Abstract
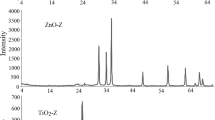
In this work, the photocatalytic degradation of amoxicillin (AMX), tetracycline(TCH), and diclofenac sodium(DCF) was studied using TiO2 and Sn/Zn/Fe-doped TiO2 as photocatalyst under ultraviolet (UV) and visible light. Photocatalysts were synthesized by sol–gel method and characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), and Fourier transform infrared spectroscopy (FTIR). Box–Behnken design (BBD) was used to achieve maximum %degradation by optimizing different parameters like the feed concentration (50–100 mg/L), feed pH (3–11), and the catalyst dosing (0.5–1.5 g/l). The study revealed that the Zn-doped TiO2 photocatalyst (band gap of 3.23 eV) was the most effective which showed 90–95% degradation of all compounds within 90 min under UV radiation. Fe-doped TiO2 (2.1 eV) and Sn-doped TiO2(2.92 eV) showed the best results in the presence of visible light as it needs lower energy. To achieve maximum degradation efficiency under UV radiation, H2O2 (550 mL/L) was used along with Zn-doped photocatalyst under acidic conditions (at pH 3) for AMX, DCF, and basic conditions (at pH 11) for TCH. COD analysis was carried out before and after the experiment. COD removal efficiencies were found to be between 70–80% and liquid chromatography–mass spectrometry (LC–MS) analysis was performed to identify intermediate compounds formed during degradation.
Article Highlights
-
Photocatalytic degradation of amoxicillin (AMX), tetracycline(TCH), and diclofenac sodium(DCF)
-
TiO2 and Sn/Zn/Fe-doped TiO2 as photocatalyst under ultraviolet (UV) and visible light.
-
Optimization of different process parameters
-
Maximum degradation efficiency using Zn-doped TiO2/UV/H2O2
-
COD and liquid chromatography–mass spectrometry (LC–MS) analysis to identify intermediate compounds formed during degradation.





Similar content being viewed by others
References
Aba-Guevara CG, Medina-Ramírez IE, Hernandez-Ramirez A, Jauregui-Rincon J, Lozano-Alvarez JA, Rodriguez-Lopez JL (2017) Comparison of two synthesis methods on the preparation of Fe, N-Co-doped TiO2 materials for degradation of pharmaceutical compounds under visible light. Ceram Int 43:5068–5079. https://doi.org/10.1016/j.ceramint.2017.01.018
Achilleos A, Hapeshi E, Xekoukoulotakis NP, Mantzavinos D, Fatta-Kassinos D (2010) Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem Eng J 161:53–59. https://doi.org/10.1016/j.cej.2010.04.020
Ahmadpour N, Sayadi MH, Sobhani S, Hajiani M (2020) Photocatalytic degradation of model pharmaceutical pollutant by novel magnetic TiO2@ZnFe2O4/Pd nanocomposite with enhanced photocatalytic activity and stability under solar light irradiation. J Environ Manage 271:110964. https://doi.org/10.1016/j.jenvman.2020.110964
Alshandoudi LM, Al Subhi AY, Al-Isaee SA, Shaltout WA, Hassan AF (2023) Static adsorption and photocatalytic degradation of amoxicillin using titanium dioxide/hydroxyapatite nanoparticles based on sea scallop shells. Environ Sci Pollut Res 30:88704–88723. https://doi.org/10.1007/s11356-023-28530-9
Ambrosetti B, Campanella L, Palmisano R (2015) Degradation of antibiotics in aqueous solution by photocatalytic process: comparing the efficiency in the use of ZnO or TiO2. J Environ Sci Eng A 4:273–281. https://doi.org/10.17265/2162-5298/2015.06.001
Ansarizadeh M, Tabatabaei T, Samaei MR, Leili M, Baneshi MM (2021) Application of catalyst / UV / PU nanocomposite for removal of Tetracycline: response surface methodology for optimization and kinetic study. J Health Sci Surveillance Sys 9:50–59. https://doi.org/10.30476/jhsss.2020.88591.1148
Benitez FJ, Acero JL, Real FJ, Roldan G, Casas F (2011) Comparison of different chemical oxidation treatments for the removal of selected pharmaceuticals in water matrices. Chem Eng J 168:1149–1156. https://doi.org/10.1016/j.cej.2011.02.001
Bjelajac A, Petrović R, Vujancevic J, Veltruska K, Matolin V, Siketic Z, Provatas G, Jaksic M, Stan GE, Socol G, Mihailescu IN, Janackovic D (2020) Sn-doped TiO2 nanotubular thin film for photocatalytic degradation of methyl orange dye. J Phys Chem Solids 147:109609. https://doi.org/10.1016/j.jpcs.2020.109609
Calza P, Sakkas VA, Medana C, Baiocchi C, Dimou A, Pelizzetti E, Albanis T (2006) Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl Catal B 67:197–205. https://doi.org/10.1016/j.apcatb.2006.04.021
Chen L, Zhang W, Wang J, Li X, Li Y, Hu X, Zhao L, Wu Y, He Y (2023) High piezo/photocatalytic efficiency of Ag/Bi5O7I nanocomposite using mechanical and solar energy for N2 fixation and methyl orange degradation. Green Energy Environ 8:283–295. https://doi.org/10.1016/j.gee.2021.04.009
Choi H, Al-Abed SR, Dionysiou DD, Stathatos E, Lianos P (2010) TiO2-based advanced oxidation nanotechnologies for water purification and reuse. Sustain Sci Eng 2:229–254. https://doi.org/10.1016/S1871-2711(09)00208-6
Czech B, Buda W (2016) Multicomponent nanocomposites for elimination of diclofenac in water based on an amorphous TiO2 active in various light sources. J Photochem Photobiol A 330:64–70. https://doi.org/10.1016/j.jphotochem.2016.07.024
De Bruin B, Boerakkar MJ, Brands JA, Donners JJJM, Donners MPJ, De Gelder R, Smits JMM, Gal AW, Spek AL (1999) Selective oxidation of [Rhl(cod)]+ by H2O2 and O2. Chem Eur J 5:2921–2936. https://doi.org/10.1002/(SICI)1521-3765(19991001)5:10%3c2921::AID-CHEM2921%3e3.0.CO;2-1
Dhaou M, Elaloui E, Khirouni K, Guermazi H, Guermazi S (2021) Enhancement of dielectric responses and conduction properties of Zn-doped TiO2 for energy storage and photosensitivity applications. J Mater Sci Mater Electron 32:13187–13204. https://doi.org/10.1007/s10854-021-05858-x
Dimitrakopoulou D, Rethemiotaki I, Frontistis Z, Xekoukoulotakis NP, Venieri D, Mantzavinos D (2012) Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J Environ Manage 98:168–174. https://doi.org/10.1016/j.jenvman.2012.01.010
Dixit A, Tirpude AJ, Mungray AK, Chakraborty M (2011) Degradation of 2,4 DCP by sequential biological-advanced oxidation process using UASB and UV/TiO2/H2O2. Desalination 272:265–269. https://doi.org/10.1016/j.desal.2011.01.035
Dutt VGV, Dubey RS (2017) Study of structural and optical properties of Zinc-doped Titanium Dioxide nanoparticles. Mech Mater Sci Eng J 9. https://hal.science/hal-01499553
Ebrahimi R, Maleki A, Rezaee R, Daraei H, Safari M, Mckay G, Lee S-M, Jafari A (2021) Synthesis and Application of Fe-Doped TiO2 nanoparticles for photodegradation of 2,4-D from aqueous solution. Arab J Sci Eng 46:6409–6422. https://doi.org/10.1007/s13369-020-05071-8
Elmolla ES, Chaudhuri M (2010) Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2 /TiO2 photocatalysis. Desalination 252:46–52. https://doi.org/10.1016/j.desal.2009.11.003
Fawzy A, Mahanna H, Mossad M (2022) Effective photocatalytic degradation of amoxicillin using MIL-53(Al)/ZnO composite. Environ Sci Pollut Res 29:68532–68546. https://doi.org/10.1007/s11356-022-20527-0
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C 1:1–21. https://doi.org/10.1016/S1389-5567(00)00002-2
Galloni MG, Cerrato G, Giordana A, Falletta E, Cl B (2022) Sustainable solar light photodegradation of diclofenac by nano- and micro- sized SrTiO3. Catalysts 12:804. https://doi.org/10.3390/catal12080804
Giahi M (2015) Photocatalytic degradation of diclofenac sodium in aqueous solution using N, S, and C-doped ZnO. Russ J Appl Chem 88:2044–2049. https://doi.org/10.1134/S10704272150120228
Gil A, García AM, Fernández M, Vicente MA, Gonzalez-Rodriguez B, Rives V, Korili SA (2017) Effect of dopants on the structure of titanium oxide used as a photocatalyst for the removal of emergent contaminants. J Ind Eng Chem 53:183–191. https://doi.org/10.1016/j.jiec.2017.04.024
Glaspell G, Manivannan A (2005) Sol-gel synthesis and magnetic studies of titanium dioxide doped with 10% M (M = Fe, Mn and Ni). J Cluster Sci 16:501–513. https://doi.org/10.1007/s10876-005-0023-z
Gómez-Pacheco CV, Sánchez-Polo M, Rivera-Utrilla L-P (2012) Tetracycline degradation in aqueous phase by ultraviolet radiation. Chem Eng J 187:89–95. https://doi.org/10.1016/j.cej.2012.01.096
Gou J, Ma Q, Cui Y, Deng X, Zhang H, Cheng X, Li X, Xie M, Cheng Q, Liu H (2017) Visible light photocatalytic removal perfromance and mechanism of diclofenac degradation by Ag3PO4 sub microcrystals through response methodology. J Ind Eng Chem 49:112–121. https://doi.org/10.1016/j.jiec.2017.01.015
Guo W, Wu Q-L, Zhou X-J, Cao H-O, Du J-S, Yin R-L, Ren N-Q (2015) Enhanced amoxicillin treatment using the electroperoxone process: key factors and degradation mechanism. RSC Adv 5:52695–52702. https://doi.org/10.1039/c5ra07951a
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469. https://doi.org/10.1016/j.jcis.2006.12.010
Heris SZ, Etemadi M, Mousavi SB, Mohammadpourfard M, Ramavandi B (2023) Preparation and characterizations of TiO2/ZnO nanohybrid and its application in photocatalytic degradation of tetracycline in wastewater. J Photochem Photobiol A 443:114893. https://doi.org/10.1016/j.jphotochem.2023.114893
Irandost M, Akbarzadeh R, Pirsaheb M, Asadi A, Mohammadi P, Sillanpaa M (2019) Fabrication of highly visible active N, S co-doped TiO2@MoS2 heterojunction with synergistic effect for photocatalytic degradation of diclofenac: Mechanisms, modeling and degradation pathway. J Mol Liq 291:111342. https://doi.org/10.1016/j.molliq.2019.111342
Jankunaite D, Tichonovas M, Buivydiene D, Radziuniene I, Racys V, Krugly E (2017) Removal of Diclofenac, Ketoprofen, and Carbamazepine from simulated drinking water by Advanced Oxidation in a model reactor. Water Air Soil Pollut 228:1–15. https://doi.org/10.1007/s11270-017-3517-z
Jiang Y, Xing C, Chen Y, Shi J, Wang S (2022) Preparation of BiFeO3 and photodegradation of tetracycline pollutant in the UV-heterogeneous Fenton-like system. Environ Sci Pollut Res 29:57656–57668. https://doi.org/10.1007/s11356-022-19806-7
John D, Rajalakshmi AS, Lopez RM, Achari VS (2020) TiO2-reduced graphene oxide nanocomposites for the trace removal of diclofenac. SN Appl Sci 2:1–16. https://doi.org/10.1007/s42452-020-2662-y
Kalash KR, Al-Furaiji MH (2020) Advanced Oxidation of Antibiotics Polluted Water Using Titanium Dioxide in Solar Photocatalysis Reactor. J. Eng. 26:1–13. https://doi.org/10.31026/j.eng.2020.02.01
Kanakaraju D, Glass BD, Oelgemoller M (2014) Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ Chem Lett 12:27–47. https://doi.org/10.1007/s10311-013-0428-0
Khan WZ, Najeeb I, Ishtiaque S, Jabeen S (2016) Photocatalytic degradation of a Real textile Wastewater using Titanium Dioxide, Zinc Oxide, and Hydrogen Peroxide during UV treatment. IOSR Journal of Engineering 6:36–46. https://doi.org/10.9790/3021-067013646
Lara-Pérez C, Leyva E, Zermeño B, Osorio I, Montalvo C, Moctezuma E (2020) Photocatalytic degradation of diclofenac sodium salt: adsorption and reaction kinetic studies. Environ Earth Sci 79:1–13. https://doi.org/10.1007/s12665-020-09017-z
Li S, Cai M, Liu Y, Wang C, Lv K, Chen X (2022) S-Scheme photocatalyst TaON/BisWO6 nanofibers with oxygen vacancies for efficient abatement of antibiotics and Cr (VI): intermediate eco-toxicity analysis and mechanistic insights. Chin J Catal 43:2652–2664. https://doi.org/10.1016/S1872-2067(22)64106-8
Li S, Wang Z, Xie X, Liang G, Cai X, Zhang X, Wang Z (2020) Fabrication of vessel-like biochar-based heterojunction catalyst Bi2S3/BiOBr/BC for diclofenac removal under visible light irradiation: Mechanisitc investigation and intermediates analysis. J Hazard Mater 391:121407. https://doi.org/10.1016/j.jhazmat.2019.121407
Lin X, Xie F, Yu X, Tang X, Guan H, Chen Y, Feng W (2019) Ultraviolet Light Assisted Hierarchical Porous Fe2O3 Catalyzing Heterogeneous Fenton Degradation of Tetracycline Under Neutral Condition with a Low Requirement of H2O2. Chem Res Chin Univ 35:304–310. https://doi.org/10.1007/s40242-019-8238-y
Loan TT, Huong VH, Tham VT, Long NN (2018) Effect of zinc doping on the bandgap and photoluminescence of Zn2+-doped TiO2 nanowires. Physica B 532:210–215. https://doi.org/10.1016/j.physb.2017.05.027
Madhavan J, Kumar PSS, Anandan S, Zhou M, Grieser F, Ashokkumar M (2010) Ultrasound assisted photocatalytic degradation of diclofenac in an aqueous environment. Chemosphere 80:747–752. https://doi.org/10.1016/j.chemosphere.2010.05.018
Malefane ME, Feleni U, Kuvarega AT (2020) Cobalt (II/III) oxide and tungsten (VI) oxide p-n heterojunction photocatalyst for photodegradation of diclofenac sodium under visible light. J Environ Chem Eng 8:103560. https://doi.org/10.1016/j.jece.2019.103560
Marami MB, Farahmandjou M, Khoshnevisan B (2018) Sol-Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J Electron Mater 47:3741–3748. https://doi.org/10.1007/s11664-018-6234-5
Michael I, Achilleos A, Lambropoulou D, Torrens VO, Perez S, Petrovic M, Barcelo D, Fatta-Kassinos D (2014) Proposed transformation pathway and evolution profile of diclofenac and ibuprofen transformation products during (sono)photocatalysis. Appl Catal B 147:1015–1027. https://doi.org/10.1016/j.apcatb.2013.10.035
Mohammadi R, Massoumi B, Eskandarloo H (2013) Preparation and characterization of Sn / Zn / TiO2 photocatalyst for enhanced amoxicillin trihydrate degradation. Desalin Water Treat 53:1995–2004. https://doi.org/10.1080/19443994.2013.862867
Mohammadi R, Massoumi B, Rabani M (2012) Photocatalytic decomposition of amoxicillin trihydrate antibiotic in aqueous solutions under UV irradiation using Sn/TiO2 Nanoparticles. Int J Photoenergy. https://doi.org/10.1155/2012/514856
Moradi H, Eshaghi A, Hosseini SR, Ghani K (2016) Fabrication of Fe-doped TiO2 nanoparticles and investigation of photocatalytic decolorization of reactive red 198 under visible light irradiation. Ultrason Sonochem 32:314–319. https://doi.org/10.1016/j.ultsonch.2016.03.025
Mozia S, Darowna D, Przepiórski J, Morawski AW (2013) Evaluation of performance of hybrid photolysis-DCMD and photocatalysis-DCMD systems utilizing UV-C radiation for removal of diclofenac sodium salt from water. Pol J Chem Technol 15:51–60. https://doi.org/10.2478/pjct-2013-0010
Mragui AE, Logvina Y, Da Silva LP, Zegaoui O, Da Silva JCGE (2019) Synthesis of Fe-and Co-doped TiO2 with improved photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 12:3874. https://doi.org/10.3390/ma12233874
Muelas-Ramous V, Sampaio MJ, Silva CG, Bedia J, Rodriguez JJ, Faria JL, Belver C (2021) Degradation of diclofenac in water under LED irradiation using combined g-C3N4/NH2-MIL-125 photocatalysts. J Hazard Mater 416:126199. https://doi.org/10.1016/j.jhazmat.2021.126199
Olama N, Dehghani M, Malakootian M (2018) The removal of amoxicillin from aquatic solutions using the TiO2/UV-C nanophotocatalytic method doped with trivalent iron. Appl Water Sci 8:1–12. https://doi.org/10.1007/s13201-018-0733-7
Papamichail P, Nannou C, Giannakoudakis DA, Bikiaris ND, Papoulia C, Pavlidou E, Lambropoulou D, Samanidou V, Deliyanni E (2023) Maximization of the photocatalytic degradation of diclofenac using polymeric g-C3N4 by tuning the precursor and the synthetic protocol. Catal Today 418:114075. https://doi.org/10.1016/j.cattod.2023.114075
Park Y, Kim S, Kim J, Khan S, Han C (2022) UV/TiO2 Photocatalysis as an efficient livestock wastewater quaternary treatment for antibiotics removal. Water (switzerland) 14:958. https://doi.org/10.3390/w14060958
Poulopoulos SG, Ulykbanova G, Philippopoulos CJ (2021) Photochemical mineralization of amoxicillin medicinal product by means of UV, hydrogen peroxide, titanium dioxide and iron. Environ Technol (united Kingdom) 42:2941–2949. https://doi.org/10.1080/09593330.2020.1720300
Radosavljević KD, Golubović AV, Radišić MM, Mladenovic AR, Mijin DZ, Petrovic SD (2017) Amoxicillin photodegradation by nanocrystalline TiO2. Chem Ind Chem Eng Q 23:187–195. https://doi.org/10.2298/CICEQ160122030R
Rajeswari R, Venugopal D, George A, Raj AD, Sundaram SJ, Bashir AKH, Maaza M, Kaviyarasu K (2021) Synthesis and characterization of Sn-doped TiO2 film for antibacterial applications. Appl Phys a: Mater Sci Proc 127:498. https://doi.org/10.1007/s00339-021-04656-w
Safari GH, Hoseini M, Seyedsalehi M, Kamani H, Jaafari J, Mahvi AH (2015) Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int J Environ Sci Technol 12:603–616. https://doi.org/10.1007/s13762-014-0706-9
Salaeh S, Juretic Perisic D, Biosic M, Kusic H, Babic S, Stangar UL, Dionysiou DD, Bozic AL (2016) Diclofenac removal by simulated solar assisted photocatalysis using TiO2-based zeolite catalyst; mechanisms, pathways and environmental aspects. Chem Eng J 304:289–302. https://doi.org/10.1016/j.cej.2016.06.083
San N, Hatipǒglu A, Koçtürk G, Cinar Z (2002) Photocatalytic degradation of 4-nitrophenol in aqueous TiO2 suspensions: theoretical prediction of the intermediates. J Photochem Photobiol A 146:189–197. https://doi.org/10.1016/S1010-6030(01)00620-7
Santhi K, Harish S, Navaneethan M, Ponnusamy S, Harish T (2021) Investigation of metal doped mesoporous TiO2 nanostructures for environmental remediation. Res Square 1–25. https://doi.org/10.21203/rs.3.rs-261249/v1
Shaykhi ZM, Zinatizadeh AAL (2014) Statistical modeling of photocatalytic degradation of synthetic amoxicillin wastewater (SAW) in an immobilized TiO2 photocatalytic reactor using response surface methodology (RSM). J Taiwan Inst Chemi Eng 45:1717–1726. https://doi.org/10.1016/j.jtice.2013.12.024
Shi H, Wu Q, Jiang L, Wang L, Huang M, Han B, Yu Z, Zuo Y (2020) Synthesis of SnO2 hollow microspheres with efficient photocatalytic activity for tetracycline hydrochloride. Int J Electrochem Sci 15:1539–1547. https://doi.org/10.20964/2020.02.41
Taherkhani S, Darvishmotevalli M, Karimyan K, Bina B, Fallahi A, Karimi H (2018) Dataset on photodegradation of tetracycline antibiotic with zinc stannate nanoflower in aqueous solution—application of response surface methodology. Data Brief 19:1997–2007. https://doi.org/10.1016/j.dib.2018.06.030
Thiruppathi M, Senthil Kumar P, Devendran P, Ramalingan C, Swaminathan M, Nagarajan ER (2018) Ce@TiO2 nanocomposites: An efficient, stable and affordable photocatalyst for the photodegradation of diclofenac sodium. J Alloys Compd 735:728–734. https://doi.org/10.1016/j.jallcom.2017.11.139
Wang H, Yao H, Pei J, Liu F, Li D (2016) Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalin Water Treat 57:19981–19987. https://doi.org/10.1080/19443994.2015.1103309
Wang T, Zhong S, Zou S, Jiang F, Feng L, Su X (2017) Novel Bi2WO6 coupled Fe3O4 Magnetic Photocatalysts: Preparation, Characterization and Photodegradation of Tetracycline Hydrochloride. Photochem Photobiol 93:1034–1042. https://doi.org/10.1111/php.12739
Wang K, Li B, Zhao C, Yuan S, Zhang C, Liang X, Wang J, Wu Y, He Y (2022) A novel NiO/BaTiO3 heterojunction for piezocatalytic water purification under ultrasonic vibration. Ultrason sonochem 92:106285. https://doi.org/10.1016/j.ultsonch.2022.106285
Yang W, Cicek N (2008) Treatment of swine wastewater by submerged membrane bioreactors with consideration of estrogenic activity removal. Desalination 231:200–208. https://doi.org/10.1016/j.desal.2007.11.047
Yuan F, Hu C, Hu X, Wei D, Chen Y, Qu J (2011) Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J Hazard Mater 185:1256–1263. https://doi.org/10.1016/j.jhazmat.2010.10.040
Yuan S, Wang J, Zhao C, Yue L, Ren X, Zeng Z, Hu X, Wu Y, He Y (2023) S-scheme Bi2O3/CdMoO4 hybrid with highly efficient charge separation for photocatalytic N2 fixation and tetracycline degradation: fabrication, catalytic Optimization, physicochemical studies. Sep Purif Technol 325:124665. https://doi.org/10.1016/j.seppur.2023.124665
Zanjanchi MA, Ebrahimian A, Arvand M (2010) Sulphonated cobalt phthalocyanine-MCM-41: An active photocatalyst for degradation of 2,4-dichlorophenol. J Hazard Mater 175:992–1000. https://doi.org/10.1016/j.jhazmat.2009.10.108
Zhang Y, Xiao Y, Zhong Y, Thye-Lim T (2019) Comparison of amoxicillin photodegradation in the UV / H2O2 and UV / persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity. Chem Eng J 372:420–428. https://doi.org/10.1016/j.cej.2019.04.160
Zhao C, Deng H, Li Y, Liu Z (2010) Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J Hazard Mater 176:884–892. https://doi.org/10.1016/j.jhazmat.2009.11.119
Zhao C, Cai L, Wang K, Li B, Yuan S, Zeng Z, Zhao L, Wu Y, He Y (2023) Novel Bi2WO6/ZnSnO3 heterojunction for the ultrasonic-vibration-driven piezocatalytic degradation of RhB. Environ Pollut 319:120982. https://doi.org/10.1016/j.envpol.2022.120982
Zhou M, Yu J, Cheng B (2006) Effects of Fe-doping on the photocatalytic activity of mesoporous TiO2 powders prepared by an ultrasonic method. J Hazard Mater 137:1838–1847. https://doi.org/10.1016/j.jhazmat.2006.05.028
Zhu X-D, Wang Y-J, Sun R-J, Zhou D-M (2013) Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 92:925–932. https://doi.org/10.1016/j.chemosphere.2013.02.066
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nema, S., Sharma, A., Rathore, V.K. et al. Removal of Pharmaceuticals from Aqueous Solutions by Photodegradation Using TiO2 and Sn/Zn/Fe-Doped TiO2 as Photocatalyst Under Ultraviolet and Visible Light. Int J Environ Res 18, 12 (2024). https://doi.org/10.1007/s41742-024-00565-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-024-00565-x