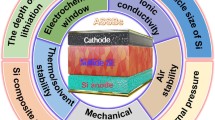
Abstract
Sulfides have been widely acknowledged as one of the most promising solid electrolytes (SEs) for all-solid-state batteries (ASSBs) due to their superior ionic conductivity and favourable mechanical properties. However, the extremely poor air stability of sulfide SEs leads to destroyed structure/performance and release of toxic H2S gas, which greatly limits mass-production/practical application of sulfide SEs and ASSBs. This review is designed to serve as an all-inclusive handbook for studying this critical issue. First, the research history and milestone breakthroughs of this field are reviewed, and this is followed by an in-depth elaboration of the theoretical paradigms that have been developed thus far, including the random network theory of glasses, hard and soft acids and bases (HSAB) theory, thermodynamic analysis and kinetics of interfacial reactions. Moreover, the characterization of air stability is reviewed from the perspectives of H2S generation, morphology evolution, mass change, component/structure variations and electrochemical performance. Furthermore, effective strategies for improving the air stabilities of sulfide SEs are highlighted, including H2S absorbents, elemental substitution, design of new materials, surface engineering and sulfide-polymer composite electrolytes. Finally, future research directions are proposed for benign development of air stability for sulfide SEs and ASSBs.
Graphical Abstract





















Similar content being viewed by others
References
Armand, M., Tarascon, J.M.: Building better batteries. Nature 451, 652–657 (2008). https://doi.org/10.1038/451652a
Xu, K.: Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004). https://doi.org/10.1021/cr030203g
Hu, Y.S.: Batteries: getting solid. Nat. Energy 1, 16042 (2016). https://doi.org/10.1038/nenergy.2016.42
Manthiram, A., Yu, X.W., Wang, S.F.: Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017). https://doi.org/10.1038/natrevmats.2016.103
Zhao, Q., Stalin, S., Zhao, C.Z., et al.: Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020). https://doi.org/10.1038/s41578-019-0165-5
Chen, R.S., Li, Q.H., Yu, X.Q., et al.: Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 120, 6820–6877 (2020). https://doi.org/10.1021/acs.chemrev.9b00268
Cheng, X.B., Zhang, R., Zhao, C.Z., et al.: A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 3, 1500213 (2016). https://doi.org/10.1002/advs.201500213
Sun, C.W., Liu, J., Gong, Y.D., et al.: Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33, 363–386 (2017). https://doi.org/10.1016/j.nanoen.2017.01.028
Bachman, J.C., Muy, S.: Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016). https://doi.org/10.1021/acs.chemrev.5b00563
Wu, Y.J., Wang, S., Li, H., et al.: Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat 3, 827–853 (2021). https://doi.org/10.1002/inf2.12224
Yan, W.L., Wu, F., Li, H., et al.: Application of Si based anodes in sulfide solid-state batteries. Energy Storage Sci. Technol. 10, 821–835 (2021). http://esst.cip.com.cn/EN/Y2021/V10/I3/821
Wu, F., Liu, L.L., Wang, S., et al.: Solid state ionics-selected topics and new directions. Prog. Mater. Sci. 126, 100921 (2022). https://doi.org/10.1016/j.pmatsci.2022.100921
Zhao, N., Khokhar, W., Bi, Z.J., et al.: Solid garnet batteries. Joule 3, 1190–1199 (2019). https://doi.org/10.1016/j.joule.2019.03.019
Abouali, S., Yim, C.H., Merati, A., et al.: Garnet-based solid-state Li batteries: from materials design to battery architecture. ACS Energy Lett. 6, 1920–1941 (2021). https://doi.org/10.1021/acsenergylett.1c00401
Wang, H.C., Sheng, L., Yasin, G., et al.: Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 33, 188–215 (2020). https://doi.org/10.1016/j.ensm.2020.08.014
Tan, S.J., Zeng, X.X., Ma, Q., et al.: Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochem. Energy Rev. 1, 113–138 (2018). https://doi.org/10.1007/s41918-018-0011-2
López-Aranguren, P., Reynaud, M., Głuchowski, P., et al.: Crystalline LiPON as a bulk-type solid electrolyte. ACS Energy Lett. 6, 445–450 (2021). https://doi.org/10.1021/acsenergylett.0c02336
Kamaya, N., Homma, K., Yamakawa, Y., et al.: A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011). https://doi.org/10.1038/nmat3066
Kato, Y., Hori, S., Saito, T., et al.: High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016). https://doi.org/10.1038/nenergy.2016.30
Han, F.D., Gao, T., Zhu, Y.J., et al.: Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1501590 (2016). https://doi.org/10.1002/aenm.201501590
Fitzhugh, W., Wu, F., Ye, L.H., et al.: A high-throughput search for functionally stable interfaces in sulfide solid-state lithium ion conductors. Adv. Energy Mater. 9, 1900807 (2019). https://doi.org/10.1002/aenm.201900807
Wu, F., Fitzhugh, W., Ye, L.H., et al.: Advanced sulfide solid electrolyte by core-shell structural design. Nat. Commun. 9, 4037 (2018). https://doi.org/10.1038/s41467-018-06123-2
Liu, L.L., Wu, F., Li, H., et al.: Advances in electrochemical stability of sulfide solid-state electrolyte. J. Chin. Ceram. Soc. 47, 1367–1385 (2019)
Fitzhugh, W., Wu, F., Ye, L.H., et al.: Strain-stabilized ceramic-sulfide electrolytes. Small 15, 1901470 (2019). https://doi.org/10.1002/smll.201901470
Haruyama, J., Sodeyama, K., Han, L.Y., et al.: Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014). https://doi.org/10.1021/cm5016959
Zhu, Y.Z., He, X.F., Mo, Y.F.: Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015). https://doi.org/10.1021/acsami.5b07517
Wang, Y., Lv, Y., Su, Y.B., et al.: 5V-class sulfurized spinel cathode stable in sulfide all-solid-state batteries. Nano Energy 90, 106589 (2021). https://doi.org/10.1016/j.nanoen.2021.106589
Richards, W.D., Miara, L.J., Wang, Y., et al.: Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016). https://doi.org/10.1021/acs.chemmater.5b04082
Wang, S., Fang, R.Y., Li, Y.T., et al.: Interfacial challenges for all-solid-state batteries based on sulfide solid electrolytes. J. Materiomics 7, 209–218 (2021). https://doi.org/10.1016/j.jmat.2020.09.003
Ye, L.H., Li, X.: A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021). https://doi.org/10.1038/s41586-021-03486-3
Xin, S., You, Y., Wang, S.F., et al.: Solid-state lithium metal batteries promoted by nanotechnology: progress and prospects. ACS Energy Lett. 2, 1385–1394 (2017). https://doi.org/10.1021/acsenergylett.7b00175
Zhang, Z.H., Wu, L.P., Zhou, D., et al.: Flexible sulfide electrolyte thin membrane with ultrahigh ionic conductivity for all-solid-state lithium batteries. Nano Lett. 21, 5233–5239 (2021). https://doi.org/10.1021/acs.nanolett.1c01344
Wan, H.L., Liu, S.F., Deng, T., et al.: Bifunctional interphase-enabled Li10GeP2S12 electrolytes for lithium-sulfur battery. ACS Energy Lett. 6, 862–868 (2021). https://doi.org/10.1021/acsenergylett.0c02617
Shi, Y.N., Zhou, D., Li, M.Q., et al.: Surface engineered Li metal anode for all-solid-state lithium metal batteries with high capacity. ChemElectroChem 8, 386–389 (2021). https://doi.org/10.1002/celc.202100010
Peng, J., Wu, D.X., Song, F.M., et al.: High current density and long cycle life enabled by sulfide solid electrolyte and dendrite-free liquid lithium anode. Adv. Funct. Mater. 32, 2105776 (2022). https://doi.org/10.1002/adfm.202105776
Muramatsu, H., Hayashi, A., Ohtomo, T., et al.: Structural change of Li2S-P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ion. 182, 116–119 (2011). https://doi.org/10.1016/j.ssi.2010.10.013
Liu, L.L., Xu, J.R., Wang, S., et al.: Practical evaluation of energy densities for sulfide solid-state batteries. eTransportation 1, 100010 (2019)
Chen, X.F., Guan, Z.Q., Chu, F.L., et al.: Air-stable inorganic solid-state electrolytes for high energy density lithium batteries: challenges, strategies, and prospects. InfoMat 4, e12248 (2022). https://doi.org/10.1002/inf2.12248
Zhao, S., Zhu, X.X., Jiang, W., et al.: Fundamental air stability in solid-state electrolytes: principles and solutions. Mater. Chem. Front. 5, 7452–7466 (2021). https://doi.org/10.1039/d1qm00951f
Galven, C., Dittmer, J., Suard, E., et al.: Instability of lithium garnets against moisture. Structural characterization and dynamics of Li7-xHxLa3Sn2O12 and Li5-xHxLa3Nb2O12. Chem. Mater. 24, 3335–3345 (2012). https://doi.org/10.1021/cm300964k
Yow, Z.F., Oh, Y.L., Gu, W.Y., et al.: Effect of Li+/H+ exchange in water treated Ta-doped Li7La3Zr2O12. Solid State Ion. 292, 122–129 (2016). https://doi.org/10.1016/j.ssi.2016.05.016
Bohnke, O., Lorant, S., Roffat, M., et al.: Fast H+/Li+ ion exchange in Li0.30La0.57TiO3 nanopowder and films in water and in ambient air. Solid State Ion. 262, 563–567 (2014). https://doi.org/10.1016/j.ssi.2013.08.008
Xia, W.H., Xu, B.Y., Duan, H.N., et al.: Reaction mechanisms of lithium garnet pellets in ambient air: the effect of humidity and CO2. J. Am. Ceram. Soc. 100, 2832–2839 (2017). https://doi.org/10.1111/jace.14865
Wang, Y., Wu, Y.J., Wang, Z.X., et al.: Doping strategy and mechanism for oxide and sulfide solid electrolytes with high ionic conductivity. J. Mater. Chem. A 10, 4517–4532 (2022). https://doi.org/10.1039/d1ta10966a
Wang, S.H., Xu, X.W., Cui, C., et al.: Air sensitivity and degradation evolution of halide solid state electrolytes upon exposure. Adv. Funct. Mater. 32, 2108805 (2022). https://doi.org/10.1002/adfm.202108805
Li, W.H., Liang, J.W., Li, M.S., et al.: Unraveling the origin of moisture stability of halide solid-state electrolytes by in situ and operando synchrotron X-ray analytical techniques. Chem. Mater. 32, 7019–7027 (2020). https://doi.org/10.1021/acs.chemmater.0c02419
Harding, J.R., Amanchukwu, C.V., Hammond, P.T., et al.: Instability of poly(ethylene oxide) upon oxidation in lithium-air batteries. J. Phys. Chem. C 119, 6947–6955 (2015). https://doi.org/10.1021/jp511794g
Hayashi, A., Muramatsu, H., Ohtomo, T., et al.: Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M = Fe, Zn, and Bi) nanoparticles. J. Mater. Chem. A 1, 6320–6326 (2013). https://doi.org/10.1039/c3ta10247e
Li, J., Chen, H.W., Shen, Y.B., et al.: Covalent interfacial coupling for hybrid solid-state Li ion conductor. Energy Stor. Mater. 23, 277–283 (2019). https://doi.org/10.1016/j.ensm.2019.05.002
Liang, J.W., Chen, N., Li, X.N., et al.: Li10Ge(P1–xSbx)2S12 lithium-ion conductors with enhanced atmospheric stability. Chem. Mater. 32, 2664–2672 (2020). https://doi.org/10.1021/acs.chemmater.9b04764
Sahu, G., Lin, Z., Li, J.C., et al.: Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4. Energy Environ. Sci. 7, 1053–1058 (2014). https://doi.org/10.1039/c3ee43357a
Park, K.H., Oh, D.Y., Choi, Y.E., et al.: Solution-processable glass LiI-Li4SnS4 superionic conductors for all-solid-state Li-ion batteries. Adv. Mater. 28, 1874–1883 (2016). https://doi.org/10.1002/adma.201505008
Saienga, J., Martin, S.W.: The comparative structure, properties, and ionic conductivity of LiI + Li2S + GeS2 glasses doped with Ga2S3 and La2S3. J. Non Cryst. Solids 354, 1475–1486 (2008). https://doi.org/10.1016/j.jnoncrysol.2007.08.058
Ohtomo, T., Hayashi, A., Tatsumisago, M., et al.: Characteristics of the Li2O-Li2S-P2S5 glasses synthesized by the two-step mechanical milling. J. Non Cryst. Solids 364, 57–61 (2013). https://doi.org/10.1016/j.jnoncrysol.2012.12.044
Hayashi, A., Muramatsu, H., Ohtomo, T., et al.: Improved chemical stability and cyclability in Li2S-P2S5-P2O5-ZnO composite electrolytes for all-solid-state rechargeable lithium batteries. J. Alloys Compd. 591, 247–250 (2014). https://doi.org/10.1016/j.jallcom.2013.12.191
Zhang, Z.X., Zhang, L., Yan, X.L., et al.: All-in-one improvement toward Li6PS5Br-based solid electrolytes triggered by compositional tune. J. Power Sources 410(411), 162–170 (2019). https://doi.org/10.1016/j.jpowsour.2018.11.016
Liu, G.Z., Xie, D.J., Wang, X.L., et al.: High air-stability and superior lithium ion conduction of Li3+3xP1−xZnxS4−xOx by aliovalent substitution of ZnO for all-solid-state lithium batteries. Energy Storage Mater. 17, 266–274 (2019). https://doi.org/10.1016/j.ensm.2018.07.008
Chen, T., Zhang, L., Zhang, Z.X., et al.: Argyrodite solid electrolyte with a stable interface and superior dendrite suppression capability realized by ZnO co-doping. ACS Appl. Mater. Interfaces 11, 40808–40816 (2019). https://doi.org/10.1021/acsami.9b13313
Ahmad, N., Zhou, L., Faheem, M., et al.: Enhanced air stability and high Li-ion conductivity of Li6.988P2.994Nb0.2S10.934O0.6 glass-ceramic electrolyte for all-solid-state lithium-sulfur batteries. ACS Appl. Mater. Interfaces 12, 21548–21558 (2020). https://doi.org/10.1021/acsami.0c00393
Pearson, R.G.: Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963). https://doi.org/10.1021/ja00905a001
Kimura, T., Kato, A., Hotehama, C., et al.: Preparation and characterization of lithium ion conductive Li3SbS4 glass and glass-ceramic electrolytes. Solid State Ion. 333, 45–49 (2019). https://doi.org/10.1016/j.ssi.2019.01.017
Wang, Y.Q., Lü, X., Zheng, C., et al.: Chemistry design towards a stable sulfide-based superionic conductor Li4Cu8Ge3S12. Angew. Chem. Int. Ed. 58, 7673–7677 (2019). https://doi.org/10.1002/anie.201901739
Zhang, Z.R., Zhang, J.X., Sun, Y.L., et al.: Li4-xSbxSn1−xS4 solid solutions for air-stable solid electrolytes. J. Energy Chem. 41, 171–176 (2020). https://doi.org/10.1016/j.jechem.2019.05.015
Kwak, H., Park, K.H., Han, D., et al.: Li+ conduction in air-stable Sb-Substituted Li4SnS4 for all-solid-state Li-ion batteries. J. Power Sources 446, 227338 (2020). https://doi.org/10.1016/j.jpowsour.2019.227338
Zhao, F.P., Liang, J.W., Yu, C., et al.: A versatile Sn-substituted argyrodite sulfide electrolyte for all-solid-state Li metal batteries. Adv. Energy Mater. 10, 1903422 (2020). https://doi.org/10.1002/aenm.201903422
Tan, D.H.S., Banerjee, A., Deng, Z., et al.: Enabling thin and flexible solid-state composite electrolytes by the scalable solution process. ACS Appl. Energy Mater. 2, 6542–6550 (2019). https://doi.org/10.1021/acsaem.9b01111
Li, Y., Arnold, W., Thapa, A., et al.: Stable and flexible sulfide composite electrolyte for high-performance solid-state lithium batteries. ACS Appl. Mater. Interfaces 12, 42653–42659 (2020). https://doi.org/10.1021/acsami.0c08261
Jung, W.D., Jeon, M., Shin, S.S., et al.: Functionalized sulfide solid electrolyte with air-stable and chemical-resistant oxysulfide nanolayer for all-solid-state batteries. ACS Omega 5, 26015–26022 (2020). https://doi.org/10.1021/acsomega.0c03453
Zhu, Y.Z., Mo, Y.F.: Materials design principles for air-stable lithium/sodium solid electrolytes. Angew. Chem. Int. Ed. 59, 17472–17476 (2020). https://doi.org/10.1002/anie.202007621
Wang, C.H., Liang, J.W., Zhao, Y., et al.: All-solid-state lithium batteries enabled by sulfide electrolytes: from fundamental research to practical engineering design. Energy Environ. Sci. 14, 2577–2619 (2021). https://doi.org/10.1039/d1ee00551k
Fukushima, A., Hayashi, A., Yamamura, H., et al.: Mechanochemical synthesis of high lithium ion conducting solid electrolytes in a Li2S-P2S5-Li3N system. Solid State Ion. 304, 85–89 (2017). https://doi.org/10.1016/j.ssi.2017.03.010
Park, K.H., Bai, Q., Kim, D.H., et al.: Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all-solid-state batteries. Adv. Energy Mater. 8, 1800035 (2018). https://doi.org/10.1002/aenm.201800035
Pearson, R.G.: Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA. 83, 8440–8441 (1986). https://doi.org/10.1073/pnas.83.22.8440
Klopman, G.: Chemical reactivity and the concept of charge- and frontier-controlled reactions. J. Am. Chem. Soc. 90, 223–234 (1968). https://doi.org/10.1021/ja01004a00210.1021/ja01004a002
Shang, S.L., Yu, Z.X., Wang, Y., et al.: Origin of outstanding phase and moisture stability in a Na3P1–xAsxS4 superionic conductor. ACS Appl. Mater. Interfaces 9, 16261–16269 (2017). https://doi.org/10.1021/acsami.7b03606
Kim, J.S., Jeon, M., Kim, S., et al.: Structural and electronic descriptors for atmospheric instability of Li-thiophosphate using density functional theory. Solid State Ion. 346, 115225 (2020). https://doi.org/10.1016/j.ssi.2020.115225
Iwasaki, R., Hori, S., Kanno, R., et al.: Weak anisotropic lithium-ion conductivity in single crystals of Li10GeP2S12. Chem. Mater. 31, 3694–3699 (2019). https://doi.org/10.1021/acs.chemmater.9b00420
Kim, J.S., Jung, W.D., Shin, S.S., et al.: Roles of polymerized anionic clusters stimulating for hydrolysis deterioration in Li7P3S11. J. Phys. Chem. C 125, 19509–19516 (2021). https://doi.org/10.1021/acs.jpcc.1c05034
Xu, M., Song, S.B., Daikuhara, S., et al.: Li10GeP2S12-type structured solid solution phases in the Li9+δP3+δ′S12–kOk system: controlling crystallinity by synthesis to improve the air stability. Inorg. Chem. 61, 52–61 (2022). https://doi.org/10.1021/acs.inorgchem.1c01748
Kanazawa, K., Yubuchi, S., Hotehama, C., et al.: Mechanochemical synthesis and characterization of metastable hexagonal Li4SnS4 solid electrolyte. Inorg. Chem. 57, 9925–9930 (2018). https://doi.org/10.1021/acs.inorgchem.8b01049
Calpa, M., Rosero-Navarro, N.C., Miura, A., et al.: Chemical stability of Li4PS4I solid electrolyte against hydrolysis. Appl. Mater. Today 22, 100918 (2021). https://doi.org/10.1016/j.apmt.2020.100918
Tufail, M.K., Zhou, L., Ahmad, N., et al.: A novel air-stable Li7Sb0.05P2.95S10.5I0.5 superionic conductor glass-ceramics electrolyte for all-solid-state lithium-sulfur batteries. Chem. Eng. J. 407, 127149 (2021). https://doi.org/10.1016/j.cej.2020.127149
Lee, Y., Jeong, J., Lee, H.J., et al.: Lithium argyrodite sulfide electrolytes with high ionic conductivity and air stability for all-solid-state Li-ion batteries. ACS Energy Lett. 7, 171–179 (2022). https://doi.org/10.1021/acsenergylett.1c02428
Lu, P.S., Liu, L.L., Wang, S., et al.: Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity. Adv. Mater. 33, 2100921 (2021). https://doi.org/10.1002/adma.202100921
Khurram Tufail, M., Ahmad, N., Zhou, L., et al.: Insight on air-induced degradation mechanism of Li7P3S11 to design a chemical-stable solid electrolyte with high Li2S utilization in all-solid-state Li/S batteries. Chem. Eng. J. 425, 130535 (2021). https://doi.org/10.1016/j.cej.2021.130535
Li, Y.Y., Li, J.W., Cheng, J., et al.: Enhanced air and electrochemical stability of Li7P3S11-based solid electrolytes enabled by aliovalent substitution of SnO2. Adv. Mater. Interfaces 8, 2100368 (2021). https://doi.org/10.1002/admi.202100368
Tian, Y.S., Sun, Y.Z., Hannah, D.C., et al.: Reactivity-guided interface design in Na metal solid-state batteries. Joule 3, 1037–1050 (2019). https://doi.org/10.1016/j.joule.2018.12.019
Xu, J.R., Li, Y.X., Lu, P.S., et al.: Water-stable sulfide solid electrolyte membranes directly applicable in all-solid-state batteries enabled by superhydrophobic Li+-conducting protection layer. Adv. Energy Mater. 12, 2102348 (2022). https://doi.org/10.1002/aenm.202102348
Joos, M., Schneider, C., Münchinger, A., et al.: Impact of hydration on ion transport in Li2Sn2S5·xH2O. J. Mater. Chem. A 9, 16532–16544 (2021). https://doi.org/10.1039/d1ta04736a
Cho, W., Kim, W.J., Lee, D.G., et al.: Enhanced air-stability of argyrodite solid electrolyte by introducing zeolite additive as H2S scavenger. Meet. Abstr. MA2020-02, 951 (2020). https://doi.org/10.1149/ma2020-025951mtgabs
Ye, L.H., Gil-González, E., Li, X.: Li9.54Si1.74(P1−xSbx)1.44S11.7Cl0.3: a functionally stable sulfide solid electrolyte in air for solid-state batteries. Electrochem. Commun. 128, 107058 (2021). https://doi.org/10.1016/j.elecom.2021.107058
Ohtomo, T., Hayashi, A., Tatsumisago, M., et al.: Suppression of H2S gas generation from the 75Li2S·25P2S5 glass electrolyte by additives. J. Mater. Sci. 48, 4137–4142 (2013). https://doi.org/10.1007/s10853-013-7226-8
Zhao, F.P., Alahakoon, S.H., Adair, K., et al.: An air-stable and Li-metal-compatible glass-ceramic electrolyte enabling high-performance all-solid-state Li metal batteries. Adv. Mater. 33, 2006577 (2021). https://doi.org/10.1002/adma.202006577
Kaib, T., Haddadpour, S., Kapitein, M., et al.: New lithium chalcogenidotetrelates, LiChT: synthesis and characterization of the Li+-conducting tetralithium ortho-sulfidostannate Li4SnS4. Chem. Mater. 24, 2211–2219 (2012). https://doi.org/10.1021/cm3011315
Zhang, Q., Cao, D.X., Ma, Y., et al.: Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries. Adv. Mater. 31, 1901131 (2019). https://doi.org/10.1002/adma.201901131
Ozekmekci, M., Salkic, G., Fellah, M.F.: Use of zeolites for the removal of H2S: a mini-review. Fuel Process. Technol. 139, 49–60 (2015). https://doi.org/10.1016/j.fuproc.2015.08.015
Sigot, L., Ducom, G., Germain, P.: Adsorption of hydrogen sulfide (H2S) on zeolite (Z): retention mechanism. Chem. Eng. J. 287, 47–53 (2016). https://doi.org/10.1016/j.cej.2015.11.010
Lee, D.G., Park, K.H., Kim, S.Y., et al.: Critical role of zeolites as H2S scavengers in argyrodite Li6PS5Cl solid electrolytes for all-solid-state batteries. J. Mater. Chem. A 9, 17311–17316 (2021). https://doi.org/10.1039/d1ta04799j
Ren, H.T., Zhang, Z.Q., Zhang, J.Z., et al.: Improvement of stability and solid-state battery performances of annealed 70Li2S–30P2S5 electrolytes by additives. Rare Met. 41, 106–114 (2022). https://doi.org/10.1007/s12598-021-01804-2
Tao, Y.C., Chen, S.J., Liu, D., et al.: Lithium superionic conducting oxysulfide solid electrolyte with excellent stability against lithium metal for all-solid-state cells. J. Electrochem. Soc. 163, A96–A101 (2015). https://doi.org/10.1149/2.0311602jes
Raj, R., Wolfenstine, J.: Current limit diagrams for dendrite formation in solid-state electrolytes for Li-ion batteries. J. Power Sources 343, 119–126 (2017). https://doi.org/10.1016/j.jpowsour.2017.01.037
Lu, Y., Zhao, C.Z., Yuan, H., et al.: Critical current density in solid-state lithium metal batteries: mechanism, influences, and strategies. Adv. Funct. Mater. 31, 2009925 (2021). https://doi.org/10.1002/adfm.202009925
Peng, L.F., Chen, S.Q., Yu, C., et al.: Enhancing moisture and electrochemical stability of the Li5.5PS4.5Cl1.5 electrolyte by oxygen doping. ACS Appl. Mater. Interfaces 14, 4179–4185 (2022). https://doi.org/10.1021/acsami.1c21561
Xu, H.J., Cao, G.Q., Shen, Y.L., et al.: Enabling argyrodite sulfides as superb solid-state electrolyte with remarkable interfacial stability against electrodes. Energy Environ. Mater (2022). https://doi.org/10.1002/eem2.12282
Xie, D.J., Chen, S.J., Zhang, Z.H., et al.: High ion conductive Sb2O5-doped β-Li3PS4 with excellent stability against Li for all-solid-state lithium batteries. J. Power Sources 389, 140–147 (2018). https://doi.org/10.1016/j.jpowsour.2018.04.021
Zhao, B.S., Wang, L., Chen, P., et al.: Congener substitution reinforced Li7P2.9Sb0.1S10.75O0.25 glass-ceramic electrolytes for all-solid-state lithium-sulfur batteries. ACS Appl. Mater. Interfaces 13, 34477–34485 (2021). https://doi.org/10.1021/acsami.1c10238
Wu, L.P., Liu, G.Z., Wan, H.L., et al.: Superior lithium-stable Li7P2S8I solid electrolyte for all-solid-state lithium batteries. J. Power Sources 491, 229565 (2021). https://doi.org/10.1016/j.jpowsour.2021.229565
Rajagopal, R., Cho, J.U., Subramanian, Y., et al.: Preparation of highly conductive metal doped/substituted Li7P2S8Br(1–x)Ix type lithium superionic conductor for all-solid-state lithium battery applications. Chem. Eng. J. 428, 132155 (2022). https://doi.org/10.1016/j.cej.2021.132155
Deiseroth, H.J., Kong, S.T., Eckert, H., et al.: Li6PS5X: a class of crystalline Li-rich solids with an unusually high li+ mobility. Angew. Chem. Int. Ed. 47, 755–758 (2008). https://doi.org/10.1002/anie.200703900
Taklu, B.W., Su, W.N., Nikodimos, Y., et al.: Dual CuCl doped argyrodite superconductor to boost the interfacial compatibility and air stability for all solid-state lithium metal batteries. Nano Energy 90, 106542 (2021). https://doi.org/10.1016/j.nanoen.2021.106542
Zhou, L., Tufail, M.K., Ahmad, N., et al.: Strong interfacial adhesion between the Li2S cathode and a functional Li7P2.9Ce0.2S10.9Cl0.3 solid-state electrolyte endowed long-term cycle stability to all-solid-state lithium-sulfur batteries. ACS Appl. Mater. Interfaces 13, 28270–28280 (2021). https://doi.org/10.1021/acsami.1c06328
Yu, Z.X., Shang, S.L., Seo, J.H., et al.: Exceptionally high ionic conductivity in Na3P0.62As0.38S4 with improved moisture stability for solid-state sodium-ion batteries. Adv. Mater. 29, 1605561 (2017). https://doi.org/10.1002/adma.201605561
Jiang, Z., Peng, H.L., Liu, Y., et al.: A versatile Li6.5In0.25P0.75S5I sulfide electrolyte triggered by ultimate-energy mechanical alloying for all-solid-state lithium metal batteries. Adv. Energy Mater. 11, 2101521 (2021). https://doi.org/10.1002/aenm.202101521
Subramanian, Y., Rajagopal, R., Ryu, K.S.: Synthesis, air stability and electrochemical investigation of lithium superionic bromine substituted argyrodite (Li6−xPS5−xCl1.0Brx) for all-solid-state lithium batteries. J. Power Sources 520, 230849 (2022). https://doi.org/10.1016/j.jpowsour.2021.230849
Min, S., Park, C., Yoon, I., et al.: Enhanced electrochemical stability and moisture reactivity of Al2S3 doped argyrodite solid electrolyte. J. Electrochem. Soc. 168, 070511 (2021). https://doi.org/10.1149/1945-7111/ac0f5c
Holzmann, T., Schoop, L.M., Ali, M.N., et al.: Li0.6 [Li0.2Sn0.8S2]: a layered lithium superionic conductor. Energy Environ. Sci. 9, 2578–2585 (2016). https://doi.org/10.1039/c6ee00633g
Kuhn, A., Holzmann, T., Nuss, J., et al.: A facile wet chemistry approach towards unilamellar tin sulfide nanosheets from Li4xSn1–xS2 solid solutions. J. Mater. Chem. A 2, 6100–6106 (2014). https://doi.org/10.1039/c3ta14190j
Brant, J.A., Massi, D.M., Holzwarth, N.A.W., et al.: Fast lithium ion conduction in Li2SnS3: synthesis, physicochemical characterization, and electronic structure. Chem. Mater. 27, 189–196 (2015). https://doi.org/10.1021/cm5037524
Choi, Y.E., Park, K.H., Kim, D.H., et al.: Coatable Li4SnS4 solid electrolytes prepared from aqueous solutions for all-solid-state lithium-ion batteries. Chemsuschem 10, 2605–2611 (2017). https://doi.org/10.1002/cssc.201700409
Matsuda, R., Kokubo, T., Phuc, N.H.H., et al.: Preparation of ambient air-stable electrolyte Li4SnS4 by aqueous ion-exchange process. Solid State Ion. 345, 115190 (2020). https://doi.org/10.1016/j.ssi.2019.115190
Xu, J., Liu, L., Yao, N., et al.: Liquid-involved synthesis and processing of sulfide-based solid electrolytes, electrodes, and all-solid-state batteries. Mater. Today Nano 8, 100048 (2019). https://doi.org/10.1016/j.mtnano.2019.100048
Schiwy, W., Pohl, S., Krebs, B.: Darstellung und struktur von Na4SnS4—14H2O. Z. Anorg. Allg. Chem. 402, 77–86 (1973). https://doi.org/10.1002/zaac.19734020110
Heo, J.W., Banerjee, A., Park, K.H., et al.: New Na-ion solid electrolytes Na4−xSn1−xSbxS4 (0.02 ≤ x ≤ 0.33) for all-solid-state Na-ion batteries. Adv. Energy Mater. 8, 1702716 (2018). https://doi.org/10.1002/aenm.201702716
Jia, H.H., Sun, Y.L., Zhang, Z.R., et al.: Group 14 element based sodium chalcogenide Na4Sn0.67Si0.33S4 as structure template for exploring sodium superionic conductors. Energy Storage Mater. 23, 508–513 (2019). https://doi.org/10.1016/j.ensm.2019.04.011
Xiong, S., Liu, Z.T., Yang, L.F., et al.: Anion and cation co-doping of Na4SnS4 as sodium superionic conductors. Mater. Today Phys. 15, 100281 (2020). https://doi.org/10.1016/j.mtphys.2020.100281
Wang, H., Chen, Y., Hood, Z.D., et al.: An air-stable Na3SbS4 superionic conductor prepared by a rapid and economic synthetic procedure. Angew. Chem. Int. Ed. 55, 8551–8555 (2016). https://doi.org/10.1002/anie.201601546
Zhang, L., Zhang, D.C., Yang, K., et al.: Vacancy-contained tetragonal Na3SbS4 superionic conductor. Adv. Sci. 3, 1600089 (2016). https://doi.org/10.1002/advs.201600089
Banerjee, A., Park, K.H., Heo, J.W., et al.: Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. Int. Ed. 55, 9634–9638 (2016). https://doi.org/10.1002/anie.201604158
Kim, T.W., Park, K.H., Choi, Y.E., et al.: Aqueous-solution synthesis of Na3SbS4 solid electrolytes for all-solid-state Na-ion batteries. J. Mater. Chem. A 6, 840–844 (2018). https://doi.org/10.1039/c7ta09242c
Hayashi, A., Masuzawa, N., Yubuchi, S., et al.: A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature. Nat. Commun. 10, 5266 (2019). https://doi.org/10.1038/s41467-019-13178-2
Fuchs, T., Culver, S.P., Till, P., et al.: Defect-mediated conductivity enhancements in Na3–xPn1–xWxS4 (Pn = P, Sb) using aliovalent substitutions. ACS Energy Lett. 5, 146–151 (2020). https://doi.org/10.1021/acsenergylett.9b02537
Yubuchi, S., Ito, A., Masuzawa, N., et al.: Aqueous solution synthesis of Na3SbS4–Na2WS4 superionic conductors. J. Mater. Chem. A 8, 1947–1954 (2020). https://doi.org/10.1039/c9ta02246e
Tsuji, F., Yubuchi, S., Sakuda, A., et al.: Preparation of sodium-ion-conductive Na3–xSbS4–xClx solid electrolytes. J. Ceram. Soc. Japan 128, 641–647 (2020). https://doi.org/10.2109/jcersj2.20089
Wang, X.L., Xiao, R.J., Li, H., et al.: Oxysulfide LiAlSO: a lithium superionic conductor from first principles. Phys. Rev. Lett. 118, 195901 (2017). https://doi.org/10.1103/physrevlett.118.195901
Kuo, D.H., Lo, R., Hsueh, T.H., et al.: LiSnOS/gel polymer hybrid electrolyte for the safer and performance-enhanced solid-state LiCoO2/Li lithium-ion battery. J. Power Sources 429, 89–96 (2019). https://doi.org/10.1016/j.jpowsour.2019.05.010
Gamon, J., Duff, B.B., Dyer, M.S., et al.: Computationally guided discovery of the sulfide Li3AlS3 in the Li-Al-S phase field: structure and lithium conductivity. Chem. Mater. 31, 9699–9714 (2019). https://doi.org/10.1021/acs.chemmater.9b03230
Sedlmaier, S.J., Indris, S., Dietrich, C., et al.: Li4PS4I: a Li+ superionic conductor synthesized by a solvent-based soft chemistry approach. Chem. Mater. 29, 1830–1835 (2017). https://doi.org/10.1021/acs.chemmater.7b00013
Zhou, L.D., Assoud, A., Zhang, Q., et al.: New family of argyrodite thioantimonate lithium superionic conductors. J. Am. Chem. Soc. 141, 19002–19013 (2019). https://doi.org/10.1021/jacs.9b08357
Sun, X., Stavola, A.M., Cao, D.X., et al.: Operando EDXRD study of all-solid-state lithium batteries coupling thioantimonate superionic conductors with metal sulfide. Adv. Energy Mater. 11, 2002861 (2021). https://doi.org/10.1002/aenm.202002861
Lee, Y., Jeong, J., Lim, H.D., et al.: Superionic Si-substituted lithium argyrodite sulfide electrolyte Li6+xSb1–xSixS5I for all-solid-state batteries. ACS Sustain. Chem. Eng. 9, 120–128 (2021). https://doi.org/10.1021/acssuschemeng.0c05549
Richards, W.D., Tsujimura, T., Miara, L.J., et al.: Design and synthesis of the superionic conductor Na10SnP2S12. Nat. Commun. 7, 11009 (2016). https://doi.org/10.1038/ncomms11009
Zhang, Z., Ramos, E., Lalère, F., et al.: Na11Sn2PS12: a new solid state sodium superionic conductor. Energy Environ. Sci. 11, 87–93 (2018). https://doi.org/10.1039/c7ee03083e
Duchardt, M., Ruschewitz, U., Adams, S., et al.: Vacancy-controlled Na+ superion conduction in Na11Sn2PS12. Angew. Chem. Int. Ed. 57, 1351–1355 (2018). https://doi.org/10.1002/anie.201712769
Ramos, E.P., Zhang, Z.Z., Assoud, A., et al.: Correlating ion mobility and single crystal structure in sodium-ion chalcogenide-based solid state fast ion conductors: Na11Sn2PnS12 (Pn = Sb, P). Chem. Mater. 30, 7413–7417 (2018). https://doi.org/10.1021/acs.chemmater.8b02077
Weng, W., Liu, G.Z., Shen, L., et al.: High ionic conductivity and stable phase Na11.5Sn2Sb0.5Ti0.5S12 for all-solid-state sodium batteries. J. Power Sources 512, 230485 (2021). https://doi.org/10.1016/j.jpowsour.2021.230485
Liu, G.Z., Sun, X.R., Yu, X.Q., et al.: Na10SnSb2S12: a nanosized air-stable solid electrolyte for all-solid-state sodium batteries. Chem. Eng. J. 420, 127692 (2021). https://doi.org/10.1016/j.cej.2020.127692
Fan, B., Xu, Y.H., Ma, R., et al.: Will sulfide electrolytes be suitable candidates for constructing a stable solid/liquid electrolyte interface? ACS Appl. Mater. Interfaces 12, 52845–52856 (2020). https://doi.org/10.1021/acsami.0c16899
Liu, G.Z., Shi, J.M., Zhu, M.T., et al.: Ultra-thin free-standing sulfide solid electrolyte film for cell-level high energy density all-solid-state lithium batteries. Energy Storage Mater. 38, 249–254 (2021). https://doi.org/10.1016/j.ensm.2021.03.017
Tsukasaki, H., Igarashi, K., Wakui, A., et al.: In situ observation of the deterioration process of sulfide-based solid electrolytes using airtight and air-flow TEM systems. Microscopy 70, 519–525 (2021). https://doi.org/10.1093/jmicro/dfab022
Kim, Y., Saienga, J., Martin, S.W.: Glass formation in and structural investigation of Li2S + GeS2 + GeO2 composition using Raman and IR spectroscopy. J. Non Cryst. Solids 351, 3716–3724 (2005). https://doi.org/10.1016/j.jnoncrysol.2005.09.028
Ohtomo, T., Hayashi, A., Tatsumisago, M., et al.: All-solid-state batteries with Li2O-Li2S-P2S5 glass electrolytes synthesized by two-step mechanical milling. J. Solid State Electrochem. 17, 2551–2557 (2013). https://doi.org/10.1007/s10008-013-2149-5
Yohannan, J.P., Vidyasagar, K.: Syntheses, structural variants and characterization of AInM’S4 (A=alkali metals, Tl; M’ = Ge, Sn) compounds; facile ion-exchange reactions of layered NaInSnS4 and KInSnS4 compounds. J. Solid State Chem. 238, 291–302 (2016). https://doi.org/10.1016/j.jssc.2016.03.045
Ohtomo, T., Hayashi, A., Tatsumisago, M., et al.: Glass electrolytes with high ion conductivity and high chemical stability in the system LiI-Li2O-Li2S-P2S5. Electrochemistry 81, 428–431 (2013). https://doi.org/10.5796/electrochemistry.81.428
Sahu, G., Rangasamy, E., Li, J.C., et al.: A high-conduction Ge substituted Li3AsS4 solid electrolyte with exceptional low activation energy. J. Mater. Chem. A 2, 10396–10403 (2014). https://doi.org/10.1039/c4ta01243g
Acknowledgements
This work is supported by the Key Program-Automobile Joint Fund of the National Natural Science Foundation of China (Grant No. U1964205), the Key R&D Project funded by the Department of Science and Technology of Jiangsu Province (Grant No. BE2020003), the General Program of the National Natural Science Foundation of China (Grant No. 51972334), the General Program of the National Natural Science Foundation of Beijing (Grant No. 2202058), the Cultivation Project of Leading Innovative Experts in Changzhou City (CQ20210003), the National Overseas High-Level Expert Recruitment Program (Grant No. E1JF021E11), the Talent Program of the Chinese Academy of Sciences, “Scientist Studio Program Funding” from the Yangtze River Delta Physics Research Center and the Tianmu Lake Institute of Advanced Energy Storage Technologies (Grant No. TIES-SS0001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Lu, P., Wu, D., Chen, L. et al. Air Stability of Solid-State Sulfide Batteries and Electrolytes. Electrochem. Energy Rev. 5, 3 (2022). https://doi.org/10.1007/s41918-022-00149-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00149-3