Abstract
As the world becomes increasingly aware of the devastating effects of climate change, the need for sustainable building materials that are both durable and environmentally friendly increases. Geopolymer and alkali-activated materials formed by a chemical reaction between an alkaline activator solution and an aluminosilicate source have gained popularity in recent years. The alkaline activator solution dissolves the aluminosilicate source, which then undergoes a polycondensation reaction to form a three-dimensional geopolymeric gel network. The development of this network ensures the strength and durability of the material. Today, this phenomenon of durability has been studied in detail to enable the development of superior construction materials, taking into account degradation mechanisms such as carbonation, leaching, shrinkage, fire, freezing and thawing, and exposure to aggressive environments (chlorides, acids, and sulphates). Although there are many unsolved problems in their engineering applications, slag-based alkali-activated materials appear to be more advantageous and are promising as alternative materials to ordinary Portland cement. First of all, it should not be ignored that the cure sensitivity is high in these systems due to compressive strength losses of up to 69%. Loss of strength of alkali-activated materials is considered an important indicator of degradation. In binary precursors, the presence of fly ash in slag can result in an improvement of over 10% in compressive strength of the binary-based alkali-activated materials after undergoing carbonation. The binary systems can provide superior resistance to many degradation mechanisms, especially exposure to high-temperature. The partial presence of class F fly ash in the slag-based precursor can overcome the poor ability of alkali-activated materials to withstand high temperatures. Due to the desired pore structure, alkali-activated materials may not be damaged even after 300 freeze–thaw cycles. Their superior permeability compared to cementitious counterparts can extend service life against chloride corrosion by more than 20 times. While traditional (ordinary Portland cement-based) concrete remains the most widely used material in construction, geopolymer concrete’s superior performance makes it an increasingly emerging option for sustainable and long-lasting infrastructure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Geopolymer and alkali-activated material technology is still in its early stages. Therefore, a lot of research is being carried out to improve the quality, economy and environmental cost-effectiveness of such materials [1,2,3,4]. Although geopolymers and alkali-activated materials are defined separately by Davidovits [5] due to their different chemistries that play a critical role in their durability, van Deventer et al. [6] define geopolymers as a special type of alkali-activated materials. Based on the data from Scopus (see Fig. 1) [7], these binders developed by CaO-dominated precursors such as slag are often referred to as alkali-activated materials rather than geopolymers [8]. In this critical review, both of these terms have been used to describe these materials as presented in the address sources.
Researchers are exploring new methods to produce geopolymer materials [9, 10] as well as new areas of application to meet the current construction needs [11,12,13]. One of the research areas focuses mainly on the development of activators for these materials that can improve their performance and reduce costs. Another area of research is focused on developing feasible applications for geopolymer materials such as 3D printing and prefabricated building components. Overall, the future looks bright for alkali-activated material technology as it continues to gain popularity as a sustainable and long-lasting (durable) construction material [14]. The issue of durability examined in contemporary materials dates back to ancient times. “De Architectura” a series of books by the architecture Marcus Vitruvius Pollio (probably dating back to between 30–20 BC), is the first printed evidence of primitive approaches to the durability of building materials [15]. Today, understanding the causes of carbonation, leaching, efflorescence, shrinkage, resistance to fire, freeze–thaw, and aggressive environments, and the solutions to deterioration mechanisms has become essential in order to ensure the durability, strength, and safety of the material. By optimizing the mix design, using appropriate curing methods, and incorporating specific additives, engineers and builders can overcome the emerging challenges and improve the specified durability performances of the material [16,17,18,19,20,21,22,23,24].
The geopolymer binders can be synthesized by a number of precursor sources in alkaline or acidic environments [25,26,27]. Slag (ground granulated blast furnace slag), a by-product of the ever-growing steelmaking industry, is becoming the most promising precursor in terms of cost, environmental and sustainability concerns in the near future when compared to residues or products from other industries such as fly ash, metakaolin and red mud. The existence of an exothermal reaction mechanism between the activator and CaO in the slag allows the geopolymerization process without thermal curing [28, 29]. Accordingly, the content of this paper is mostly addressed with data obtained from the test results of slag-based alkali-activated materials cured at ambient temperatures. This content has been supported by the partial use of other aluminosilicate sources, which have a much larger crystalline phase, reactive alumina, and lower CaO content, requiring a special curing regime [19, 30,31,32]. In contrast to the advantage of alkali-activated slag to easily gain strength at ambient temperatures, the stability of the resulting geopolymerization gels such as C-(N)-A-S–H and weakly linked Si networks (≤ Q2) for leaching out in contact with water is highly questionable [33]. Many studies have been conducted to understand its degradation mechanism, especially on carbonation [34,35,36,37], leaching [38,39,40,41], shrinkage [42,43,44,45], fire [46,47,48,49], freezing and thawing [50,51,52,53], and exposure to aggressive environments (chlorides, acids, and sulphates) [54,55,56,57,58,59,60,61].
The durability aspects of alkali-activated slag pose challenges for researchers working in cement and concrete technology due to the complexity of their design compared to the well-established chemistry of ordinary Portland cement. Although today’s research may seem like a small step, all scientific initiatives are necessary to contribute to overcoming these current challenges. The author believes that this critical review on the durability of geopolymer and alkali-activated material will encourage researchers to produce these sustainable materials from blast furnace slag, a by-product of vital industry (iron and steel), rather than by-products of other industries (e.g., coal-fired thermal power plants), which is under discussion due to global warming.
Objectives, methodology and data analysis
The term “durability” has recently gained popularity in many fields of science, especially in engineering, as seen in Fig. 2 [62]. Only 3.4% of these documents (in engineering field) are review documents. The number of review studies addressing research articles on durability has become critical to responding to the world’s sustainability goals. This critical review aims to investigate the degradation of alkali-activated slag/slag-based geopolymer when exposed to the deterioration mechanisms commonly encountered in the construction industry.
Considering the large number of relevant publications, a specific methodology was followed to consider the most suitable documents to be included in the literature search to achieve the above-mentioned aims of the critical review. Accordingly, a systematic approach was adopted: (I) identifying the most relevant keywords; (II) listing relevant publications obtained from Google Scholar, Web of Science, and Scopus databases; (III) ignoring documents that are out of scope or unnecessary after a preliminary analysis focusing on the title, abstract and concluding remarks; (IV) second screening to focus more on the most relevant documents, taking into account more details of the available documents; (V) deciding on the final list of documents and outline of the paper; (VI) writing the first draft; (VII) determining weak parts of the draft; (VIII) researching additional documents with new keywords to strengthen weak sections; (IX) adequately addressing durability aspects of alkali-activated slag/slag-based geopolymer in the text; (X) critically discuss and develop results, and identify the need for future perspective; (XI) concluding the final text of this study. Accordingly, a total of 188 documents obtained from journals, conferences, books, and the Internet were examined and discussed in this critical review. A significant part of these documents was taken from reputable journals. In order to better contribute to the collective knowledge in the field, 66% of the references are current sources published in the last five years (2018–2023). Table 1 lists the category, source, and number of these documents.
Carbonation, leaching, and efflorescence
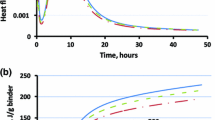
As a quantitative indicator of durability, the link between strength loss and degradation mechanism is mainly investigated in studies for geopolymers [63,64,65]. In addition to environmental variables, some compositional characteristics, including microcracks, porosity (determining the diffusivity of CO2 into the matrix), and water availability in the pores, are the well-known parameters affecting the carbonation rate of composites [66]. The presence of unbonded and solved alkalis (Na and K) and calcium ions is the main reason for these types of degradation in geopolymers when exposed to certain environmental conditions. A reduction in strength results from the carbonation of free alkalis (Na, and K) not playing a role in geopolymerization reactions [67]. In addition, a lower pH below 11 may induce the risk of corrosion of the reinforcing steel [68]. Due to the role of CaO in C-(N)-A-S–H gels and pH, Ca-rich slag content contributes to the carbonation resistance of geopolymers, and even to some extent improvement in strength can be achieved at various carbonation exposures [69]. An increment in the initial pH (from 10.8 to 12.2) of geopolymers, including slag in terms of fly ash, can be documented to be another reason for the lower carbonation level and its benefit in mechanical results, as given in Fig. 3.
Carbonation statement (a) and strength variations (b) of geopolymer samples at various amounts of GGBS [69]
The leaching potential of geopolymer composites has not been reported enough, while numerous studies evaluate the leaching potential of ordinary Portland cement composites [70]. Besides, the leaching of geopolymers requires more attention than cement counterparts due to additional concerns. Not only does its durability suffer from the dissolution of aluminosilicate gel and free alkali ions, but geopolymer can create a further threat for the environment as it contains high-volume industrial by-products with concentrated toxic heavy metal contents [71, 72]. Upcycling these industrial wastes into composites can limit the leakage potential of toxic metals into the ground by capturing them in geopolymeric products. In fact, Sun and Vollpracht [73] stated that As, Ba, Cd, Co, Cr, Cu, Ni, Pb, Sb, Se, Tl, and Zn are in similar leaching amounts with the cement-based counterparts except V and Mo. These leachable toxic contents of geopolymers are directly proportional to the leachable contents in precursors [71]. On the other hand, when specimens are exposed to water, leaching of unreacted sodium silicate can cause up to a 25% strength loss [74]. Under open curing conditions, interruption of geopolymeric reactions by water evaporation may result in a higher strength loss reaching 69% due to the solid body’s increased porosity (around 50%) [75].
While a high concentration of alkalis is required for favorable strength development of geopolymers, it increases the solubility and absorption rate of CO2 and accelerates efflorescence [76]. The visible deposits of efflorescence on the surface can or cannot result in the degradation of geopolymeric structures depending on the original strength, porosity, and crystallization pressure [77, 78]. Silica modulus (Ms, SiO2/Na2O ratio) is one of the most pronounced characteristics of geopolymers’ strength, porosity, and leachable alkalis [79,80,81]. Thus, original strength and its loss can be significantly affected by its variation, as seen in Fig. 4. To be a deleterious and internal part of the efflorescence mechanism, subflorescence in pores below 360 nm creates excessive pressure compared to the tensile strength capability of geopolymer products during the evaporation of water [82, 83]. According to Zhang et al. [84], even if the relationship between subflorescence and tensile strength has not been investigated by experiments, its impact cannot be ignored for understanding the fracture mechanism of geopolymers.
Mechanical deterioration of geopolymer samples with various silica modulus (Ms) ranging from 0 to 1.5 [85]
Shrinkage
One of the biggest challenges that engineers and builders face is the issue of shrinkage when working with geopolymer and alkali-activated materials. Shrinkage is the reduction in the size or volume of a material, which can lead to cracking, reduced strength, and ultimately compromise the structural integrity of a building [86, 87]. Four types of shrinkage can occur in geopolymer and alkali-activated materials: chemical, autogenous, drying, and carbonation shrinkage. The chemical shrinkage, which occurs when the volume of the reaction products is less than its ingredients, and the autogenous shrinkage, which results from the increase in capillary pressure in the matrix, are defined in the same way up to the initial setting [88]. Autogenous shrinkage occurs when the material undergoes self-desiccation during the setting and hardening process. The water consumed during the chemical reaction is not replaced, which can result in shrinkage. This shrinkage typically occurs in the early stages of the hardening process. Drying shrinkage occurs when the material loses moisture due to evaporation. This can occur during the curing process, or after the material has been placed in its final position. Drying shrinkage can be particularly problematic in hot and dry climates, where evaporation rates are high. Carbonation shrinkage occurs when carbon dioxide in the atmosphere reacts with the alkaline activator solution in the material. This can cause the material to expand initially, but over time, the reaction can lead to shrinkage. Carbonation shrinkage is typically a long-term issue and can take years to manifest [89].
Understanding the causes of shrinkage is crucial to finding effective solutions to this problem. One of the leading causes of shrinkage in geopolymer and alkali-activated materials is the chemical reaction that occurs during the setting and hardening process. These materials are typically made by mixing an alkaline activator solution, such as sodium hydroxide or potassium hydroxide, with an aluminosilicate source, such as slag, fly ash, or clay. This results in the formation of a three-dimensional network of geopolymeric gels that provide the strength and durability of the material [90, 91]. However, during the chemical reaction, water is consumed, which can result in shrinkage. Another factor that can contribute to shrinkage is the microstructure of the material. Geopolymer and alkali-activated materials have a unique microstructure different from traditional cement-based materials. In particular, geopolymer slurries cured at high temperatures result in higher chemical shrinkage than ordinary Portland cement [92]. The gel structure of these materials is more porous and has a higher surface area, which can lead to more shrinkage. Additionally, the pore size distribution and the presence of unreacted particles can also have an impact on the amount of shrinkage that occurs. A smaller critical pore size and a higher fraction of mesopores (2.5–50 nm) result in a higher shrinkage [93]. An increment in the amount of slag and NaOH contributes to the pore structure of geopolymers, resulting in lower drying and autogenous shrinkage but higher chemical shrinkage, as seen in Fig. 5 [94].
Effect of (a) slag (0–50%) and (b) NaOH content (8–12%) on the shrinkage results of geopolymers [94]
There are several solutions to shrinkage in geopolymer and alkali-activated materials. One solution is to add certain additives, such as silica fume, to the mix [95]. These additives can help reduce the amount of water consumed during the chemical reaction, which can reduce shrinkage. The use of nanomaterials as additives in the mix, such as nanosilica, nanoalumina, and carbon nanotubes, has unique properties that can improve the microstructure of the material and reduce shrinkage [96, 97]. Another area of research is the usage of alternative aluminosilicate sources, such as rice husk ash and zeolite [98, 99]. These sources have the potential to reduce the carbon footprint of the material, as well as improve its durability and strength. Other additives, such as fibers, can help to improve the toughness and ductility of the material, which can reduce cracking [100, 101]. Curing done using various methods, such as steam curing, water curing, or ambient curing, is also an essential factor in reducing shrinkage [102, 103]. Proper curing can help to control the amount of water consumed during the chemical reaction. It can reduce the amount of drying shrinkage that occurs, as well as improve the durability and strength of the material [104, 105]. Mix design is also important in reducing shrinkage. The amount of alkaline activator solution, the type of aluminosilicate source, and the water content can all impact the shrinkage that occurs [106,107,108]. By optimizing the mix design, engineers and builders can reduce the amount of shrinkage that occurs and improve the durability and strength of the material. Another kind of shrinkage to be considered is that the geopolymer specimens demonstrate a significant volumetric shrinkage reaching 20% at exposure to elevated temperatures [109].
Fire resistance
While traditional cement-based concrete can spall and crack when exposed to high temperatures, reducing its ability to resist fire, geopolymers and alkali-activated materials have shown excellent fire resistance properties [110, 111]. One study found that geopolymer concrete had fire resistance without any exhibited spalling at various fire regimes compared to traditional concrete due to its high connected pore structure allowing water vapor escape [112]. Similarly, Duan et al. [113] exposed samples of geopolymer and OPC to temperatures of up to 1000 °C for several hours and found that geopolymers maintained their structural integrity and did not exhibit any signs of spalling, unlike the OPC samples. In fact, the desirable fire behavior are controlled by two opposite phenomena: (1) damage to the matrix due to thermal incompatibility of ingredients, and (2) further hydration or geopolymerization that densifies the matrix [114]. Le et al. [115] reported that up to 2 times, a significant improvement in strength can be achieved for geopolymer foams after exposure to a temperature of 1200 ℃. These results suggest that geopolymers and alkali-activated materials have the potential to be used in a wide range of applications where fire resistance is a critical consideration. These include:
-
Fire-resistant cladding and facades for buildings [116]
-
Fire-resistant insulation materials [117]
-
Fire-resistant coatings for steel structures [118]
-
Fire-resistant flooring and paving materials [119]
-
Fire-resistant radiation shields [11]
Many factors can affect the ability of geopolymers to resist fire. These include the type of precursor used, the type and concentration of activator used, fibers, and the curing conditions of the material [115, 120,121,122,123]. For example, the concentration of the activator used can also affect the material’s fire resistance, with higher concentrations generally resulting in better fire resistance [120]. In addition, studies have shown that using slag as a precursor can decrease fire resistance compared to other types of precursors, such as fly ash [120, 124]. As documented by Luo et al. [125], a minor use (5%) of slag within siliceous (class F) fly ash-based geopolymers can cause higher crack intensities reaching five times in the matrix after exposure to elevated temperatures. To understand the fire resistance of geopolymers in detail, not only the crack density, crack rehabilitation, transformation of phase chemistry, and pore structure are needed further investigations [126, 127]. The loss in compressive strength of fly ash-based geopolymers can reach 65% by using slag instead of the siliceous precursor at elevated temperatures, as observed in Fig. 6 [125, 127]. Although a significant loss of compressive strength was observed with increasing slag content in the current literature, Junru et al. [128] reported that 100% slag-based geopolymer concrete has superior fire resistance than OPC concrete.
In addition to their fire resistance properties, geopolymers and alkali-activated materials also have several other desirable properties that make them a good choice for sustainable construction, including their low carbon footprint and high durability [14, 129]. While there is still much research and development needed to fully understand the potential of these materials, they offer a glimpse into a more sustainable and fire-resistant future for the construction industry.
Freeze–thaw resistance
In addition to superior durability and heat resistance, geopolymers’ ability to resist freeze–thaw damage, a common problem that plagues conventional concrete and other building materials, is truly fascinating [130]. Before delving into how geopolymer resists freeze–thaw damage, it’s essential to understand the effects of freeze–thaw cycles on traditional concrete. When water freezes in voids, it expands by about 9%, and this expansion exerts pressure on the surrounding materials. When this freeze–thaw cycle is repeated, the pressure becomes too much for the concrete to bear, and it begins to crack and break apart. This damage not only affects the appearance of the building but also weakens the structural integrity of the concrete. Once the concrete is damaged, it becomes more porous, allowing water to seep in and exacerbate the freeze–thaw cycle. This vicious cycle can lead to costly repairs, and in extreme cases, it can even lead to the total failure of the structures. In this respect, air-entraining admixtures are often studied in conventional concrete products [131]. Unlike OPC, these additives can impair the freeze–thaw performance of geopolymers [132, 133]. So, it is clear that finding a solution to this problem in a material scale is of utmost importance. Compared to low-calcium geopolymers, including fly ash and natural zeolite, slag-based geopolymers present better resistance to freeze–thaw cycles [123, 134, 135]. Slag-based concrete can withstand even after 300 freeze–thaw cycles [51]. This is possibly caused by their favorable pore structure in denser microstructure and high-strength matrix proportional to the slag content, as seen in Fig. 7.
Effect of slag content on (a) pore structure and microstructure of specimens, (b) without slag, and (c) with 50% slag [136]
Geopolymers are formed by the reaction between aluminosilicate and alkali solutions, which results in the formation of a three-dimensional network of Si–O-Al bonds [12]. This network is highly stable and resistant to both physical and chemical degradation [137]. One of the main reasons why geopolymer is able to resist freeze–thaw damage is its low permeability, as known in concrete technology [138]. Unlike cement paste, geopolymer has a much lower porosity, which means that it is less likely to absorb water [139]. When water does come into contact with the material, it is less likely to penetrate deep into the structure, and this reduces the risk of freeze–thaw damage. Another factor that contributes to the durability of geopolymer is its microstructure [136]. Geopolymer has a unique microstructure that is made up of a dense matrix of interlocking crystals. These crystals are tightly packed together, leaving very little space for water to seep in. This dense structure makes it difficult for water to penetrate the material, making it more resistant to freeze–thaw damage. The curing temperature of geopolymer is another factor that affects its ability to resist freeze–thaw damage [140]. Studies have shown that geopolymer cured at higher temperatures is more resistant to freeze–thaw damage than geopolymer cured at lower temperatures [141]. This is because higher curing temperatures result in a more compact microstructure, which makes it even more difficult for water to penetrate the material.
Resistance to aggressive solutions
The most aggressive challenges that traditional construction materials face are known as chloride permeability, sulfate, and acid attack for long-lasting performance [142]. While reported to be more resistant than OPC mortar [143], these degradation mechanisms are mostly also questioned for geopolymers as basic durability aspects to provide a comprehensive breakdown of how they are changing the game in the construction industry. The potential for use of geopolymer concrete in various applications worldwide has been investigated. In Australia, geopolymer mortar has been used in the construction of a wastewater treatment plant, where it has been found to provide superior resistance to chemical attack compared to sulphate-resistant traditional mortar [144]. In India, geopolymer concrete can be promising for constructing a bridge, high-rise buildings, highways, tunnels, dams, and hydraulic structures, to reduce the economic and environmental costs and natural resources, to utilize waste materials, to ensure long-life infrastructure construction, societal income, and employment generation compared to traditional concrete [145]. In Malaysia and Germany, geopolymer concrete has been used in the construction of tunnel segments, where it has been found to provide superior resistance to sulphate and acid attacks and chloride ion ingress [146]. Overall, geopolymer concrete has been found to be a versatile and effective material for use in various construction projects.
Chlorides are commonly found in seawater, de-icing salts, and other sources, and they can penetrate concrete over time, leading to corrosion of embedded steel reinforcement. The corrosion statement can cause structural damage and shorten the lifespan of concrete structures [147]. Geopolymers, made from a combination of industrial waste and natural materials, have been found to be highly resistant to chloride penetration [148]. The structure of geopolymers is based on a three-dimensional network of covalently bonded tetrahedral units, which makes them highly resistant to water and chloride permeability. A chloride level of more than 0.07% by weight of concrete can be considered critical to initiate the corrosion of embedded steel [149]. Given this threshold chloride level in a certain depth (40 mm) of concrete, Tennakoon et al. [150] reported that it is required a significantly higher exposure time, reaching 21 times for high-volume slag-based geopolymer concrete compared to OPC concrete (please see Fig. 8). With increasing slag content, geopolymers have been found to have a low permeability to chloride ions, which means they are less susceptible to corrosion caused by chloride penetration [136]. The longer lifespan of geopolymers than traditional concrete reduces the need for frequent repairs and replacements [151]. This makes geopolymers an ideal material for use in coastal areas or other environments where chloride exposure is a concern.
Chloride content of various concrete series (a) at 5-week and (b) 500-day exposure time, (c) corrosion statement at 150-day exposure time, and (d) predicted service life (exposure time) of concrete series for chloride threshold level at 25- and 50-mm concrete depth [150]
In addition to chloride permeability, geopolymer products are also less susceptible to sulfate and acid attack compared to traditional cement products [152, 153]. Sulfate attack occurs when sulfate ions in water or soil react with the calcium hydroxide in concrete, forming calcium sulfate [154]. This can cause the concrete to expand and crack, leading to structural damage [155]. Unlike cementitious composites, a better sulfate performance has been reported for geopolymers thanks to the contribution of sodium and magnesium sulfate solutions to a more stable cross-linked aluminosilicate polymer structure [156]. Compared to sodium sulfate, magnesium sulfate can cause higher strength losses, reaching ten times for slag-based geopolymers due to degradation of gypsum formation and transformation of C-A-S–H binding gels to non-cementitious and fibrous M-A-S–H products (please see Fig. 9) [157].
The effect of (a) sodium and (b) magnesium sulfate on the microstructural deterioration of slag-based geopolymers [157]
Acid attack occurs when acid rain or other acidic solutions come into contact with concrete, causing the surface to erode and weaken [158]. Geopolymers have been found to be highly resistant to both sulfate and acid attack [159]. This is because the chemical structure of geopolymers makes them less susceptible to chemical attack than OPC composites [153]. Using slag in geopolymers improves the pore structure, mass loss, and deterioration caused by acid attack, as seen in Fig. 10 [160]. These deteriorations are proportionally followed by strength loss of geopolymer composites. Overall, slag-based geopolymers have been found to provide superior protection against both sulfate and acid attack, making them an ideal material for use in harsh environments. While traditional concrete remains the most widely used material in construction, the superior performance of geopolymer concrete is making it an increasingly popular choice for sustainable and long-lasting infrastructure.
Effect of slag content on (a) the porosity, (b) surface deterioration, and (c) the schematical deterioration mechanism of geopolymers [160]
Basic and recent approaches for improving durability aspects
The inherent complexity of alkali-activated materials can pose a major challenge in overcoming their poor durability aspects. Unlike its cementitious counterparts, it is not possible to improve these durability properties by controlling limited parameters such as water/cement ratio, binder dosage and binder type. The degradation mechanisms of these binders are significantly affected by many factors, including the mixing details, the type, chemistry, fineness, and amorphous content of precursors, the type and molarity of activator, silicate modulus, curing regime, pressing, etc. [28, 161,162,163,164,165,166,167,168,169,170,171,172,173,174,175]. Alkali-activated materials can be made into a more durable material by appropriate selection of precursors and alkaline activators, optimization of mixing ratios, and appropriate curing [176]. Enhancing the reactivity of aluminosilicates contributes to the development of geopolymeric network and strength [177]. SiO2 and Al2O3, the main components of zeolitic structures, are the most important oxides to be considered in geopolymerization [178]. For superior mechanical properties, it is recommended by many researchers that the SiO2/Al2O3 ratio be in the range of 3.2–3.8 [179, 180]. In addition, a suitable CaO/SiO2 ratio, which is mainly controlled by the slag ratio in the mixture precursors, can result in better durability of alkali-activated materials [54, 181]. A strong connection between strength and durability is expected due to the presence of stable reaction products in alkali-activated and geopolymer systems [182]. Therefore, all attempts increasing the strength can contribute the development of durability. Unlike cementitious systems, threshold content of aggregate has been found to be a specific approach to increase the strength of geopolymer concrete [183]. This is probably due to the strong bond between the aggregate and the matrix [184]. The mechanical performance of geopolymers is related to their porosity structure. When a pre-setting pressure is applied to geopolymer mixtures, air bubbles are removed and macroporosity is significantly reduced, resulting in high mechanical strength [1]. Zivica et al. [185] developed a geopolymer material with low alkaline activator content by pressing the mixtures under 300 MPa pressure for 1 min. It was announced that 500 times higher mechanical results were obtained compared to the reference sample, and this increase was achieved by the decrease in porosity and pore size. Another parameter that affects this process with the hot press method is the temperature applied. As the applied temperature and application time increased, mechanical performances also increased. Ranjbar et al. [186] showed that samples with a compressive strength of up to 185 MPa were produced with a temperature between 110–400 °C and a pressing pressure of 74 MPa for a period of 10–40 min. In this application, it is understood that the microstructure improves significantly as the temperature increases from 110 °C to 400 °C (Fig. 11).
Samples cured at (a) 110 °C (b) 400 °C for 30 min [186]
Recently, hot-pressed geopolymers have been introduced as a ceramic-like material that can be produced in a very short time, has a low amount of alkaline activator, and has high strength [187]. This method uses a simultaneous combination of continuous heating and pressing to eliminate the macropore structure, facilitate the dissolution of the reacted oxides, and accelerate the subsequent geopolymerization reaction. The material properties of hot-pressed geopolymers largely depend on the parameters of temperature, pressure and curing time. In a study, it was observed that increasing the temperature accelerated the dissolution of amorphous phases, the removal of water and the densification process of geopolymers [186]. The densification regarding the formation amount of geopolymer bonds under the influence of temperature and pressure parameters is visually presented in Fig. 12.
Image analysis of the hot-pressed geopolymer produced at (a) and (b) 110 °C-6.1 MPa, (c) and (d) 400 °C-73.2 MPa; (e) phase distribution of the specimens processed at 110 °C-6.1 MPa, 200 °C-24.4 MPa, 300 °C-48.7 MPa, and 350 °C-73.2 MPa; and (f) geopolymer gel to solid ratio [188]
Conclusions and future perspective
As the demand for sustainable and low-carbon footprint materials continues to grow, it seems important that we continue to innovate and advance the science of geopolymers and alkali-activated materials. Recently, a lot of research has done on the durability of these materials to great success. However, compared with the current progress of ordinary Portland cement, there is still a lot of work to be done in the coming years to overcome inherent problems and difficulties. The following conclusions and future recommendations can be drawn from this critical review on the durability of slag-based alkali-activated materials:
-
The complexity in durability of alkali-activated systems appears by researchers as the most significant obstacle to becoming commercial-off-the-shelf products of them. Mechanical loss, a quantitative indicator for durability, is mostly used in order to evaluate the degradation level of alkali-activated and geopolymer systems. However, overcoming durability deficiencies is not as easy to manage as mechanical aspects because of complex chemistry, strongly affected by many factors, including compositional details, curing regimes, pre-setting pressure, environmental degradation mechanisms, etc. For this reason, recently, durability studies have come to the fore in research rather than their mechanical, cost and environmental properties.
-
Inappropriate design of these systems can cause dimensional stability problems, including micro and macro cracks that appear during setting and hardening. Therefore, unbound and dissolved alkalis (Na and K) and calcium ions in the matrix are easily leached and carbonated. Not only is durability compromised by the dissolution of the aluminosilicate gel and free alkaline ions during leaching, but the alkali-activated materials may also pose a further threat to the environment as it contains high volumes of industrial byproducts with concentrated toxic heavy metal contents. When specimens are exposed to water, leaching of sodium silicate can produce a strength loss of up to 25%, while interruption of geopolymeric reactions by evaporation of water can result in a higher strength loss of up to 69% due to the increased porosity (around 50%) of the solid body. A high concentration of alkalis is required for favorable strength development of alkali-activated materials. However, their increased solubility and absorption rate of CO2 accelerate efflorescence. An increment of slag content instead of fly ash contributes to the carbonation resistance of them, and even some extent improvement in strength can be achieved at various carbonation exposures.
-
One of the main reasons for shrinkage in geopolymer and alkali-activated materials is the chemical reaction that occurs during the setting and hardening process. Water is consumed during the chemical reaction, which can cause shrinkage. Another factor that can contribute to shrinkage is the microstructure of the material. The gel structure of these materials is more porous and has a higher surface area than OPC, which can lead to greater shrinkage. The increase in the amount of slag and NaOH contributes to the pore structure of alkali-activated materials, resulting in lower drying and autogenous shrinkage but higher chemical shrinkage. By optimizing the mix design, engineers and builders can reduce the amount of shrinkage and improve the durability and strength of the material.
-
These materials are fire resistant without showing any spalling in various fire regimes compared to conventional concrete, due to the highly interconnected pore structure that allows water vapor to escape. Alkali-activated materials exposed to temperatures of up to 1000 °C for several hours can maintain their structural integrity and show no signs of spalling, unlike OPC samples. Current results demonstrate that geopolymers and alkali-activated materials have the potential to be used in a wide variety of applications where fire resistance is critical: (1) fire-resistant cladding and facades for buildings, (2) fire-resistant insulation materials, (3) fire-resistant coatings for steel structures, (4) fire-resistant flooring and paving materials, and (5) fire-resistant radiation shields. Studies show that the use of slag as a precursor can lead to a decrease in fire resistance compared to other types of precursors, such as fly ash. Even the use of small amounts (5%) of slag in siliceous fly ash-based materials can cause higher crack densities in the matrix, up to fivefold, after exposure to high temperatures. To understand the fire resistance of alkali-activated materials in detail, further research is needed not only on topics such as crack density, crack rehabilitation, transformation of phase chemistry, and pore structure.
-
Unlike OPC, air-entraining admixtures can impair the freeze–thaw performance of alkali-activated materials. Therefore, it is clear that finding a solution to this problem at a material scale is of utmost importance. Compared to low-calcium ones, including fly ash and natural zeolite, slag-based materials have better resistance to freeze–thaw cycles. This is probably due to suitable pore structures with denser microstructure and high strength matrix proportional to the slag content. One of the main reasons why these materials are resistant to freeze–thaw damage is their low permeability, as is known in concrete technology. Unlike cement paste, alkali-activated materials have much lower porosity, meaning they are less likely to absorb water. Another factor is a unique microstructure consisting of a dense matrix of interlocking crystals. These crystals are packed tightly together, leaving little space for water to seep in. This dense structure makes it harder for water to penetrate the material, making it more resistant to freeze–thaw damage.
-
These degradation mechanisms examined in the article are mostly interrogated as key durability considerations for alkali-activated materials to provide a comprehensive breakdown of how they are changing the game in the construction industry. Overall, it is understood that this concrete will be a versatile and effective material for use in a variety of construction projects, such as wastewater treatment plants, bridges, high-rise buildings, highways, tunnels, dams, hydraulic structures, and tunnel segments. A higher exposure time of up to 21 times is reported for high-volume slag-based material concrete compared to OPC concrete in terms of threshold chloride level. With increasing slag content, these materials were found to have lower permeability to chloride ions; this means they are less susceptible to corrosion caused by chloride penetration. This makes alkali-activated materials an ideal material for use in coastal areas or other environments where chloride exposure is a concern. Unlike cementitious composites, a better sulfate performance has been reported for these materials, thanks to the contribution of sodium and magnesium sulfate solutions to a more stable cross-linked aluminosilicate polymer structure. Compared to sodium sulfate, magnesium sulfate can cause higher strength losses, up to tenfold for slag-based materials due to the degradation of gypsum formation and transformation of C-A-S–H binding gels to non-cementitious and fibrous M-A-S–H products. The favorable chemical structure of alkali-activated materials makes them less susceptible to chemical attack than OPC composites. The use of slag improves the pore structure, mass loss, and deterioration caused by acid attack. In general, slag-based materials have been found to provide superior protection against both sulfate and acid attack, making them an ideal material for use in harsh environments.
References
Gökçe, H.S., Tuyan, M., Ramyar, K., Nehdi, M.L.: Development of eco-efficient fly ash–based alkali-activated and geopolymer composites with reduced alkaline activator dosage. J. Mater. Civ. Eng. 32, 04019350 (2020). https://doi.org/10.1061/(asce)mt.1943-5533.0003017
Krishna, R.S., Mishra, J., Zribi, M., Adeniyi, F., Saha, S., Baklouti, S., Shaikh, F.U.A., Gökçe, H.S.: A review on developments of environmentally friendly geopolymer technology. Materialia 20, 101212 (2021). https://doi.org/10.1016/j.mtla.2021.101212
Ünal, M.T., Gökçe, H.S., Ayough, P., Alnahhal, A.M., Şimşek, O., Nehdi, M.L.: Nanomaterial and fiber-reinforced sustainable geopolymers: A systematic critical review. Constr. Build. Mater. 404, 133325 (2023). https://doi.org/10.1016/j.conbuildmat.2023.133325
Tekin, İ, Ciza, B., Gökçe, H.S.: Development of alkali-activated binders from recycling regional tuff and marble wastes. El-Cezeri J. Sci. Eng. 10, 371–387 (2023). https://doi.org/10.31202/ecjse.1225457
Davidovits, J.: Geopolymer Cement a review. Geopolymer Sci. Tech., Geopolymer Institute Library, Technical Paper 21(1), 11 (2013). www.geopolymer.org
Van Deventer, J.S.J., Provis, J.L., Duxson, P., Brice, D.G.: Chemical research and climate change as drivers in the commercial adoption of alkali activated materials. Waste and Biomass Valorization 1, 145–155 (2010). https://doi.org/10.1007/s12649-010-9015-9
Scopus: Number of documents from 1984 to 2024 on “alkali-activated slag” and “slag-based geopolymer” (accessed 1 Mar 2024). https://www.scopus.com/search/form.uri?display=basic#basic/ (2024)
Provis, J.L., Bernal, S.A.: Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 44, 299–327 (2014). https://doi.org/10.1146/annurev-matsci-070813-113515
Gökçe, H.S., Güngör, O., Öksüzer, N.: A novel internal curing method for 3D-printed geopolymer structures reinforced with a steel cable: Electro-heating. Mater. Lett. 309, 131364 (2022). https://doi.org/10.1016/j.matlet.2021.131364
Qian, L.P., Xu, L.Y., Huang, B.T., Dai, J.G.: Pelletization and properties of artificial lightweight geopolymer aggregates (GPA): One-part vs two-part geopolymer techniques. J. Clean. Prod. 374, 133933 (2022). https://doi.org/10.1016/j.jclepro.2022.133933
Kök, S., Türetken, M.S., Öksüzer, N., Gökçe, H.S.: Effect of elevated temperature on radiation shielding properties of cement and geopolymer mortars including barite aggregate and colemanite powder. Materialia 27, 101693 (2023). https://doi.org/10.1016/j.mtla.2023.101693
Gökçe, H.S., Tuyan, M., Nehdi, M.L.: Alkali-activated and geopolymer materials developed using innovative manufacturing techniques: A critical review. Constr. Build. Mater. 303, 124483 (2021). https://doi.org/10.1016/j.conbuildmat.2021.124483
Wan, J., Zhang, F., Han, Z., Song, L., Zhang, C., Zhang, J.: Adsorption of Cd2+ and Pb2+ by biofuel ash-based geopolymer synthesized by one-step hydrothermal method. Arab. J. Chem. 14, 103234 (2021). https://doi.org/10.1016/j.arabjc.2021.103234
Wasim, M., Ngo, T.D., Law, D.: A state-of-the-art review on the durability of geopolymer concrete for sustainable structures and infrastructure. Constr. Build. Mater. 291, 123381 (2021). https://doi.org/10.1016/j.conbuildmat.2021.123381
Pollio, M.V., Morgan, M.H.: The Ten Books on Architecture. Harvard University Press, Cambridge (1914)
Ling, Y., Wang, K., Wang, X., Hua, S.: Effects of mix design parameters on heat of geopolymerization, set time, and compressive strength of high calcium fly ash geopolymer. Constr. Build. Mater. 228, 116763 (2019). https://doi.org/10.1016/j.conbuildmat.2019.116763
Naghizadeh, A., Ekolu, S.O.: Effects of compositional and physico – chemical mix design parameters on properties of fly ash geopolymer mortars. SILICON 13, 4669–4680 (2021). https://doi.org/10.1007/s12633-020-00799-2
Mayhoub, O.A., Mohsen, A., Alharbi, Y.R., Abadel, A.A., Habib, A.O., Kohail, M.: Effect of curing regimes on chloride binding capacity of geopolymer. Ain Shams Eng. J. 12, 3659–3668 (2021). https://doi.org/10.1016/j.asej.2021.04.032
Nodehi, M., Ozbakkaloglu, T., Gholampour, A., Mohammed, T., Shi, X.: The effect of curing regimes on physico-mechanical, microstructural and durability properties of alkali-activated materials: A review. Constr. Build. Mater. 321, 126335 (2022). https://doi.org/10.1016/j.conbuildmat.2022.126335
Verma, N.K., Rao, M.C., Kumar, S.: Effect of curing regime on compressive strength of geopolymer concrete. IOP Conf. Ser. Earth Environ. Sci. 982, 012031 (2022). https://doi.org/10.1088/1755-1315/982/1/012031
John, S.K., Nadir, Y., Girija, K.: Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 280, 122443 (2021). https://doi.org/10.1016/j.conbuildmat.2021.122443
Archez, J., Farges, R., Gharzouni, A., Rossignol, S.: Influence of the geopolymer formulation on the endogeneous shrinkage. Constr. Build. Mater. 298, 123813 (2021). https://doi.org/10.1016/j.conbuildmat.2021.123813
Kaya, M.: The effect of micro-SiO2 and micro-Al2O3 additive on the strength properties of ceramic powder-based geopolymer pastes. J. Mater. Cycles Waste Manag. 24, 333–350 (2022). https://doi.org/10.1007/s10163-021-01323-3
Kantarcı, F., Türkmen, İ, Ekinci, E.: Enhancing acid resistance of geopolymer concrete composites by utilising styrene-butadiene latex, nano-silica and micro-silica powder. Eur. J. Environ. Civ. Eng. 27, 4416–4434 (2023). https://doi.org/10.1080/19648189.2023.2191675
Zhang, B., Guo, H., Yuan, P., Deng, L., Zhong, X., Li, Y., Wang, Q., Liu, D.: Novel acid-based geopolymer synthesized from nanosized tubular halloysite: The role of precalcination temperature and phosphoric acid concentration. Cem. Concr. Compos. 110, 103601 (2020). https://doi.org/10.1016/j.cemconcomp.2020.103601
Jin, M., Zheng, Z., Sun, Y., Chen, L., Jin, Z.: Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J. Non Cryst. Solids 450, 116–122 (2016). https://doi.org/10.1016/j.jnoncrysol.2016.07.036
Tennakoon, C., De Silva, P., Sagoe-Crentsil, K., Sanjayan, J.G.: Influence and role of feedstock Si and Al content in geopolymer synthesis. J. Sustain. Cem. Mater. 4, 129–139 (2016). https://doi.org/10.1080/21650373.2014.979264
Phoo-ngernkham, T., Maegawa, A., Mishima, N., Hatanaka, S., Chindaprasirt, P.: Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 91, 1–8 (2015). https://doi.org/10.1016/j.conbuildmat.2015.05.001
Khan, M.Z.N., Shaikh, F., Uddin, A., Hao, Y., Hao, H.: Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 125, 809–820 (2016). https://doi.org/10.1016/j.conbuildmat.2016.08.097
Zhang, Y., Xiao, R., Jiang, X., Li, W., Zhu, X., Huang, B.: Effect of particle size and curing temperature on mechanical and microstructural properties of waste glass-slag-based and waste glass-fly ash-based geopolymers. J. Clean. Prod. 273, 122970 (2020). https://doi.org/10.1016/j.jclepro.2020.122970
Zhang, R., He, H., Song, Y., Zhi, X., Fan, F.: Influence of mix proportioning parameters and curing regimes on the properties of ultra-high strength alkali-activated concrete. Constr. Build. Mater. 393, 132139 (2023). https://doi.org/10.1016/j.conbuildmat.2023.132139
Alam, S., Das, S.K., Rao, B.H.: Strength and durability characteristic of alkali activated GGBS stabilized red mud as geo-material. Constr. Build. Mater. 211, 932–942 (2019). https://doi.org/10.1016/j.conbuildmat.2019.03.261
Davidovits, J.: Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 8, 335–350 (2017). https://doi.org/10.4416/JCST2017-00038
Bernal, S.A., San Nicolas, R., Provis, J.L., Mejía De Gutiérrez, R., Van Deventer, J.S.J.: Natural carbonation of aged alkali-activated slag concretes. Mater. Struct. Constr. 47, 693–707 (2014). https://doi.org/10.1617/s11527-013-0089-2
Behfarnia, K., Rostami, M.: An assessment on parameters affecting the carbonation of alkali-activated slag concrete. J. Clean. Prod. 157, 1–9 (2017). https://doi.org/10.1016/j.jclepro.2017.04.097
Shi, Z., Shi, C., Wan, S., Li, N., Zhang, Z.: Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 113, 55–64 (2018). https://doi.org/10.1016/j.cemconres.2018.07.005
Li, N., Farzadnia, N., Shi, C.: Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 100, 214–226 (2017). https://doi.org/10.1016/j.cemconres.2017.07.008
Saludung, A., Azeyanagi, T., Ogawa, Y., Kawai, K.: Effect of silica fume on efflorescence formation and alkali leaching of alkali-activated slag. J. Clean. Prod. 315, 128210 (2021). https://doi.org/10.1016/j.jclepro.2021.128210
Keulen, A., van Zomeren, A., Dijkstra, J.J.: Leaching of monolithic and granular alkali activated slag-fly ash materials, as a function of the mixture design. Waste Manag. 78, 497–508 (2018). https://doi.org/10.1016/j.wasman.2018.06.019
Srinivasamurthy, L., Chevali, V.S., Zhang, Z., Wang, H.: Effect of fly ash to slag ratio and Na2O content on leaching behaviour of fly Ash/Slag based alkali activated materials. Constr. Build. Mater. 383, 131234 (2023). https://doi.org/10.1016/j.conbuildmat.2023.131234
Zhu, Y., Longhi, M.A., Wang, A., Hou, D., Wang, H., Zhang, Z.: Alkali leaching features of 3-year-old alkali activated fly ash-slag-silica fume: For a better understanding of stability. Compos. Part B Eng. 230, 109469 (2022). https://doi.org/10.1016/j.compositesb.2021.109469
Zhang, B., Zhu, H., Cheng, Y., Huseien, G.F., Shah, K.W.: Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 318, 125993 (2022). https://doi.org/10.1016/j.conbuildmat.2021.125993
Duran Atiş, C., Bilim, C., Çelik, Ö., Karahan, O.: Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 23, 548–555 (2009). https://doi.org/10.1016/j.conbuildmat.2007.10.011
Li, Z., Lu, T., Liang, X., Dong, H., Ye, G.: Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 135, 106107 (2020). https://doi.org/10.1016/j.cemconres.2020.106107
Cartwright, C., Rajabipour, F., Radlińska, A.: Shrinkage characteristics of alkali-activated slag cements. J. Mater. Civ. Eng. 27, B4014007 (2015). https://doi.org/10.1061/(asce)mt.1943-5533.0001058
Karahan, O., Yakupoǧlu, A.: Resistance of alkali-activated slag mortar to abrasion and fire. Adv. Cem. Res. 23, 289–297 (2011). https://doi.org/10.1680/adcr.2011.23.6.289
Shoaei, P., Ghassemi, P., Ameri, F., Musaeei, H.R., Chee Ban, C., Ozbakkaloglu, T.: Comparative study on the effect of fiber type and content on the fire resistance of alkali-activated slag composites. Constr. Build. Mater. 288, 123136 (2021). https://doi.org/10.1016/j.conbuildmat.2021.123136
Huang, W., Wang, Y., Zhang, Y., Zheng, W.: Experimental study of high-temperature resistance of alkali-activated slag crushed aggregate mortar. J. Mater. Res. Technol. 23, 3961–3973 (2023). https://doi.org/10.1016/j.jmrt.2023.02.072
Zuda, L., Rovnaník, P., Bayer, P., Černý, R.: Thermal properties of alkali-activated slag subjected to high temperatures. J. Build. Phys. 30, 337–350 (2007). https://doi.org/10.1177/1744259106075234
Shahrajabian, F., Behfarnia, K.: The effects of nano particles on freeze and thaw resistance of alkali-activated slag concrete. Constr. Build. Mater. 176, 172–178 (2018). https://doi.org/10.1016/j.conbuildmat.2018.05.033
Fu, Y., Cai, L., Yonggen, W.: Freeze–thaw cycle test and damage mechanics models of alkali-activated slag concrete. Constr. Build. Mater. 25, 3144–3148 (2011). https://doi.org/10.1016/j.conbuildmat.2010.12.006
Zhu, H., Zhai, M., Liang, G., Li, H., Wu, Q., Zhang, C., Hua, S.: Experimental study on the freezing resistance and microstructure of alkali-activated slag in the presence of rice husk ash. J. Build. Eng. 38, 102173 (2021). https://doi.org/10.1016/j.jobe.2021.102173
Zhang, Y., He, Y., Cui, X., Liu, L.: Enhancing freeze-thaw resistance of alkali-activated slag by metakaolin. ACS Omega 8, 20869–20880 (2023). https://doi.org/10.1021/acsomega.3c01600
Lee, N.K., Lee, H.K.: Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 72, 168–179 (2016). https://doi.org/10.1016/j.cemconcomp.2016.06.004
Zhang, J., Shi, C., Zhang, Z., Ou, Z.: Durability of alkali-activated materials in aggressive environments: A review on recent studies. Constr. Build. Mater. 152, 598–613 (2017). https://doi.org/10.1016/j.conbuildmat.2017.07.027
Wang, X., Kong, L., Zhao, W., Liu, Y.: Chloride transport resistance of alkali-activated concrete exposed to combined chloride, sulfate and carbonation environment. Constr. Build. Mater. 367, 130353 (2023). https://doi.org/10.1016/j.conbuildmat.2023.130353
Vu Huyen, T., Dang, L.C., Kang, G., Sirivivatnanon, V.: Chloride induced corrosion of steel reinforcement in alkali activated slag concretes: A critical review. Case Stud. Constr. Mater. 16, e01112 (2022). https://doi.org/10.1016/j.cscm.2022.e01112
Manjunath, R., Narasimhan, M.C., Umesha, K.M.: Studies on high performance alkali activated slag concrete mixes subjected to aggressive environments and sustained elevated temperatures. Constr. Build. Mater. 229, 116887 (2019). https://doi.org/10.1016/j.conbuildmat.2019.116887
Thunuguntla, C.S., Gunneswara Rao, T.D.: Effect of mix design parameters on mechanical and durability properties of alkali activated slag concrete. Constr. Build. Mater. 193, 173–188 (2018). https://doi.org/10.1016/j.conbuildmat.2018.10.189
Awoyera, P., Adesina, A.: Durability properties of alkali activated slag composites: short overview. SILICON 12, 987–996 (2020). https://doi.org/10.1007/s12633-019-00199-1
Kantarcı, F.: Influence of fiber characteristics on sulfate resistance of ambient-cured geopolymer concrete. Struct. Concr. 23, 775–790 (2022). https://doi.org/10.1002/suco.202100540
Scopus: Analyzing number of documents on “durability” (accessed 1 Mar 2024). https://www.scopus.com/search/form.uri?display=basic#basic/. (2024)
Li, Z., Li, S.: Carbonation resistance of fly ash and blast furnace slag based geopolymer concrete. Constr. Build. Mater. 163, 668–680 (2018). https://doi.org/10.1016/j.conbuildmat.2017.12.127
Pouhet, R., Cyr, M.: Carbonation in the pore solution of metakaolin-based geopolymer. Cem. Concr. Res. 88, 227–235 (2016). https://doi.org/10.1016/j.cemconres.2016.05.008
Pandey, B., Kinrade, S.D., Catalan, L.J.J.: Effects of carbonation on the leachability and compressive strength of cement-solidified and geopolymer-solidified synthetic metal wastes. J. Environ. Manage. 101, 59–67 (2012). https://doi.org/10.1016/j.jenvman.2012.01.029
Hills, T.P., Gordon, F., Florin, N.H., Fennell, P.S.: Statistical analysis of the carbonation rate of concrete. Cem. Concr. Res. 72, 98–107 (2015). https://doi.org/10.1016/j.cemconres.2015.02.007
Komnitsas, K., Zaharaki, D.: Utilisation of low-calcium slags to improve the strength and durability of geopolymers. In Geopolymers (Elsevier), pp. 343–375. https://doi.org/10.1533/9781845696382.2.343. (2009)
Law, D.W., Adam, A.A., Molyneaux, T.K., Patnaikuni, I., Wardhono, A.: Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 48, 721–731 (2015). https://doi.org/10.1617/s11527-014-0268-9
Pasupathy, K., Sanjayan, J., Rajeev, P.: Evaluation of alkalinity changes and carbonation of geopolymer concrete exposed to wetting and drying. J. Build. Eng. 35, 102029 (2021). https://doi.org/10.1016/j.jobe.2020.102029
Azarsa, P., Gupta, R.: Durability and leach-ability evaluation of K-based geopolymer concrete in real environmental conditions. Case Stud. Constr. Mater. 13, e00366 (2020). https://doi.org/10.1016/j.cscm.2020.e00366
Izquierdo, M., Querol, X., Davidovits, J., Antenucci, D., Nugteren, H., Fernández-Pereira, C.: Coal fly ash-slag-based geopolymers: Microstructure and metal leaching. J. Hazard. Mater. 166, 561–566 (2009). https://doi.org/10.1016/j.jhazmat.2008.11.063
Xu, J.Z., Zhou, Y.L., Chang, Q., Qu, H.Q.: Study on the factors of affecting the immobilization of heavy metals in fly ash-based geopolymers. Mater. Lett. 60, 820–822 (2006). https://doi.org/10.1016/j.matlet.2005.10.019
Sun, Z., Vollpracht, A.: Leaching of monolithic geopolymer mortars. Cem. Concr. Res. 136, 106161 (2020). https://doi.org/10.1016/j.cemconres.2020.106161
Guerrieri, M., Sanjayan, J., Mohd Ali, A.Z.: Geopolymer Damage Due to Leaching When Exposed to Water. In International RILEM Workshop on Concrete Durability and Service Life Planning (ConcreteLife) (Cham.: Springer), pp. 74–78. https://doi.org/10.1007/978-3-030-43332-1_15. (2020)
Izquierdo, M., Querol, X., Phillipart, C., Antenucci, D., Towler, M.: The role of open and closed curing conditions on the leaching properties of fly ash-slag-based geopolymers. J. Hazard. Mater. 176, 623–628 (2010). https://doi.org/10.1016/j.jhazmat.2009.11.075
Dow, C., Glasser, F.: Calcium carbonate efflorescence on Portland cement and building materials. Cem. Concr. Res. 33, 147–154 (2003). https://doi.org/10.1016/S0008-8846(02)00937-7
Allahverdi, A., Najafi Kani, E., Shaverdi, B.: Carbonation versus efflorescence in alkali-activated blast-furnace slag in relation with chemical composition of activator. Int. J. Civ. Eng. 15, 565–573 (2017). https://doi.org/10.1007/s40999-017-0225-4
Simão, L., Fernandes, E., Hotza, D., Ribeiro, M.J., Montedo, O.R.K., Raupp-Pereira, F.: Controlling efflorescence in geopolymers: A new approach. Case Stud. Constr. Mater. 15, e00740 (2021). https://doi.org/10.1016/j.cscm.2021.e00740
Gado, R.A., Hebda, M., Łach, M., Mikuła, J.: Alkali activation of waste clay bricks: Influence of the silica modulus, SiO2/Na2O, H2O/Na2O molar ratio, and liquid/solid ratio. Materials (Basel). 13, 383 (2020). https://doi.org/10.3390/ma13020383
Longhi, M.A., Walkley, B., Rodríguez, E.D., Kirchheim, A.P., Zhang, Z., Wang, H.: New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos. Part B Eng. 176, 107172 (2019). https://doi.org/10.1016/j.compositesb.2019.107172
Longhi, M.A., Rodríguez, E.D., Walkley, B., Zhang, Z., Kirchheim, A.P.: Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 182, 107671 (2020). https://doi.org/10.1016/j.compositesb.2019.107671
Yellaiah, P., Sharma, S.K., Gunneswara Rao, T.D.: Tensile strength of fly ash based geopolymer mortar. ARPN J. Eng. Appl. Sci. 9, 2297–2301 (2014)
Scherer, G.W.: Stress from crystallization of salt. Cem. Concr. Res. 34, 1613–1624 (2004). https://doi.org/10.1016/j.cemconres.2003.12.034
Zhang, Z., Provis, J.L., Ma, X., Reid, A., Wang, H.: Efflorescence and subflorescence induced microstructural and mechanical evolution in fly ash-based geopolymers. Cem. Concr. Compos. 92, 165–177 (2018). https://doi.org/10.1016/j.cemconcomp.2018.06.010
Longhi, M.A., Rodríguez, E.D., Walkley, B., Eckhard, D., Zhang, Z., Provis, J.L., Kirchheim, A.P.: Metakaolin-based geopolymers: Efflorescence and its effect on microstructure and mechanical properties. Ceram. Int. 48, 2212–2229 (2022). https://doi.org/10.1016/j.ceramint.2021.09.313
Shen, J., Li, Y., Lin, H., Li, Y.: Development of autogenous shrinkage prediction model of alkali-activated slag-fly ash geopolymer based on machine learning. J. Build. Eng. 71, 106538 (2023). https://doi.org/10.1016/j.jobe.2023.106538
Khan, I., Xu, T., Castel, A., Gilbert, R.I., Babaee, M.: Risk of early age cracking in geopolymer concrete due to restrained shrinkage. Constr. Build. Mater. 229, 116840 (2019). https://doi.org/10.1016/j.conbuildmat.2019.116840
Panchmatia, P., Olvera, R., Genedy, M., Juenger, M.C.G., van Oort, E.: Shrinkage behavior of Portland and geopolymer cements at elevated temperature and pressure. J. Pet. Sci. Eng. 195, 107884 (2020). https://doi.org/10.1016/j.petrol.2020.107884
Humad, A.M., Provis, J.L., Habermehl-Cwirzen, K., Rajczakowska, M., Cwirzen, A.: Creep and long-term properties of alkali-activated swedish-slag concrete. J. Mater. Civ. Eng. 33, 04020475 (2021). https://doi.org/10.1061/(asce)mt.1943-5533.0003381
Hou, Y., Wang, D., Zhou, W., Lu, H., Wang, L.: Effect of activator and curing mode on fly ash-based geopolymers. J. Wuhan Univ. Technol. Sci. Ed. 24, 711–715 (2009). https://doi.org/10.1007/s11595-009-5711-3
Lingyu, T., Dongpo, H., Jianing, Z., Hongguang, W.: Durability of geopolymers and geopolymer concretes: A review. Rev. Adv. Mater. Sci. 60, 1–14 (2021). https://doi.org/10.1515/rams-2021-0002
Olvera, R., Panchmatia, P., Juenger, M., Aldin, M., and van Oort, E.: Long-term oil well zonal isolation control using geopolymers: an analysis of shrinkage behavior. In Day 3 Thu, March 07, 2019 (The Hague, The Netherlands: SPE). https://doi.org/10.2118/194092-MS. (2019)
Ling, Y., Wang, K., Fu, C.: Shrinkage behavior of fly ash based geopolymer pastes with and without shrinkage reducing admixture. Cem. Concr. Compos. 98, 74–82 (2019). https://doi.org/10.1016/j.cemconcomp.2019.02.007
Zhan, J., Li, H., Pan, Q., Cheng, Z., Li, H., Fu, B.: Effect of slag on the strength and shrinkage properties of metakaolin-based geopolymers. Materials (Basel). 15, 2944 (2022). https://doi.org/10.3390/ma15082944
Zhang, Y., Liu, H., Ma, T., Gu, G., Chen, C., Hu, J.: Understanding the changes in engineering behaviors and microstructure of FA-GBFS based geopolymer paste with addition of silica fume. J. Build. Eng. 70, 106450 (2023). https://doi.org/10.1016/j.jobe.2023.106450
Dheyaaldin, M.H., Mosaberpanah, M.A., Alzeebaree, R.: Shrinkage behavior and mechanical properties of alkali activated mortar incorporating nanomaterials and polypropylene fiber. Ceram. Int. 48, 23159–23171 (2022). https://doi.org/10.1016/j.ceramint.2022.04.297
Khater, H.M., Abd el Gawaad, H.A.: Characterization of alkali activated geopolymer mortar doped with MWCNT. Constr. Build. Mater. 102, 329–337 (2016). https://doi.org/10.1016/j.conbuildmat.2015.10.121
da Silva Nuernberg, N.B., Niero, D.F., Bernardin, A.M.: Valorization of rice husk ash and aluminum anodizing sludge as precursors for the synthesis of geopolymers. J. Clean. Prod. 298, 126770 (2021). https://doi.org/10.1016/j.jclepro.2021.126770
Nikolov, A., Nugteren, H., Rostovsky, I.: Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr. Build. Mater. 243, 118257 (2020). https://doi.org/10.1016/j.conbuildmat.2020.118257
Zahid, M., Shafiq, N., Razak, S.N.A., Tufail, R.F.: Investigating the effects of NaOH molarity and the geometry of PVA fibers on the post-cracking and the fracture behavior of engineered geopolymer composite. Constr. Build. Mater. 265, 120295 (2020). https://doi.org/10.1016/j.conbuildmat.2020.120295
Zhang, N., Yan, C., Li, L., Khan, M.: Assessment of fiber factor for the fracture toughness of polyethylene fiber reinforced geopolymer. Constr. Build. Mater. 319, 126130 (2022). https://doi.org/10.1016/j.conbuildmat.2021.126130
Chen, W., Li, B., Guo, M.Z., Wang, J., Chen, Y.T.: Impact of heat curing regime on the compressive strength and drying shrinkage of alkali-activated slag mortar. Dev. Built Environ. 14, 100123 (2023). https://doi.org/10.1016/j.dibe.2023.100123
Sadeghian, G., Behfarnia, K., Teymouri, M.: Drying shrinkage of one-part alkali-activated slag concrete. J. Build. Eng. 51, 104263 (2022). https://doi.org/10.1016/j.jobe.2022.104263
Kaplan, G., Yavuz Bayraktar, O., Bayrak, B., Celebi, O., Bodur, B., Oz, A., Aydin, A.C.: Physico-mechanical, thermal insulation and resistance characteristics of diatomite and attapulgite based geopolymer foam concrete: Effect of different curing regimes. Constr. Build. Mater. 373, 130850 (2023). https://doi.org/10.1016/j.conbuildmat.2023.130850
Ridtirud, C., Chindaprasirt, P., Pimraksa, K.: Factors affecting the shrinkage of fly ash geopolymers. Int. J. Miner. Metall. Mater. 18, 100–104 (2011). https://doi.org/10.1007/s12613-011-0407-z
Hwang, C.-L., Huynh, T.-P.: Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 101, 1–9 (2015). https://doi.org/10.1016/j.conbuildmat.2015.10.025
Singh, B., Rahman, M.R., Paswan, R., Bhattacharyya, S.K.: Effect of activator concentration on the strength, ITZ and drying shrinkage of fly ash/slag geopolymer concrete. Constr. Build. Mater. 118, 171–179 (2016). https://doi.org/10.1016/j.conbuildmat.2016.05.008
Zahid, M., Shafiq, N.: Effects of sand/fly ash and the water/solid ratio on the mechanical properties of engineered geopolymer composite and mix design optimization. Minerals 10, 333 (2020). https://doi.org/10.3390/min10040333
Thokchom, S., Mandal, K.K., Ghosh, S.: Effect of Si/Al ratio on performance of fly ash geopolymers at elevated temperature. Arab. J. Sci. Eng. 37, 977–989 (2012). https://doi.org/10.1007/s13369-012-0230-5
Amran, M., Huang, S.S., Debbarma, S., Rashid, R.S.M.: Fire resistance of geopolymer concrete: A critical review. Constr. Build. Mater. 324, 126722 (2022). https://doi.org/10.1016/j.conbuildmat.2022.126722
Alehyen, S., Zerzouri, M., El Alouani, M., El Achouri, M., Taibi, M.: Porosity and fire resistance of fly ash based geopolymer. J. Mater. Environ. Sci. 8, 3676–3689 (2017)
Zhao, R., Sanjayan, J.G.: Geopolymer and Portland cement concretes in simulated fire. Mag. Concr. Res. 63, 163–173 (2011). https://doi.org/10.1680/macr.9.00110
Duan, P., Yan, C., Zhou, W., Luo, W.: Thermal behavior of portland cement and fly ash–metakaolin-based geopolymer cement pastes. Arab. J. Sci. Eng. 40, 2261–2269 (2015). https://doi.org/10.1007/s13369-015-1748-0
Zhang, P., Zheng, Y., Wang, K., and Zhang, J.: A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 152. https://doi.org/10.1016/j.compositesb.2018.06.031. (2018)
Le, V.S., Louda, P., Tran, H.N., Nguyen, P.D., Bakalova, T., Ewa Buczkowska, K., Dufkova, I.: Study on temperature-dependent properties and fire resistance of metakaolin-based geopolymer foams. Polymers (Basel). 12, 2994 (2020). https://doi.org/10.3390/polym12122994
Le, V.S., Nguyen, V.V., Sharko, A., Ercoli, R., Nguyen, T.X., Tran, D.H., Łoś, P., Buczkowska, K.E., Mitura, S., Špirek, T., et al.: Fire resistance of geopolymer foams layered on polystyrene boards. Polymers (Basel). 14, 1945 (2022). https://doi.org/10.3390/polym14101945
Chindaprasirt, P., Lao-un, J., Zaetang, Y., Wongkvanklom, A., Phoo-ngernkham, T., Wongsa, A., Sata, V.: Thermal insulating and fire resistance performances of geopolymer mortar containing auto glass waste as fine aggregate. J. Build. Eng. 60, 105178 (2022). https://doi.org/10.1016/j.jobe.2022.105178
Wang, K., Le, H.: The development of cement-based, intumescent and geopolymer fire-retardation coatings for metal structures: A review. Coatings 13, 495 (2023). https://doi.org/10.3390/coatings13030495
Logesh Kumar, M., Ramalingam, M., Dharamaraj, R.: WITHDRAWN: Alkali activated systems of geopolymer based paver blocks under different curing conditions. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2023.03.724. (2023)
Sarıdemir, M., Çelikten, S.: Effects of Ms modulus, Na concentration and fly ash content on properties of vapour-cured geopolymer mortars exposed to high temperatures. Constr. Build. Mater. 363, 129868 (2023). https://doi.org/10.1016/j.conbuildmat.2022.129868
He, R., Dai, N., Wang, Z.: Thermal and mechanical properties of geopolymers exposed to high temperature: A literature review. Adv. Civ. Eng. 2020, 1–17 (2020). https://doi.org/10.1155/2020/7532703
Behera, P., Baheti, V., Militky, J., Naeem, S.: Microstructure and mechanical properties of carbon microfiber reinforced geopolymers at elevated temperatures. Constr. Build. Mater. 160, 733–743 (2018). https://doi.org/10.1016/j.conbuildmat.2017.11.109
Degirmenci, F.N.: Freeze-Thaw and fire resistance of geopolymer mortar based on natural and waste pozzolans. Ceram. - Silikaty 62, 41–49 (2018). https://doi.org/10.13168/cs.2017.0043
Hager, I., Sitarz, M., Mróz, K.: Fly-ash based geopolymer mortar for high-temperature application – Effect of slag addition. J. Clean. Prod. 316, 128168 (2021). https://doi.org/10.1016/j.jclepro.2021.128168
Luo, Y., Li, S.H., Klima, K.M., Brouwers, H.J.H., Yu, Q.: Degradation mechanism of hybrid fly ash/slag based geopolymers exposed to elevated temperatures. Cem. Concr. Res. 151, 106649 (2022). https://doi.org/10.1016/j.cemconres.2021.106649
Aziz, I.H., Abdullah, M.M.A.B., Heah, C.-Y., Liew, Y.-M.: Behaviour changes of ground granulated blast furnace slag geopolymers at high temperature. Adv. Cem. Res. 32, 465–475 (2020). https://doi.org/10.1680/jadcr.18.00162
Pan, Z., Tao, Z., Cao, Y.F., Wuhrer, R., Murphy, T.: Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 86, 9–18 (2018). https://doi.org/10.1016/j.cemconcomp.2017.09.011
Junru, R., Huiguo, C., Ruixi, D., Tao, S.: Behavior of combined fly ash/GBFS-based geopolymer concrete after exposed to elevated temperature. IOP Conf. Ser. Earth Environ. Sci. 267, 032056 (2019). https://doi.org/10.1088/1755-1315/267/3/032056
Shehata, N., Mohamed, O.A., Sayed, E.T., Abdelkareem, M.A., Olabi, A.G.: Geopolymer concrete as green building materials: Recent applications, sustainable development and circular economy potentials. Sci. Total. Environ. 836, 155577 (2022). https://doi.org/10.1016/j.scitotenv.2022.155577
Pilehvar, S., Szczotok, A.M., Rodríguez, J.F., Valentini, L., Lanzón, M., Pamies, R., Kjøniksen, A.-L.: Effect of freeze-thaw cycles on the mechanical behavior of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change materials. Constr. Build. Mater. 200, 94–103 (2019). https://doi.org/10.1016/j.conbuildmat.2018.12.057
Tunstall, L.E., Ley, M.T., Scherer, G.W.: Air entraining admixtures: Mechanisms, evaluations, and interactions. Cem. Concr. Res. 150, 106557 (2021). https://doi.org/10.1016/j.cemconres.2021.106557
Sun, P., Wu, H.-C.: Chemical and freeze–thaw resistance of fly ash-based inorganic mortars. Fuel 111, 740–745 (2013). https://doi.org/10.1016/j.fuel.2013.04.070
Brooks, R., Bahadory, M., Tovia, F., Rostami, H.: Properties of alkali-activated fly ash: High performance to lightweight. Int. J. Sustain. Eng. 3, 211–218 (2010). https://doi.org/10.1080/19397038.2010.487162
Özdal, M., Karakoç, M.B., Özcan, A.: Investigation of the properties of two different slag-based geopolymer concretes exposed to freeze–thaw cycles. Struct. Concr. 22, E332–E340 (2021). https://doi.org/10.1002/suco.201900441
Zhao, R., Yuan, Y., Cheng, Z., Wen, T., Li, J., Li, F., Ma, Z.J.: Freeze-thaw resistance of Class F fly ash-based geopolymer concrete. Constr. Build. Mater. 222, 474–483 (2019). https://doi.org/10.1016/j.conbuildmat.2019.06.166
Zhang, B., Yan, B., Li, Y.: Study on mechanical properties, freeze–thaw and chlorides penetration resistance of alkali activated granulated blast furnace slag-coal gangue concrete and its mechanism. Constr. Build. Mater. 366, 130218 (2023). https://doi.org/10.1016/j.conbuildmat.2022.130218
Ahn, S.W., Lee, H.S., Yang, W.H., Lee, J.W., and Jeong, Y.K.: 27Al MAS-NMR study of inorganic polymer formation at ambient temperature. Trans. Nonferrous Met. Soc. China (English Ed. 21, s182–s187. https://doi.org/10.1016/S1003-6326(11)61085-6. (2011)
Kropp, J., and Hilsdorf, H.: Performance Criteria for Concrete Durability H. Hilsdorf and J. Kropp, eds. (London: CRC Press) https://doi.org/10.1201/9781482271522. (1995)
Razak, S., Zainal, F.F., Shamsudin, S.R.: Effect of porosity and water absorption on compressive strength of fly ash based geopolymer and OPC paste. IOP Conf. Ser. Mater. Sci. Eng. 957, 012035 (2020). https://doi.org/10.1088/1757-899X/957/1/012035
Jiao, Z., Li, X., Yu, Q.: Effect of curing conditions on freeze-thaw resistance of geopolymer mortars containing various calcium resources. Constr. Build. Mater. 313, 125507 (2021). https://doi.org/10.1016/j.conbuildmat.2021.125507
Zada Farhan, K., Johari, A.M.M., Demirboğa, R.: Evaluation of properties of steel fiber reinforced GGBFS-based geopolymer composites in aggressive environments. Constr. Build. Mater. 345, 128339 (2022). https://doi.org/10.1016/j.conbuildmat.2022.128339
Snehal, K., Das, B.B.: Acid, alkali and chloride resistance of binary, ternary and quaternary blended cementitious mortar integrated with nano-silica particles. Cem. Concr. Compos. 123, 104214 (2021). https://doi.org/10.1016/j.cemconcomp.2021.104214
Okoye, F.N., Prakash, S., Singh, N.B.: Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 149, 1062–1067 (2017). https://doi.org/10.1016/j.jclepro.2017.02.176
Khan, H.A., Castel, A., Khan, M.S.H.: Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 168, 108586 (2020). https://doi.org/10.1016/j.corsci.2020.108586
Verma, M., Dev, N., Rahman, I., Nigam, M., Ahmed, M., Mallick, J.: Geopolymer concrete: A material for sustainable development in Indian construction industries. Crystals 12, 514 (2022). https://doi.org/10.3390/cryst12040514
Glasby, T., Day, J., Genrich, R., Kemp, M.: Commercial scale geopolymer concrete construction. In: The Saudi International Building and Constructions Technology Conference, Riyadh, Saudi Arabia, pp. 1–11. (2015)
Vidal, T., Castel, A., François, R.: Corrosion process and structural performance of a 17 year old reinforced concrete beam stored in chloride environment. Cem. Concr. Res. 37, 1551–1561 (2007). https://doi.org/10.1016/j.cemconres.2007.08.004
Zhang, Y., Chen, J., Xia, J.: Compressive strength and chloride resistance of slag/metakaolin-based ultra-high-performance geopolymer concrete. Materials (Basel). 16, 181 (2023). https://doi.org/10.3390/ma16010181
Arskøg, V., Ferreira, M., Gjørv, O.E.: Durability analysis and performance of concrete barges. In: CONCES’04, Oh, B.H., et al. (eds.) Concrete under Severe Conditions: Environment & Loading. Seoul, Korea (2004)
Tennakoon, C., Shayan, A., Sanjayan, J.G., Xu, A.: Chloride ingress and steel corrosion in geopolymer concrete based on long term tests. Mater. Des. 116, 287–299 (2017). https://doi.org/10.1016/j.matdes.2016.12.030
Amorim Júnior, N.S., Andrade Neto, J.S., Santana, H.A., Cilla, M.S., Ribeiro, D.V.: Durability and service life analysis of metakaolin-based geopolymer concretes with respect to chloride penetration using chloride migration test and corrosion potential. Constr. Build. Mater. 287, 122970 (2021). https://doi.org/10.1016/j.conbuildmat.2021.122970
Elyamany, H.E., Abd Elmoaty, A.E.M., Elshaboury, A.M.: Magnesium sulfate resistance of geopolymer mortar. Constr. Build. Mater. 184, 111–127 (2018). https://doi.org/10.1016/j.conbuildmat.2018.06.212
Sata, V., Sathonsaowaphak, A., Chindaprasirt, P.: Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Compos. 34, 700–708 (2012). https://doi.org/10.1016/j.cemconcomp.2012.01.010
Mehta, P.K.: Mechanism of sulfate attack on portland cement concrete — Another look. Cem. Concr. Res. 13, 401–406 (1983). https://doi.org/10.1016/0008-8846(83)90040-6
Basista, M., Weglewski, W.: Chemically assisted damage of concrete: A model of expansion under external sulfate attack. Int. J. Damage Mech 18, 155–175 (2009). https://doi.org/10.1177/1056789508097540
Bakharev, T.: Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 35, 1233–1246 (2005). https://doi.org/10.1016/j.cemconres.2004.09.002
Yang, T., Gao, X., Zhang, J., Zhuang, X., Wang, H., Zhang, Z.: Sulphate resistance of one-part geopolymer synthesized by calcium carbide residue-sodium carbonate-activation of slag. Compos. Part B Eng. 242, 110024 (2022). https://doi.org/10.1016/j.compositesb.2022.110024
Wang, W., Shen, A., He, Z., Guo, Y., Li, D.: Mechanism and erosion resistance of internally cured concrete including super absorbent polymers against coupled effects of acid rain and fatigue load. Constr. Build. Mater. 290, 123252 (2021). https://doi.org/10.1016/j.conbuildmat.2021.123252
Kumar, R., Verma, M., Dev, N.: Investigation on the effect of seawater condition, sulphate attack, acid attack, freeze–thaw condition, and wetting–drying on the geopolymer concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 46, 2823–2853 (2022). https://doi.org/10.1007/s40996-021-00767-9
Aiken, T.A., Kwasny, J., Sha, W., Soutsos, M.N.: Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 111, 23–40 (2018). https://doi.org/10.1016/j.cemconres.2018.06.011
Ahmad, M., Rashid, K., Hameed, R., Ul Haq, E., Farooq, H., Ju, M.: Physico-mechanical performance of fly ash based geopolymer brick: Influence of pressure − temperature − time. J. Build. Eng. 50, 104161 (2022). https://doi.org/10.1016/j.jobe.2022.104161
Alexander, A.E., Shashikala, A.P.: Studies on the microstructure and durability characteristics of ambient cured FA-GGBS based geopolymer mortar. Constr. Build. Mater. 347, 128538 (2022). https://doi.org/10.1016/j.conbuildmat.2022.128538
Paruthi, S., Husain, A., Alam, P., Husain Khan, A., Abul Hasan, M., Magbool, H.M.: A review on material mix proportion and strength influence parameters of geopolymer concrete: Application of ANN model for GPC strength prediction. Constr. Build. Mater. 356, 129253 (2022). https://doi.org/10.1016/j.conbuildmat.2022.129253
Tale Masoule, M.S., Bahrami, N., Karimzadeh, M., Mohasanati, B., Shoaei, P., Ameri, F., Ozbakkaloglu, T.: Lightweight geopolymer concrete: A critical review on the feasibility, mixture design, durability properties, and microstructure. Ceram. Int. 48, 10347–10371 (2022). https://doi.org/10.1016/j.ceramint.2022.01.298
Sahoo, S., Prasad Singh, S.: Strength and durability properties of expansive soil treated with geopolymer and conventional stabilizers. Constr. Build. Mater. 328, 127078 (2022). https://doi.org/10.1016/j.conbuildmat.2022.127078
Jegan, M., Annadurai, R., Kannan Rajkumar, P.R.: A state of the art on effect of alkali activator, precursor, and fibers on properties of geopolymer composites. Case Stud. Constr. Mater. 18, e01891 (2023). https://doi.org/10.1016/j.cscm.2023.e01891
P, M., and V, V.: Potential utilization of waste glass powder as a precursor material in synthesizing ecofriendly ternary blended geopolymer matrix. J. Clean. Prod. 355, 131860 (2022). https://doi.org/10.1016/j.jclepro.2022.131860
Prusty, J.K., Pradhan, B.: Investigation on effect of precursor materials and sand-to-binder ratio on strength development, acid resistance and microstructure evolution of geopolymer mortar. Constr. Build. Mater. 346, 128501 (2022). https://doi.org/10.1016/j.conbuildmat.2022.128501
Swathi, B., Vidjeapriya, R.: Influence of precursor materials and molar ratios on normal, high, and ultra-high performance geopolymer concrete – A state of art review. Constr. Build. Mater. 392, 132006 (2023). https://doi.org/10.1016/j.conbuildmat.2023.132006
Singh, R.P., Reddy, P.S., Vanapalli, K.R., and Mohanty, B.: Influence of binder materials and alkali activator on the strength and durability properties of geopolymer concrete: A review. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2023.05.226. (2023)
Ghazy, M.F., Abd Elaty, M.A., Taman, M., Eissa, M.E.: Durability performance of geopolymer ferrocement panels prepared by different alkaline activators. Structures 38, 168–183 (2022). https://doi.org/10.1016/j.istruc.2022.01.087
Raut, A.N., Murmu, A.L., Alomayri, T.: Physico-Mechanical and thermal behavior of prolong heat Cured geopolymer blocks. Constr. Build. Mater. 370, 130309 (2023). https://doi.org/10.1016/j.conbuildmat.2023.130309
Shen, Y., Kang, S., Cheng, G., Wang, J., Wu, W., Wang, X., Zhao, Y., Li, Q.: Effects of silicate modulus and alkali dosage on the performance of one-part electric furnace nickel slag-based geopolymer repair materials. Case Stud. Constr. Mater. 19, e02224 (2023). https://doi.org/10.1016/j.cscm.2023.e02224
Ekinci, E.: The effects of different production parameters (NaOH concentration, curing regime and replacement ratio of recycled aggregate) on fresh, hardened and elevated temperature performance of geopolymer mortar samples. J. Build. Eng. 76, 107052 (2023). https://doi.org/10.1016/j.jobe.2023.107052
Wang, A., Zheng, Y., Zhang, Z., Liu, K., Li, Y., Shi, L., Sun, D.: The durability of alkali-activated materials in comparison with ordinary portland cements and concretes: A review. Engineering 6, 695–706 (2020). https://doi.org/10.1016/j.eng.2019.08.019
Li, W., Tang, Z., Tam, V.W.Y., Zhao, X., Wang, K.: A review on durability of alkali-activated system from sustainable construction materials to engineering infrastructures. ES Mater. Manuf. 4, 2–19 (2019). https://doi.org/10.30919/esmm5f204
Tchadjie, L.N., Ekolu, S.O.: Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J. Mater. Sci. 53, 4709–4733 (2018). https://doi.org/10.1007/s10853-017-1907-7
Reddy, M.S., Dinakar, P., Rao, B.H.: A review of the influence of source material’s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater. 234, 12–23 (2016). https://doi.org/10.1016/j.micromeso.2016.07.005
Silva, P.D., Sagoe-Crenstil, K., Sirivivatnanon, V.: Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 37, 512–518 (2007). https://doi.org/10.1016/j.cemconres.2007.01.003
Chindaprasirt, P., De Silva, P., Sagoe-Crentsil, K., Hanjitsuwan, S.: Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 47, 4876–4883 (2012). https://doi.org/10.1007/s10853-012-6353-y
Sugama, T., Brothers, L.E., Van de Putte, T.R.: Acid-resistant cements for geothermal wells: Sodium silicate activated slag/fly ash blends. Adv. Cem. Res. 17, 65–75 (2005). https://doi.org/10.1680/adcr.2005.17.2.65
van Deventer, J.S.J., Nicolas, R.S., Ismail, I., Bernal, S.A., Brice, D.G., Provis, J.L.: Microstructure and durability of alkali-activated materials as key parameters for standardization. J. Sustain. Cem. Mater. 4, 116–128 (2014). https://doi.org/10.1080/21650373.2014.979265
Joseph, B., Mathew, G.: Influence of aggregate content on the behavior of fly ash based geopolymer concrete. Sci. Iran. 19, 1188–1194 (2012). https://doi.org/10.1016/j.scient.2012.07.006
Temuujin, J., Van Riessen, A., MacKenzie, K.J.D.: Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 24, 1906–1910 (2010). https://doi.org/10.1016/j.conbuildmat.2010.04.012
Živica, V., Balkovic, S., Drabik, M.: Properties of metakaolin geopolymer hardened paste prepared by high-pressure compaction. Constr. Build. Mater. 25, 2206–2213 (2011). https://doi.org/10.1016/j.conbuildmat.2010.11.004
Ranjbar, N., Kashefi, A., Maheri, M.R.: Hot-pressed geopolymer: Dual effects of heat and curing time. Cem. Concr. Compos. 86, 1–8 (2018). https://doi.org/10.1016/j.cemconcomp.2017.11.004
Ranjbar, N., Mehrali, M., Maheri, M.R., Mehrali, M.: Hot-pressed geopolymer. Cem. Concr. Res. 100, 14–22 (2017). https://doi.org/10.1016/j.cemconres.2017.05.010
Ranjbar, N., Kashefi, A., Ye, G., Mehrali, M.: Effects of heat and pressure on hot-pressed geopolymer. Constr. Build. Mater. 231, 117106 (2020). https://doi.org/10.1016/j.conbuildmat.2019.117106
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gökçe, H.S. Durability of slag-based alkali-activated materials: A critical review. J Aust Ceram Soc 60, 885–903 (2024). https://doi.org/10.1007/s41779-024-01011-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-024-01011-z