Abstract
The most commonly used electrode materials in lithium organic batteries (LOBs) are redox-active organic materials, which have the advantages of low cost, environmental safety, and adjustable structures. Although the use of organic materials as electrodes in LOBs has been reported, these materials have not attained the same recognition as inorganic electrode materials, mainly due to their slight electronic conductivity and possible solubility in organic electrolytes, resulting in a low reversible capacity. However, over the past 10 years, organic materials have achieved outstanding results when used as battery electrodes, and an increasing number of researchers have realized their significance. This review summarizes the recent progress in organic electrodes for use in rechargeable LOBs. By classifying Li-storage mechanisms with various functional organic groups and designing molecules for next-generation advanced lithium organic systems, we attempt to analyze the working principle and the effect of various organic functionalities on electrochemical performance, to reveal the advantages and disadvantages of various organic molecules and to propose possible design principles and development trends for future LOBs. In addition, we highlight the recently reported two-dimensional covalent organic framework that is unique in its extensive π conjugated structure and Li-storage mechanisms based on benzene and N-containing rings; this framework is considered to be the most promising alternative to metal-based electrode materials with comparable large reversible capacities and long cycle lives.
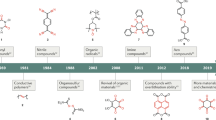
Graphical abstract




Copyright © 2016, Wiley–VCH

Copyright © 2017, Wiley–VCH

Copyright © 2015, Wiley–VCH

Copyright © 2015, Wiley–VCH

Copyright © 2018, Nature Group

Copyright © 2018, Wiley–VCH

Copyright © 2020, American Chemical Society

Copyright © 2018, Wiley–VCH

Similar content being viewed by others
References
Li, S., Cheng, C., Thomas, A.: Carbon-based microbial-fuel-cell electrodes: from conductive supports to active catalysts. Adv. Mater. 29, 1602547 (2017). https://doi.org/10.1002/adma.201602547
Singh, R., Polu, A.R., Bhattacharya, B., et al.: Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew. Sustain. Energy Rev. 65, 1098–1117 (2016). https://doi.org/10.1016/j.rser.2016.06.026
Chen, X.D., Zhang, H., Ci, C.G., et al.: Few-layered boronic ester based covalent organic frameworks/carbon nanotube composites for high-performance K-organic batteries. ACS Nano 13, 3600–3607 (2019). https://doi.org/10.1021/acsnano.9b00165
Chen, X.D., Xu, Y.J., Du, F.H., et al.: Covalent organic framework derived boron/oxygen codoped porous carbon on CNTs as an efficient sulfur host for lithium-sulfur batteries. Small Methods 3, 1900338 (2019). https://doi.org/10.1002/smtd.201900338
Choi, J.W., Aurbach, D.: Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016). https://doi.org/10.1038/natrevmats.2016.13
Wang, J., He, X., Paillard, E., et al.: Lithium- and manganese-rich oxide cathode materials for high-energy lithium ion batteries. Adv. Energy Mater. 6, 1600906 (2016). https://doi.org/10.1002/aenm.201600906
Li, W., Song, B., Manthiram, A.: High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017). https://doi.org/10.1039/c6cs00875e
Zheng, J.M., Myeong, S., Cho, W., et al.: Li- and Mn-rich cathode materials: challenges to commercialization. Adv. Energy Mater. 7, 1601284 (2017). https://doi.org/10.1002/aenm.201601284
Zhao, Y., Wang, L.P., Sougrati, M.T., et al.: A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance Li and Na ion battery anodes. Adv. Energy Mater. 7, 1601424 (2017). https://doi.org/10.1002/aenm.201601424
Gu, Y., Wu, F.D., Wang, Y.: Confined volume change in Sn-Co-C ternary tube-in-tube composites for high-capacity and long-life lithium storage. Adv. Funct. Mater. 23, 893–899 (2013). https://doi.org/10.1002/adfm.201202136
Zou, Y., Wang, Y.: Sn@CNT nanostructures rooted in graphene with high and fast Li-storage capacities. ACS Nano 5, 8108–8114 (2011). https://doi.org/10.1021/nn2027159
Keppeler, M., Shen, N., Nageswaran, S., et al.: Synthesis of α-Fe2O3/carbon nanocomposites as high capacity electrodes for next generation lithium ion batteries: a review. J. Mater. Chem. A 4, 18223–18239 (2016). https://doi.org/10.1039/c6ta08456g
Hu, X.S., Li, C., Lou, X.B., et al.: Hierarchical CuO octahedra inherited from copper metal-organic frameworks: high-rate and high-capacity lithium-ion storage materials stimulated by pseudocapacitance. J. Mater. Chem. A 5, 12828–12837 (2017). https://doi.org/10.1039/c7ta02953e
Jiang, T., Bu, F., Feng, X., et al.: Porous Fe2O3 nanoframeworks encapsulated within three-dimensional graphene as high-performance flexible anode for lithium-ion battery. ACS Nano 11, 5140–5147 (2017). https://doi.org/10.1021/acsnano.7b02198
Dong, S., Li, C., Ge, X., et al.: ZnS-Sb2S3@C core-double shell polyhedron structure derived from metal-organic framework as anodes for high performance sodium ion batteries. ACS Nano 11, 6474–6482 (2017). https://doi.org/10.1021/acsnano.7b03321
Li, H., Su, Y., Sun, W.W., et al.: Carbon nanotubes rooted in porous ternary metal sulfide@N/S-doped carbon dodecahedron: bimetal-organic-frameworks derivation and electrochemical application for high-capacity and long-life lithium-ion batteries. Adv. Funct. Mater. 26, 8345–8353 (2016). https://doi.org/10.1002/adfm.201601631
Xia, G.L., Su, J.W., Li, M.S., et al.: A MOF-derived self-template strategy toward cobalt phosphide electrodes with ultralong cycle life and high capacity. J. Mater. Chem. A 5, 10321–10327 (2017). https://doi.org/10.1039/c7ta02600e
Lou, P., Cui, Z., Jia, Z., et al.: Monodispersed carbon-coated cubic NiP2 nanoparticles anchored on carbon nanotubes as ultra-long-life anodes for reversible lithium storage. ACS Nano 11, 3705–3715 (2017). https://doi.org/10.1021/acsnano.6b08223
Wang, X.F., Kong, D.Z., Huang, Z.X., et al.: Nontopotactic reaction in highly reversible sodium storage of ultrathin Co9Se8/rGO hybrid nanosheets. Small 13, 1603980 (2017). https://doi.org/10.1002/smll.201603980
Chen, X.D., Lv, L.P., Sun, W.W., et al.: Ultrasmall MoC nanoparticles embedded in 3D frameworks of nitrogen-doped porous carbon as anode materials for efficient lithium storage with pseudocapacitance. J. Mater. Chem. A 6, 13705–13716 (2018). https://doi.org/10.1039/c8ta03176b
Huang, H., Gao, S., Wu, A.M., et al.: Fe3N constrained inside C nanocages as an anode for Li-ion batteries through post-synthesis nitridation. Nano Energy 31, 74–83 (2017). https://doi.org/10.1016/j.nanoen.2016.10.059
Rahman, M.A., Song, G.S., Bhatt, A.I., et al.: Nanostructured silicon anodes for high-performance lithium-ion batteries. Adv. Funct. Mater. 26, 647–678 (2016). https://doi.org/10.1002/adfm.201502959
Choi, S., Kwon, T.W., Coskun, A., et al.: Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 357, 279–283 (2017). https://doi.org/10.1126/science.aal4373
Du, F.H., Wang, K.X., Chen, J.S.: Strategies to succeed in improving the lithium-ion storage properties of silicon nanomaterials. J. Mater. Chem. A 4, 32–50 (2016). https://doi.org/10.1039/c5ta06962a
Zhou, L., Zhuang, Z.C., Zhao, H.H., et al.: Intricate hollow structures: controlled synthesis and applications in energy storage and conversion. Adv. Mater. 29, 1602914 (2017). https://doi.org/10.1002/adma.201602914
Guo, W.X., Sun, W.W., Wang, Y.: Multilayer CuO@NiO hollow spheres: microwave-assisted metal-organic-framework derivation and highly reversible structure-matched stepwise lithium storage. ACS Nano 9, 11462–11471 (2015). https://doi.org/10.1021/acsnano.5b05610
Zhang, K., Li, P., Ma, M., et al.: Core-shelled low-oxidation state oxides@reduced graphene oxides cubes via pressurized reduction for highly stable lithium ion storage. Adv. Funct. Mater. 26, 2959–2965 (2016). https://doi.org/10.1149/ma2016-02/3/377
Tan, G.Q., Wu, F., Yuan, Y.F., et al.: Freestanding three-dimensional core–shell nanoarrays for lithium-ion battery anodes. Nat. Commun. 7, 11774 (2016). https://doi.org/10.1038/ncomms11774
Ma, L.B., Yan, P.J., Wu, S.K., et al.: Engineering tin phosphides@carbon yolk–shell nanocube structures as a highly stable anode material for sodium-ion batteries. J. Mater. Chem. A 5, 16994–17000 (2017). https://doi.org/10.1039/c7ta04900e
Zhang, M., Liu, E.Z., Cao, T.T., et al.: Sandwiched graphene inserted with graphene-encapsulated yolk–shell γ-Fe2O3 nanoparticles for efficient lithium ion storage. J. Mater. Chem. A 5, 7035–7042 (2017). https://doi.org/10.1039/c7ta01239j
Nevers, D.R., Brushett, F.R., Wheeler, D.R.: Engineering radical polymer electrodes for electrochemical energy storage. J. Power Sources 352, 226–244 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.077
Flamme, B., Rodriguez Garcia, G., Weil, M., et al.: Guidelines to design organic electrolytes for lithium-ion batteries: environmental impact, physicochemical and electrochemical properties. Green Chem. 19, 1828–1849 (2017). https://doi.org/10.1039/c7gc00252a
Xie, J., Gu, P.Y., Zhang, Q.C.: Nanostructured conjugated polymers: toward high-performance organic electrodes for rechargeable batteries. ACS Energy Lett. 2, 1985–1996 (2017). https://doi.org/10.1021/acsenergylett.7b00494
Liang, Y.L., Tao, Z.L., Chen, J.: Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2, 742–769 (2012). https://doi.org/10.1002/aenm.201100795
Muench, S., Wild, A., Friebe, C., et al.: Polymer-based organic batteries. Chem. Rev. 116, 9438–9484 (2016). https://doi.org/10.1021/acs.chemrev.6b00070
Häupler, B., Wild, A., Schubert, U.S.: Carbonyls: powerful organic materials for secondary batteries. Adv. Energy Mater. 5, 1402034 (2015). https://doi.org/10.1002/aenm.201402034
Williams, D.L., Byrne, J.J., Driscoll, J.S.: A high energy density lithium/dichloroisocyanuric acid battery system. J. Electrochem. Soc. 116, 2 (1969). https://doi.org/10.1149/1.2411755
Wu, Y.W., Zeng, R.H., Nan, J.M., et al.: Quinone electrode materials for rechargeable lithium/sodium ion batteries. Adv. Energy Mater. 7, 1700278 (2017). https://doi.org/10.1002/aenm.201700278
Xie, J., Zhang, Q.C.: Recent progress in rechargeable lithium batteries with organic materials as promising electrodes. J. Mater. Chem. A 4, 7091–7106 (2016). https://doi.org/10.1039/c6ta01069e
Zhao, Q., Guo, C.Y., Lu, Y., et al.: Rechargeable lithium batteries with electrodes of small organic carbonyl salts and advanced electrolytes. Ind. Eng. Chem. Res. 55, 5795–5804 (2016). https://doi.org/10.1021/acs.iecr.6b01462
Zhu, Z.Q., Chen, J.: Review: advanced carbon-supported organic electrode materials for lithium (sodium)-ion batteries. J. Electrochem. Soc. 162, A2393–A2405 (2015). https://doi.org/10.1149/2.0031514jes
Zhong, Y.R., Yang, M., Zhou, X.L., et al.: Structural design for anodes of lithium-ion batteries: emerging horizons from materials to electrodes. Mater. Horiz. 2, 553–566 (2015). https://doi.org/10.1039/c5mh00136f
Bresser, D., Passerini, S., Scrosati, B.: Leveraging valuable synergies by combining alloying and conversion for lithium-ion anodes. Energy Environ. Sci. 9, 3348–3367 (2016). https://doi.org/10.1039/c6ee02346k
Song, Z.P., Zhou, H.S.: Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ. Sci. 6, 2280–2301 (2013). https://doi.org/10.1039/c3ee40709h
Miroshnikov, M., Divya, K.P., Babu, G., et al.: Power from nature: designing green battery materials from electroactive quinone derivatives and organic polymers. J. Mater. Chem. A 4, 12370–12386 (2016). https://doi.org/10.1039/c6ta03166h
Janoschka, T., Hager, M.D., Schubert, U.S.: Powering up the future: radical polymers for battery applications. Adv. Mater. 24, 6397–6409 (2012). https://doi.org/10.1002/adma.201203119
Zhang, Y.G., Wang, J.Q., Riduan, S.N.: Strategies toward improving the performance of organic electrodes in rechargeable lithium (sodium) batteries. J. Mater. Chem. A 4, 14902–14914 (2016). https://doi.org/10.1039/c6ta05231b
Schon, T.B., McAllister, B.T., Li, P.F., et al.: The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 45, 6345–6404 (2016). https://doi.org/10.1039/c6cs00173d
Lu, Y., Zhang, Q., Li, L., et al.: Design strategies toward enhancing the performance of organic electrode materials in metal-ion batteries. Chem 4, 2786–2813 (2018). https://doi.org/10.1016/j.chempr.2018.09.005
Lu, Y., Chen, J.: Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 4, 127–142 (2020). https://doi.org/10.1038/s41570-020-0160-9
Wang, Y., Deng, Y.H., Qu, Q.T., et al.: Ultrahigh-capacity organic anode with high-rate capability and long cycle life for lithium-ion batteries. ACS Energy Lett. 2, 2140–2148 (2017). https://doi.org/10.1021/acsenergylett.7b00622
Chen, L., Liu, S.H., Zhao, L., et al.: OH-substituted 2,3-dichloro-5,6-dicyano-1,4-benzoquinone as highly stable organic electrode for lithium ion battery. Electrochim. Acta 258, 677–683 (2017). https://doi.org/10.1016/j.electacta.2017.11.113
Chen, D.Y., Avestro, A.J., Chen, Z.H., et al.: A rigid naphthalenediimide triangle for organic rechargeable lithium-ion batteries. Adv. Mater. 27, 2907–2912 (2015). https://doi.org/10.1002/adma.201405416
Kim, D.J., Hermann, K.R., Prokofjevs, A., et al.: Redox-active macrocycles for organic rechargeable batteries. J. Am. Chem. Soc. 139, 6635–6643 (2017). https://doi.org/10.1021/jacs.7b01209
Wu, D.H., Xie, Z.J., Zhou, Z., et al.: Designing high-voltage carbonyl-containing polycyclic aromatic hydrocarbon cathode materials for Li-ion batteries guided by Clar’s theory. J. Mater. Chem. A 3, 19137–19143 (2015). https://doi.org/10.1039/c5ta05437k
Iordache, A., Maurel, V., Mouesca, J.M., et al.: Monothioanthraquinone as an organic active material for greener lithium batteries. J. Power Sources 267, 553–559 (2014). https://doi.org/10.1016/j.jpowsour.2014.05.050
Liang, Y.L., Zhang, P., Yang, S.Q., et al.: Fused heteroaromatic organic compounds for high-power electrodes of rechargeable lithium batteries. Adv. Energy Mater. 3, 600–605 (2013). https://doi.org/10.1002/aenm.201200947
Yokoji, T., Matsubara, H., Satoh, M.: Rechargeable organic lithium-ion batteries using electron-deficient benzoquinones as positive-electrode materials with high discharge voltages. J. Mater. Chem. A 2, 19347–19354 (2014). https://doi.org/10.1039/c4ta02812k
Chen, J., Zhang, Q., Zeng, M., et al.: Carboxyl-conjugated phthalocyanines used as novel electrode materials with high specific capacity for lithium-ion batteries. J. Solid State Electrochem. 20, 1285–1294 (2016). https://doi.org/10.1007/s10008-016-3126-6
Zeng, R.H., Xing, L.D., Qiu, Y.C., et al.: Polycarbonyl(quinonyl) organic compounds as cathode materials for sustainable lithium ion batteries. Electrochim. Acta 146, 447–454 (2014). https://doi.org/10.1016/j.electacta.2014.09.082
Yokoji, T., Kameyama, Y., Sakaida, S., et al.: Steric effects on the cyclability of benzoquinone-type organic cathode active materials for rechargeable batteries. Chem. Lett. 44, 1726–1728 (2015). https://doi.org/10.1246/cl.150836
Kim, D.J., Je, S.H., Sampath, S., et al.: Effect of N-substitution in naphthalenediimides on the electrochemical performance of organic rechargeable batteries. RSC Adv. 2, 7968–7970 (2012). https://doi.org/10.1039/c2ra21239k
Yao, M., Ando, H., Kiyobayashi, T.: Polycyclic quinone fused by a sulfur-containing ring as an organic positive-electrode material for use in rechargeable lithium batteries. Energy Procedia 89, 222–230 (2016). https://doi.org/10.1016/j.egypro.2016.05.029
Yokoji, T., Kameyama, Y., Maruyama, N., et al.: High-capacity organic cathode active materials of 2,2′-bis-p-benzoquinone derivatives for rechargeable batteries. J. Mater. Chem. A 4, 5457–5466 (2016). https://doi.org/10.1039/c5ta10713j
Zhang, Y.S., Murtaza, I., Liu, D., et al.: Understanding the mechanism of improvement in practical specific capacity using halogen substituted anthraquinones as cathode materials in lithium batteries. Electrochim. Acta 224, 622–627 (2017). https://doi.org/10.1016/j.electacta.2016.12.065
Ma, T., Zhao, Q., Wang, J.B., et al.: A sulfur heterocyclic quinone cathode and a multifunctional binder for a high-performance rechargeable lithium-ion battery. Angew. Chem. Int. Ed. 55, 6428–6432 (2016). https://doi.org/10.1002/anie.201601119
Wang, J., Wang, X.M., Li, H.F., et al.: Intrinsic factors attenuate the performance of anhydride organic cathode materials of lithium battery. J. Electroanal. Chem. 773, 22–26 (2016). https://doi.org/10.1016/j.jelechem.2016.04.038
Lee, J., Park, M.J.: Tattooing dye as a green electrode material for lithium batteries. Adv. Energy Mater. 7, 1602279 (2017). https://doi.org/10.1002/aenm.201602279
Bhosale, M.E., Krishnamoorthy, K.: Chemically reduced organic small-molecule-based lithium battery with improved efficiency. Chem. Mater. 27, 2121–2126 (2015). https://doi.org/10.1021/cm5046786
Lee, M., Hong, J., Kim, H., et al.: Organic nanohybrids for fast and sustainable energy storage. Adv. Mater. 26, 2558–2565 (2014). https://doi.org/10.1002/adma.201305005
Li, H., Duan, W.C., Zhao, Q., et al.: 2,2′-Bis(3-hydroxy-1,4-naphthoquinone)/CMK-3 nanocomposite as cathode material for lithium-ion batteries. Inorg. Chem. Front. 1, 193–199 (2014). https://doi.org/10.1039/c3qi00076a
Cui, D.M., Tian, D., Chen, S.S., et al.: Graphene wrapped 3,4,9,10-perylenetetracarboxylic dianhydride as a high-performance organic cathode for lithium ion batteries. J. Mater. Chem. A 4, 9177–9183 (2016). https://doi.org/10.1039/c6ta02880b
Ai, W., Zhou, W.W., Du, Z.Z., et al.: Toward high energy organic cathodes for Li-ion batteries: a case study of vat dye/graphene composites. Adv. Funct. Mater. 27, 1603603 (2017). https://doi.org/10.1002/adfm.201603603
Zhang, G.F., Xu, Z.X., Liu, P., et al.: A facile in situ polymerization strategy towards polyimide/carbon black composites as high performance lithium ion battery cathodes. Electrochim. Acta 260, 598–605 (2018). https://doi.org/10.1016/j.electacta.2017.12.075
Wang, Y., Zheng, X.Y., Qu, Q.T., et al.: A novel maleic acid/graphite composite anode for lithium ion batteries with high energy and power density. Carbon 132, 420–429 (2018). https://doi.org/10.1016/j.carbon.2018.02.043
Zou, Q.L., Wang, W.K., Wang, A.B., et al.: Preparation of the tetrahydro-hexaquinone as a novel cathode material for rechargeable lithium batteries. Mater. Lett. 117, 290–293 (2014). https://doi.org/10.1016/j.matlet.2013.12.027
Liang, Y.L., Zhang, P., Chen, J.: Function-oriented design of conjugated carbonyl compound electrodes for high energy lithium batteries. Chem. Sci. 4, 1330–1337 (2013). https://doi.org/10.1039/c3sc22093a
Xie, J., Chen, W.Q., Wang, Z.L., et al.: Synthesis and exploration of ladder-structured large aromatic dianhydrides as organic cathodes for rechargeable lithium-ion batteries. Chem. Asian J. 12, 868–876 (2017). https://doi.org/10.1002/asia.201700070
Goriparti, S., Harish, M.N.K., Sampath, S.: Ellagic acid: a novel organic electrode material for high capacity lithium ion batteries. Chem. Commun. 49, 7234–7236 (2013). https://doi.org/10.1039/c3cc43194k
Deng, Q.J., Xue, J., Zou, W., et al.: The electrochemical behaviors of Li2C8H4O6 and its corresponding organic acid C8H6O6 as anodes for Li-ion batteries. J. Electroanal. Chem. 761, 74–79 (2016). https://doi.org/10.1016/j.jelechem.2015.12.005
Zhu, H., Yin, J., Zhao, X., et al.: Humic acid as promising organic anodes for lithium/sodium ion batteries. Chem. Commun. 51, 14708–14711 (2015). https://doi.org/10.1039/c5cc04772b
Luo, Z.Q., Liu, L.J., Zhao, Q., et al.: An insoluble benzoquinone-based organic cathode for use in rechargeable lithium-ion batteries. Angew. Chem. Int. Ed. 56, 12561–12565 (2017). https://doi.org/10.1002/anie.201706604
Sieuw, L., Jouhara, A., Quarez, É., et al.: A H-bond stabilized quinone electrode material for Li-organic batteries: the strength of weak bonds. Chem. Sci. 10, 418–426 (2019). https://doi.org/10.1039/c8sc02995d
Yao, M., Numoto, T., Araki, M., et al.: Long cycle-life organic electrode material based on an ionic naphthoquinone derivative for rechargeable batteries. Energy Procedia 56, 228–236 (2014). https://doi.org/10.1016/j.egypro.2014.07.153
Veerababu, M., Kothandaraman, R.: Rational functionalization of perylene diimide for stable capacity and long-term cycling performance for Li-ion batteries. Electrochim. Acta 232, 244–253 (2017). https://doi.org/10.1016/j.electacta.2017.02.152
Veerababu, M., Varadaraju, U.V., Kothandaraman, R.: Improved electrochemical performance of lithium/sodium perylene-3,4,9,10-tetracarboxylate as an anode material for secondary rechargeable batteries. Int. J. Hydrog. Energy 40, 14925–14931 (2015). https://doi.org/10.1016/j.ijhydene.2015.09.001
Han, X.Y., Yi, F., Sun, T.L., et al.: Synthesis and electrochemical performance of Li and Ni 1,4,5,8-naphthalenetetracarboxylates as anodes for Li-ion batteries. Electrochem. Commun. 25, 136–139 (2012). https://doi.org/10.1016/j.elecom.2012.09.014
Medabalmi, V., Wang, G.X., Ramani, V.K., et al.: Lithium salt of biphenyl tetracarboxylate as an anode material for Li/Na-ion batteries. Appl. Surf. Sci. 418, 9–16 (2017). https://doi.org/10.1016/j.apsusc.2016.12.041
Cahyadi, H.S., William, W., Verma, D., et al.: Enhanced lithium storage capacity of a tetralithium 1,2,4,5-benzenetetracarboxylate (Li4C10H2O8) salt through crystal structure transformation. ACS Appl. Mater. Interfaces 10, 17183–17194 (2018). https://doi.org/10.1021/acsami.8b03323
Lee, H.H., Park, Y., Shin, K.H., et al.: Abnormal excess capacity of conjugated dicarboxylates in lithium-ion batteries. ACS Appl. Mater. Interfaces 6, 19118–19126 (2014). https://doi.org/10.1021/am505090p
Veerababu, M., Varadaraju, U.V., Kothandaraman, R.: Reversible lithium storage behaviour of aromatic diimide dilithium carboxylates. Electrochim. Acta 193, 80–87 (2016). https://doi.org/10.1016/j.electacta.2016.02.030
Lakraychi, A.E., Dolhem, F., Djedaïni-Pilard, F., et al.: Decreasing redox voltage of terephthalate-based electrode material for Li-ion battery using substituent effect. J. Power Sources 359, 198–204 (2017). https://doi.org/10.1016/j.jpowsour.2017.05.046
Fédèle, L., Sauvage, F., Gottis, S., et al.: 2D-layered lithium carboxylate based on biphenyl core as negative electrode for organic lithium-ion batteries. Chem. Mater. 29, 546–554 (2017). https://doi.org/10.1021/acs.chemmater.6b03524
Gou, L., Zhang, H.X., Fan, X.Y., et al.: Lithium based coordination polymer as anode for Li-ion battery. Inorg. Chim. Acta 394, 10–14 (2013). https://doi.org/10.1016/j.ica.2012.07.024
Rajshekar Shetty, V., Gurukar, S.S., Marriappa, R., et al.: Novel synthetic approach for 1,4-dihydroxyanthraquinone and the development of its lithiated salts as anode materials for aqueous rechargeable lithium-ion batteries. New J. Chem. 39, 8534–8544 (2015). https://doi.org/10.1039/C5NJ01300C
Wang, S., Wang, L., Zhang, K., et al.: Organic Li4C8H2O6 nanosheets for lithium-ion batteries. Nano Lett. 13, 4404–4409 (2013). https://doi.org/10.1021/nl402239p
Maiti, S., Pramanik, A., Dhawa, T., et al.: Redox-active organic molecular salt of 1,2,4-benzenetricarboxylic acid as lithium-ion battery anode. Mater. Lett. 209, 613–617 (2017). https://doi.org/10.1016/j.matlet.2017.08.112
Li, L., Hong, Y.J., Chen, D.Y., et al.: A laterally extended perylene hexacarboxylate via Diels-Alder reaction for high-performance organic lithium-ion batteries. Electrochim. Acta 254, 255–261 (2017). https://doi.org/10.1016/j.electacta.2017.09.119
Renault, S., Brandell, D., Gustafsson, T., et al.: Improving the electrochemical performance of organic Li-ion battery electrodes. Chem. Commun. 49, 1945–1947 (2013). https://doi.org/10.1039/c3cc39065a
Zhao, Q., Wang, J.B., Chen, C.C., et al.: Nanostructured organic electrode materials grown on graphene with covalent-bond interaction for high-rate and ultra-long-life lithium-ion batteries. Nano Res. 10, 4245–4255 (2017). https://doi.org/10.1007/s12274-017-1580-9
Luo, C., Huang, R., Kevorkyants, R., et al.: Self-assembled organic nanowires for high power density lithium ion batteries. Nano Lett 14, 1596–1602 (2014). https://doi.org/10.1021/nl500026j
Wu, X.Y., Ma, J., Hu, Y.S., et al.: Nano-sized carboxylates as anode materials for rechargeable lithium-ion batteries. J. Energy Chem. 23, 269–273 (2014). https://doi.org/10.1016/S2095-4956(14)60146-7
Wang, J.W., Zhao, H.Y., Xu, L.T., et al.: Three-electron redox enabled dithiocarboxylate electrode for superior lithium storage performance. ACS Appl. Mater. Interfaces 10, 35469–35476 (2018). https://doi.org/10.1021/acsami.8b11485
Wan, W., Lee, H., Yu, X.Q., et al.: Tuning the electrochemical performances of anthraquinone organic cathode materials for Li-ion batteries through the sulfonic sodium functional group. RSC Adv. 4, 19878–19882 (2014). https://doi.org/10.1039/c4ra01166j
Lu, Y., Zhao, Q., Miao, L.C., et al.: Flexible and free-standing organic/carbon nanotubes hybrid films as cathode for rechargeable lithium-ion batteries. J. Phys. Chem. C 121, 14498–14506 (2017). https://doi.org/10.1021/acs.jpcc.7b04341
Lakraychi, A.E., Fahsi, K., Aymard, L., et al.: Carboxylic and sulfonic N-substituted naphthalene diimide salts as highly stable non-polymeric organic electrodes for lithium batteries. Electrochem. Commun. 76, 47–50 (2017). https://doi.org/10.1016/j.elecom.2017.01.019
Deng, Q.J., Fan, C., Wang, L.P., et al.: Organic potassium terephthalate (K2C8H4O4) with stable lattice structure exhibits excellent cyclic and rate capability in Li-ion batteries. Electrochim. Acta 222, 1086–1093 (2016). https://doi.org/10.1016/j.electacta.2016.11.079
Wang, L.P., Zhang, H.Q., Mou, C.X., et al.: Dicarboxylate CaC8H4O4 as a high-performance anode for Li-ion batteries. Nano Res. 8, 523–532 (2015). https://doi.org/10.1007/s12274-014-0666-x
Fei, H.L., Liu, X., Li, Z.W.: Hollow cobalt coordination polymer microspheres: a promising anode material for lithium-ion batteries with high performance. Chem. Eng. J. 281, 453–458 (2015). https://doi.org/10.1016/j.cej.2015.06.082
Fei, H.L., Li, Z.W., Liu, X.: Manganese pyridinedicarboxylates: new anode materials for lithium-ion batteries with good cycling performance. J. Alloy. Compd. 640, 118–121 (2015). https://doi.org/10.1016/j.jallcom.2015.04.044
Fei, H.L., Feng, W.J., Xu, T.: Zinc naphthalenedicarboxylate coordination complex: a promising anode material for lithium and sodium-ion batteries with good cycling stability. J. Colloid Interface Sci. 488, 277–281 (2017). https://doi.org/10.1016/j.jcis.2016.11.010
Fei, H.L., Lin, Y.Q.: Zinc pyridinedicarboxylate micro-nanostructures: promising anode materials for lithium-ion batteries with excellent cycling performance. J. Colloid Interface Sci. 481, 256–262 (2016). https://doi.org/10.1016/j.jcis.2016.07.056
Wu, D.B., Li, H., Li, R.G., et al.: In situ growth of copper rhodizonate complexes on reduced graphene oxide for high-performance organic lithium-ion batteries. Chem. Commun. 54, 11415–11418 (2018). https://doi.org/10.1039/c8cc06317f
Schmidt, D., Häupler, B., Stolze, C., et al.: Poly[N-(10-oxo-2-vinylanthracen-9(10H)-ylidene)cyanamide] as a novel cathode material for Li-organic batteries. J. Polym. Sci. Part A Polym. Chem. 53, 2517–2523 (2015). https://doi.org/10.1002/pola.27716
Lyu, H.L., Liu, J.R., Mahurin, S., et al.: Polythiophene coated aromatic polyimide enabled ultrafast and sustainable lithium ion batteries. J. Mater. Chem. A 5, 24083–24090 (2017). https://doi.org/10.1039/c7ta07893e
Pirnat, K., Mali, G., Gaberscek, M., et al.: Quinone-formaldehyde polymer as an active material in Li-ion batteries. J. Power Sources 315, 169–178 (2016). https://doi.org/10.1016/j.jpowsour.2016.03.010
Song, Z.P., Qian, Y.M., Gordin, M.L., et al.: Polyanthraquinone as a reliable organic electrode for stable and fast lithium storage. Angew. Chem. Int. Ed. 54, 13947–13951 (2015). https://doi.org/10.1002/anie.201506673
Nauroozi, D., Pejic, M., Schwartz, P.O., et al.: Synthesis and solvent-free polymerisation of vinyl terephthalate for application as an anode material in organic batteries. RSC Adv. 6, 111350–111357 (2016). https://doi.org/10.1039/c6ra24064j
Li, Z.P., Zhong, W.H., Cheng, A., et al.: Novel hyper-crosslinked polymer anode for lithium-ion batteries with highly reversible capacity and long cycling stability. Electrochim. Acta 281, 162–169 (2018). https://doi.org/10.1016/j.electacta.2018.05.149
Jähnert, T., Hager, M.D., Schubert, U.S.: Assorted phenoxyl-radical polymers and their application in lithium-organic batteries. Macromol. Rapid Commun. 37, 725–730 (2016). https://doi.org/10.1002/marc.201500702
Xie, J., Wang, Z.L., Gu, P.Y., et al.: A novel quinone-based polymer electrode for high performance lithium-ion batteries. Sci. China Mater. 59, 6–11 (2016). https://doi.org/10.1007/s40843-016-0112-3
Wang, S., Wang, Q.Y., Shao, P.P., et al.: Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J. Am. Chem. Soc. 139, 4258–4261 (2017). https://doi.org/10.1021/jacs.7b02648
Xu, F., Jin, S.B., Zhong, H., et al.: Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 5, 8225 (2015). https://doi.org/10.1038/srep08225
Yang, D.H., Yao, Z.Q., Wu, D.H., et al.: Structure-modulated crystalline covalent organic frameworks as high-rate cathodes for Li-ion batteries. J. Mater. Chem. A 4, 18621–18627 (2016). https://doi.org/10.1039/c6ta07606h
Liu, K., Zheng, J.M., Zhong, G.M., et al.: Poly(2,5-dihydroxy-1,4-benzoquinonyl sulfide) (PDBS) as a cathode material for lithium ion batteries. J. Mater. Chem. 21, 4125–4131 (2011). https://doi.org/10.1039/c0jm03127e
Xu, W., Read, A., Koech, P.K., et al.: Factors affecting the battery performance of anthraquinone-based organic cathode materials. J. Mater. Chem. 22, 4032–4039 (2012). https://doi.org/10.1039/c2jm15764k
Wei, W.F., Li, L., Zhang, L., et al.: A benzoquinone-based cathode for Li-organic batteries. Mater. Lett. 213, 126–130 (2018). https://doi.org/10.1016/j.matlet.2017.11.035
Vizintin, A., Bitenc, J., Kopač Lautar, A., et al.: Probing electrochemical reactions in organic cathode materials via in operando infrared spectroscopy. Nat. Commun. 9, 661 (2018). https://doi.org/10.1038/s41467-018-03114-1
Song, Z.P., Qian, Y.M., Zhang, T., et al.: Poly(benzoquinonyl sulfide) as a high-energy organic cathode for rechargeable Li and Na batteries. Adv. Sci. 2, 1500124 (2015). https://doi.org/10.1002/advs.201500124
Jing, Y., Liang, Y.L., Gheytani, S., et al.: Cross-conjugated oligomeric quinones for high performance organic batteries. Nano Energy 37, 46–52 (2017). https://doi.org/10.1016/j.nanoen.2017.04.055
Speer, M.E., Kolek, M., Jassoy, J.J., et al.: Thianthrene-functionalized polynorbornenes as high-voltage materials for organic cathode-based dual-ion batteries. Chem. Commun. 51, 15261–15264 (2015). https://doi.org/10.1039/c5cc04932f
Zhang, Y., Huang, Y.S., Yang, G.H., et al.: Dispersion-assembly approach to synthesize three-dimensional graphene/polymer composite aerogel as a powerful organic cathode for rechargeable Li and Na batteries. ACS Appl. Mater. Interfaces 9, 15549–15556 (2017). https://doi.org/10.1021/acsami.7b03687
Huang, Y.S., Li, K., Liu, J.J., et al.: Three-dimensional graphene/polyimide composite-derived flexible high-performance organic cathode for rechargeable lithium and sodium batteries. J. Mater. Chem. A 5, 2710–2716 (2017). https://doi.org/10.1039/c6ta09754e
Lyu, H., Li, P., Liu, J., et al.: Aromatic polyimide/graphene composite organic cathodes for fast and sustainable lithium-ion batteries. Chemsuschem 11, 763–772 (2018). https://doi.org/10.1002/cssc.201702001
Wu, H.P., Wang, K., Meng, Y.N., et al.: An organic cathode material based on a polyimide/CNT nanocomposite for lithium ion batteries. J. Mater. Chem. A 1, 6366–6372 (2013). https://doi.org/10.1039/c3ta10473g
Wu, H.P., Shevlin, S.A., Meng, Q.H., et al.: Flexible and binder-free organic cathode for high-performance lithium-ion batteries. Adv. Mater. 26, 3338–3343 (2014). https://doi.org/10.1002/adma.201305452
Wu, H.P., Meng, Q.H., Yang, Q., et al.: Large-area polyimide/SWCNT nanocable cathode for flexible lithium-ion batteries. Adv. Mater. 27, 6504–6510 (2015). https://doi.org/10.1002/adma.201502241
Ahmad, A., Wu, H.P., Guo, Y.F., et al.: A graphene supported polyimide nanocomposite as a high performance organic cathode material for lithium ion batteries. RSC Adv. 6, 33287–33294 (2016). https://doi.org/10.1039/c5ra27471k
Liu, T.Y., Lee, B., Kim, B.G., et al.: In situ polymerization of dopamine on graphene framework for charge storage applications. Small 14, 1801236 (2018). https://doi.org/10.1002/smll.201801236
Luo, Z.Q., Liu, L.J., Ning, J.X., et al.: A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew. Chem. Int. Ed. 57, 9443–9446 (2018). https://doi.org/10.1002/anie.201805540
Wu, H.P., Yang, Q., Meng, Q.H., et al.: A polyimide derivative containing different carbonyl groups for flexible lithium ion batteries. J. Mater. Chem. A 4, 2115–2121 (2016). https://doi.org/10.1039/c5ta07246h
Tian, B.B., Ning, G.H., Tang, W., et al.: Polyquinoneimines for lithium storage: more than the sum of its parts. Mater. Horiz. 3, 429–433 (2016). https://doi.org/10.1039/c6mh00072j
Tian, D., Zhang, H.Z., Zhang, D.S., et al.: Li-ion storage and gas adsorption properties of porous polyimides (PIs). RSC Adv. 4, 7506–7510 (2014). https://doi.org/10.1039/c3ra45563g
Ahmad, A., Meng, Q.H., Melhi, S., et al.: A hierarchically porous hypercrosslinked and novel quinone based stable organic polymer electrode for lithium-ion batteries. Electrochim. Acta 255, 145–152 (2017). https://doi.org/10.1016/j.electacta.2017.09.017
Zindy, N., Blaskovits, J.T., Beaumont, C., et al.: Pyromellitic diimide-based copolymers and their application as stable cathode active materials in lithium and sodium-ion batteries. Chem. Mater. 30, 6821–6830 (2018). https://doi.org/10.1021/acs.chemmater.8b02862
Petronico, A., Bassett, K.L., Nicolau, B.G., et al.: Toward a four-electron redox quinone polymer for high capacity lithium ion storage. Adv. Energy Mater. 8, 1700960 (2018). https://doi.org/10.1002/aenm.201700960
Song, Z.P., Qian, Y.M., Liu, X.Z., et al.: A quinone-based oligomeric lithium salt for superior Li-organic batteries. Energy Environ. Sci. 7, 4077–4086 (2014). https://doi.org/10.1039/c4ee02575j
Hong, J., Lee, M., Lee, B., et al.: Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 5, 5335 (2014). https://doi.org/10.1038/ncomms6335
Tian, B.B., Ding, Z.J., Ning, G.H., et al.: Amino group enhanced phenazine derivatives as electrode materials for lithium storage. Chem. Commun. 53, 2914–2917 (2017). https://doi.org/10.1039/c6cc09084b
Ghosh, A., Mitra, S.: Facile synthesis of viologen and its reversible lithium storage property in organic lithium-ion batteries. RSC Adv. 5, 105632–105635 (2015). https://doi.org/10.1039/c5ra20301e
Peng, C.X., Ning, G.H., Su, J., et al.: Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2, 17074 (2017). https://doi.org/10.1038/nenergy.2017.74
Wang, J.Q., Chen, C.S., Zhang, Y.G.: Hexaazatrinaphthylene-based porous organic polymers as organic cathode materials for lithium-ion batteries. ACS Sustain. Chem. Eng. 6, 1772–1779 (2018). https://doi.org/10.1021/acssuschemeng.7b03165
Wang, J.Q., Tee, K.Z., Lee, Y., et al.: Hexaazatriphenylene derivatives/GO composites as organic cathodes for lithium ion batteries. J. Mater. Chem. A 6, 2752–2757 (2018). https://doi.org/10.1039/c7ta10232a
Chen, Z.X., Su, C., Zhu, X.G., et al.: Micro-/Mesoporous conjugated polymer based on star-shaped triazine-functional triphenylamine framework as the performance-improved cathode of Li-organic battery. J. Polym. Sci. Part A: Polym. Chem. 56, 2574–2583 (2018). https://doi.org/10.1002/pola.29239
Bai, L.Y., Gao, Q., Zhao, Y.L.: Two fully conjugated covalent organic frameworks as anode materials for lithium ion batteries. J. Mater. Chem. A 4, 14106–14110 (2016). https://doi.org/10.1039/c6ta06449c
Feng, S., Xu, H., Zhang, C., et al.: Bicarbazole-based redox-active covalent organic frameworks for ultrahigh-performance energy storage. Chem. Commun. 53, 11334–11337 (2017). https://doi.org/10.1039/c7cc07024a
Wu, M.M., Zhao, Y., Sun, B.Q., et al.: A 2D covalent organic framework as a high-performance cathode material for lithium-ion batteries. Nano Energy 70, 104498 (2020). https://doi.org/10.1016/j.nanoen.2020.104498
Xu, S.Q., Wang, G., Biswal, B.P., et al.: A nitrogen-rich 2D sp2-carbon-linked conjugated polymer framework as a high-performance cathode for lithium-ion batteries. Angew. Chem. Int. Ed. 58, 849–853 (2019). https://doi.org/10.1002/anie.201812685
Xu, L.H., Ji, L., Wang, G.S., et al.: A novel nitroxide radical polymer-containing conductive polyaniline as molecular skeleton: its synthesis and electrochemical properties as organic cathode. Ionics 22, 1377–1385 (2016). https://doi.org/10.1007/s11581-016-1663-8
Xu, L.H., Guo, P.J., He, H.H., et al.: Preparation of TEMPO-contained pyrrole copolymer by in situ electrochemical polymerization and its electrochemical performances as cathode of lithium ion batteries. Ionics 23, 1375–1382 (2017). https://doi.org/10.1007/s11581-016-1965-x
Jiménez, P., Levillain, E., Alévêque, O., et al.: Lithium n-doped polyaniline as a high-performance electroactive material for rechargeable batteries. Angew. Chem. Int. Ed. 56, 1553–1556 (2017). https://doi.org/10.1002/anie.201607820
Su, C., He, H.H., Xu, L.H., et al.: A mesoporous conjugated polymer based on a high free radical density polytriphenylamine derivative: its preparation and electrochemical performance as a cathode material for Li-ion batteries. J. Mater. Chem. A 5, 2701–2709 (2017). https://doi.org/10.1039/c6ta10127e
Su, C., Ji, L., Xu, L.H., et al.: A polytriphenylamine derivative exhibiting a four-electron redox center as a high free radical density organic cathode. RSC Adv. 6, 22989–22995 (2016). https://doi.org/10.1039/c6ra03248f
Kim, J., Park, H.S., Kim, T.H., et al.: An inter-tangled network of redox-active and conducting polymers as a cathode for ultrafast rechargeable batteries. Phys. Chem. Chem. Phys. 16, 5295–5300 (2014). https://doi.org/10.1039/c3cp54624a
Yao, M., Senoh, H., Sakai, T., et al.: Redox active poly(N-vinylcarbazole) for use in rechargeable lithium batteries. J. Power Sources 202, 364–368 (2012). https://doi.org/10.1016/j.jpowsour.2011.11.035
Su, C., Yang, F., Ji, L., et al.: Polytriphenylamine derivative with high free radical density as the novel organic cathode for lithium ion batteries. J. Mater. Chem. A 2, 20083–20088 (2014). https://doi.org/10.1039/c4ta03413a
Kim, Y., Jo, C., Lee, J., et al.: An ordered nanocomposite of organic radicalpolymer and mesocellular carbon foam as cathode material in lithium ion batteries. J. Mater. Chem. 22, 1453–1458 (2012). https://doi.org/10.1039/c1jm15053g
Bahceci, S., Esat, B.: A polyacetylene derivative with pendant TEMPO group as cathode material for rechargeable batteries. J. Power Sources 242, 33–40 (2013). https://doi.org/10.1016/j.jpowsour.2013.05.051
Hansen, K.A., Nerkar, J., Thomas, K., et al.: New spin on organic radical batteries: an isoindoline nitroxide-based high-voltage cathode material. ACS Appl. Mater. Interfaces 10, 7982–7988 (2018). https://doi.org/10.1021/acsami.7b18252
Zhang, K., Hu, Y.X., Wang, L.Z., et al.: Pyrene-functionalized PTMA by NRC for greater π–π stacking with rGO and enhanced electrochemical properties. ACS Appl. Mater. Interfaces 9, 34900–34908 (2017). https://doi.org/10.1021/acsami.7b09604
Xiong, J.Q., Wei, Z., Xu, T., et al.: Polytriphenylamine derivative with enhanced electrochemical performance as the organic cathode material for rechargeable batteries. Polymer 130, 135–142 (2017). https://doi.org/10.1016/j.polymer.2017.10.004
Wang, Z.H., Xu, C., Tammela, P., et al.: Conducting polymer paper-based cathodes for high-areal-capacity lithium-organic batteries. Energy Technol. 3, 563–569 (2015). https://doi.org/10.1002/ente.201402224
Deng, W.W., Shen, Y.F., Liang, X.M., et al.: Redox-active organics/polypyrrole composite as a cycle-stable cathode for Li ion batteries. Electrochim. Acta 147, 426–431 (2014). https://doi.org/10.1016/j.electacta.2014.09.103
Qie, L., Yuan, L.X., Zhang, W.X., et al.: Revisit of polypyrrole as cathode material for lithium-ion battery. J. Electrochem. Soc. 159, A1624–A1629 (2012). https://doi.org/10.1149/2.042210jes
Godet-Bar, T., Leprêtre, J.C., Le Bacq, O., et al.: Electrochemical and ab initio investigations to design a new phenothiazine based organic redox polymeric material for metal-ion battery cathodes. Phys. Chem. Chem. Phys. 17, 25283–25296 (2015). https://doi.org/10.1039/c5cp01495f
Vlad, A., Arnould, K., Ernould, B., et al.: Exploring the potential of polymer battery cathodes with electrically conductive molecular backbone. J. Mater. Chem. A 3, 11189–11193 (2015). https://doi.org/10.1039/c5ta01500f
Pirnat, K., Bitenc, J., Vizintin, A., et al.: Indirect synthesis route toward cross-coupled polymers for high voltage organic positive electrodes. Chem. Mater. 30, 5726–5732 (2018). https://doi.org/10.1021/acs.chemmater.8b02329
Truong, T.T., Coates, G.W., Abruña, H.D.: High power organic cathodes using thin films of electropolymerized benzidine polymers. Chem. Commun. 51, 14674–14677 (2015). https://doi.org/10.1039/c5cc05134g
Lee, M., Hong, J., Lee, B., et al.: Multi-electron redox phenazine for ready-to-charge organic batteries. Green Chem. 19, 2980–2985 (2017). https://doi.org/10.1039/c7gc00849j
Dai, G.L., Wang, X.L., Qian, Y.M., et al.: Manipulation of conjugation to stabilize N redox-active centers for the design of high-voltage organic battery cathode. Energy Storage Mater. 16, 236–242 (2019). https://doi.org/10.1016/j.ensm.2018.06.005
Lakraychi, A.E., Deunf, E., Fahsi, K., et al.: An air-stable lithiated cathode material based on a 1,4-benzenedisulfonate backbone for organic Li-ion batteries. J. Mater. Chem. A 6, 19182–19189 (2018). https://doi.org/10.1039/c8ta07097k
Patil, N., Aqil, A., Ouhib, F., et al.: Bioinspired redox-active catechol-bearing polymers as ultrarobust organic cathodes for lithium storage. Adv. Mater. 29, 1703373 (2017). https://doi.org/10.1002/adma.201703373
Otteny, F., Kolek, M., Becking, J., et al.: Unlocking full discharge capacities of poly(vinylphenothiazine) as battery cathode material by decreasing polymer mobility through cross-linking. Adv. Energy Mater. 8, 1802151 (2018). https://doi.org/10.1002/aenm.201802151
Zhang, C., Yang, X., Ren, W.F., et al.: Microporous organic polymer-based lithium ion batteries with improved rate performance and energy density. J. Power Sources 317, 49–56 (2016). https://doi.org/10.1016/j.jpowsour.2016.03.080
Wang, X., Zhang, C., Xu, Y.F., et al.: Conjugated microporous polytetra(2-thienyl)ethylene as high performance anode material for lithium- and sodium-ion batteries. Macromol. Chem. Phys. 219, 1700524 (2018). https://doi.org/10.1002/macp.201700524
Zhang, C., He, Y.W., Mu, P., et al.: Toward high performance thiophene-containing conjugated microporous polymer anodes for lithium-ion batteries through structure design. Adv. Funct. Mater. 28, 1705432 (2018). https://doi.org/10.1002/adfm.201705432
Han, X.Y., Qing, G.Y., Sun, J.T., et al.: How many lithium ions can be inserted onto fused C6 aromatic ring systems? Angew. Chem. Int. Ed. 51, 5147–5151 (2012). https://doi.org/10.1002/anie.201109187
Wu, J.S., Rui, X.H., Long, G.K., et al.: Pushing up lithium storage through nanostructured polyazaacene analogues as anode. Angew. Chem. Int. Ed. 54, 7354–7358 (2015). https://doi.org/10.1002/anie.201503072
Wu, J.S., Rui, X.H., Wang, C.Y., et al.: Nanostructured conjugated ladder polymers for stable and fast lithium storage anodes with high-capacity. Adv. Energy Mater. 5, 1402189 (2015). https://doi.org/10.1002/aenm.201402189
Xie, J., Rui, X.H., Gu, P.Y., et al.: Novel conjugated ladder-structured oligomer anode with high lithium storage and long cycling capability. ACS Appl. Mater. Interfaces 8, 16932–16938 (2016). https://doi.org/10.1021/acsami.6b04277
Sun, T., Li, Z.J., Wang, H.G., et al.: A biodegradable polydopamine-derived electrode material for high-capacity and long-life lithium-ion and sodium-ion batteries. Angew. Chem. Int. Ed. 55, 10662–10666 (2016). https://doi.org/10.1002/anie.201604519
Mukherjee, D., Gowda, Y.K.G., Makri Nimbegondi Kotresh, H., et al.: Porous, hyper-cross-linked, three-dimensional polymer as stable, high rate capability electrode for lithium-ion battery. ACS Appl. Mater. Interfaces 9, 19446–19454 (2017). https://doi.org/10.1021/acsami.6b09575
Lei, Z.D., Yang, Q.S., Xu, Y., et al.: Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat. Commun. 9, 576 (2018). https://doi.org/10.1038/s41467-018-02889-7
Lei, Z.D., Chen, X.D., Sun, W.W., et al.: Exfoliated triazine-based covalent organic nanosheets with multielectron redox for high-performance lithium organic batteries. Adv. Energy Mater. 9, 1801010 (2019). https://doi.org/10.1002/aenm.201801010
Chen, X.D., Li, Y.S., Wang, L., et al.: High-lithium-affinity chemically exfoliated 2D covalent organic frameworks. Adv. Mater. 31, 1901640 (2019). https://doi.org/10.1002/adma.201901640
Haldar, S., Roy, K., Kushwaha, R., et al.: Chemical exfoliation as a controlled route to enhance the anodic performance of COF in LIB. Adv. Energy Mater. 9, 1902428 (2019). https://doi.org/10.1002/aenm.201902428
Yang, H.Q., Liu, S.W., Cao, L.H., et al.: Superlithiation of non-conductive polyimide toward high-performance lithium-ion batteries. J. Mater. Chem. A 6, 21216–21224 (2018). https://doi.org/10.1039/c8ta05109g
Kang, H.W., Liu, H.L., Li, C.X., et al.: Polyanthraquinone-triazine: a promising anode material for high-energy lithium-ion batteries. ACS Appl. Mater. Interfaces 10, 37023–37030 (2018). https://doi.org/10.1021/acsami.8b12888
Ryu, J., Park, B., Kang, J., et al.: Three-dimensional monolithic organic battery electrodes. ACS Nano 13, 14357–14367 (2019). https://doi.org/10.1021/acsnano.9b07807
Lin, Z.Q., Xie, J., Zhang, B.W., et al.: Solution-processed nitrogen-rich graphene-like holey conjugated polymer for efficient lithium ion storage. Nano Energy 41, 117–127 (2017). https://doi.org/10.1016/j.nanoen.2017.08.038
Yang, H., Zhang, S.L., Han, L.H., et al.: High conductive two-dimensional covalent organic framework for lithium storage with large capacity. ACS Appl. Mater. Interfaces 8, 5366–5375 (2016). https://doi.org/10.1021/acsami.5b12370
Haldar, S., Roy, K., Nandi, S., et al.: High and reversible lithium ion storage in self-exfoliated triazole-triformyl phloroglucinol-based covalent organic nanosheets. Adv. Energy Mater. 8, 1702170 (2018). https://doi.org/10.1002/aenm.201702170
Zhang, H., Sun, W., Chen, X., et al.: Few-layered fluorinated triazine-based covalent organic nanosheets for high-performance alkali organic batteries. ACS Nano 13, 14252–14261 (2019). https://doi.org/10.1021/acsnano.9b07360
Luo, C., Ji, X., Hou, S., et al.: Azo compounds derived from electrochemical reduction of nitro compounds for high performance Li-ion batteries. Adv. Mater. 30, 1706498 (2018). https://doi.org/10.1002/adma.201706498
Zhao, G.F., Zhang, Y.H., Gao, Z.H., et al.: Dual active site of the azo and carbonyl-modified covalent organic framework for high-performance Li storage. ACS Energy Lett. 5, 1022–1031 (2020). https://doi.org/10.1021/acsenergylett.0c00069
Renault, S., Oltean, V.A., Araujo, C.M., et al.: Superlithiation of organic electrode materials: the case of dilithium benzenedipropiolate. Chem. Mater. 28, 1920–1926 (2016). https://doi.org/10.1021/acs.chemmater.6b00267
Park, J., Lee, C.W., Park, J.H., et al.: Capacitive organic anode based on fluorinated-contorted hexabenzocoronene: applicable to lithium-ion and sodium-ion storage cells. Adv. Sci. 5, 1801365 (2018). https://doi.org/10.1002/advs.201801365
Deng, Q.J., He, S.J., Pei, J.F., et al.: Exploitation of redox-active 1,4-dicyanobenzene and 9,10-dicyanoanthracene as the organic electrode materials in rechargeable lithium battery. Electrochem. Commun. 75, 29–32 (2017). https://doi.org/10.1016/j.elecom.2016.12.005
Schon, T.B., Tilley, A.J., Kynaston, E.L., et al.: Three-dimensional arylene diimide frameworks for highly stable lithium ion batteries. ACS Appl. Mater. Interfaces 9, 15631–15637 (2017). https://doi.org/10.1021/acsami.7b02336
Zhang, H.C., Xie, Y.P., Chen, X.J., et al.: Naphthalene diimide-ethylene conjugated copolymer as cathode material for lithium ion batteries. J. Electrochem. Soc. 164, A290–A294 (2016). https://doi.org/10.1149/2.1011702jes
Sharma, P., Damien, D., Nagarajan, K., et al.: Perylene-polyimide-based organic electrode materials for rechargeable lithium batteries. J. Phys. Chem. Lett. 4, 3192–3197 (2013). https://doi.org/10.1021/jz4017359
Acknowledgements
Xiudong Chen and Xiaojie Yin contributed equally to this work. This work was generously funded by the National Natural Science Foundation of China (52073170, 22065017), the Project funded by China Postdoctoral Science Foundation (BX2021029, 2021M700353), the Start-Up Grant and Scientific Research Project of Chaohu University (Nos. KYQD-202008 and XLY-202012), the Shanghai Municipal Education Commission (Innovation Program 2019-01-07-00-09-E00021), and the Creative Research Team of High-level Local Universities in Shanghai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Xiudong Chen and Xiaojie Yin contribute equally to this work.
Rights and permissions
About this article
Cite this article
Chen, X., Yin, X., Aslam, J. et al. Recent Progress and Design Principles for Rechargeable Lithium Organic Batteries. Electrochem. Energy Rev. 5, 12 (2022). https://doi.org/10.1007/s41918-022-00135-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00135-9