.org/10.1016/j.bone.2014.06.015
Uehara M, Nakamura Y, Nakano M, Miyazaki A, Suzuki T, Takahashi J (2022) Efficacy of romosozumab for osteoporosis in a patient with osteogenesis imperfecta: a case report. Mod Rheumatol Case Rep 6(1):128–133. https://doi.org/10.1093/mrcr/rxab018
Carpenter KA, Ross RD (2020) Sclerostin antibody treatment increases bone mass and normalizes circulating phosphate levels in growing Hyp mice. J Bone Miner Res 35(3):596–607. https://doi.org/10.1002/jbmr.3923
Ren Y, Han X, Jing Y, Yuan B, Ke H, Liu M, Feng JQ (2016) Sclerostin antibody (Scl-Ab) improves osteomalacia phenotype in dentin matrix protein 1(Dmp1) knockout mice with little impact on serum levels of phosphorus and FGF23. Matrix Biol 52–54:151–161. https://doi.org/10.1016/j.matbio.2015.12.009
McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, Pettitt JA, Simic MK, Cheng TL, Morse A, Le LMT, Abi-Hanna D, Kramer I, Falank C, Fairfield H, Ghobrial IM, Baldock PA, Little DG, Kneissel M, Vanderkerken K, Bassett JHD, Williams GR, Oyajobi BO, Hose D, Phan TG, Croucher PI (2017) Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 129(26):3452–3464. https://doi.org/10.1182/blood-2017-03-773341
Hesse E, Schroder S, Brandt D, Pamperin J, Saito H, Taipaleenmaki H (2019) Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight 5(9). https://doi.org/10.1172/jci.insight.125543
Marenzana M, Greenslade K, Eddleston A, Okoye R, Marshall D, Moore A, Robinson MK (2011) Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum 63(8):2385–2395. https://doi.org/10.1002/art.30385
Marenzana M, Vugler A, Moore A, Robinson M (2013) Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: a microCT study. Arthritis Res Ther 15(5):R125. https://doi.org/10.1186/ar4305
Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, Ghosh P, Bassit ACF, Borst SE, Yarrow JF (2018) Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS ONE 13(3):e0194440. https://doi.org/10.1371/journal.pone.0194440
Shin YK, Yoon YK, Chung KB, Rhee Y, Cho SR (2017) Patients with non-ambulatory cerebral palsy have higher sclerostin levels and lower bone mineral density than patients with ambulatory cerebral palsy. Bone 103:302–307. https://doi.org/10.1016/j.bone.2017.07.015
Takase R, Tsubouchi Y, Otsu T, Kataoka T, Iwasaki T, Kataoka M, Tsumura H (2022) The effects of romosozumab combined with active vitamin D(3) on fracture healing in ovariectomized rats. J Orthop Surg Res 17(1):384. https://doi.org/10.1186/s13018-022-03276-1
Schemitsch EH, Miclau T, Karachalios T, Nowak LL, Sancheti P, Poolman RW, Caminis J, Daizadeh N, Dent-Acosta RE, Egbuna O, Chines A, Maddox J, Grauer A, Bhandari M (2020) A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J Bone Joint Surg Am 102(8):693–702. https://doi.org/10.2106/JBJS.19.00790
Bhandari M, Schemitsch EH, Karachalios T, Sancheti P, Poolman RW, Caminis J, Daizadeh N, Dent-Acosta RE, Egbuna O, Chines A, Miclau T (2020) Romosozumab in skeletally mature adults with a fresh unilateral tibial diaphyseal fracture: a randomized phase-2 study. J Bone Joint Surg Am 102(16):1416–1426. https://doi.org/10.2106/JBJS.19.01008
Kaneuchi Y, Iwabuchi M, Hakozaki M, Yamada H, Konno SI (2022) Pregnancy and lactation-associated osteoporosis successfully treated with romosozumab: a case report. Medicina (Kaunas) 59(1). https://doi.org/10.3390/medicina59010019
Author information
Authors and Affiliations
Contributions
Conception of the hypothesis of the study: ADA and ΕΤ. Design of the study: ADA and ΕΤ. Acquisition, analysis, and interpretation of data: ADA, ΕΤ. Drafting the manuscript: ADA and ΕΤ. Revising the work critically for important intellectual content: ADA and ΕΤ. Final approval of the submitted version: ADA and ΕΤ. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: ADA and ΕΤ.
Corresponding author
Ethics declarations
Ethical approval and informed consent
Since this is a review article, no approval or informed consent was required.
Competing interests
Athanasios D. Anastasilakis reports lecture fees from Amgen, Bianex, Eli-Lilly, Galenica, ITF, Unifarma, and UCB; E. Tsourdi received honoraria for lectures and advisory boards from Amgen, UCB, Takeda, Kyowa Kirin, and educational grants from Takeda and UCB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anastasilakis, A.D., Tsourdi, E. Τhe story of sclerostin inhibition: the past, the present, and the future. Hormones (2024). https://doi.org/10.1007/s42000-023-00521-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42000-023-00521-y