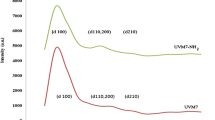
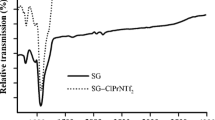
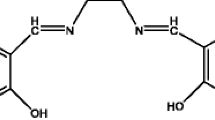
Abstract
In this study, a novel method for determination of Pd(II) ions at trace level was developed using on-line preconcentration onto polyamine group bonded silica (PA-SG) with flame atomic absorption spectrometric determination. Pd(II) uptake dynamics of PA-SG was studied batchwise by investigating acidity and chloride concentration of aqueous phase, contact time and initial concentration of Pd(II) ions. The Pd(II) adsorption capacity of PA-SG was found to be 158.7 mg g−1 from aqueous phase containing 0.1 M HCl. The on-line preconcentration procedure of Pd(II) was optimized with main analytical parameters including sample and eluent flow rate, eluent type and volume and matrix ions. The optimum eluent type and flow rate of sample and eluent were found to be 1.0% thiourea in 1.0 mol L−1 HCl and 7.5 mL min−1, respectively. The preconcentration factor and sampling frequency were calculated to be 23.9 and 20 h−1, respectively. The calibration graph was linear over the range 10–200 µg L−1. The limits of detection (3σ) and quantification (10σ) values were computed to be 3 µg L−1 and 10 µg L−1, respectively. The RSD, % was found to be 4.6% for five measurement of 25 µg L−1 of Pd(II) ions. The accuracy of the developed method was successfully checked by determination of Pd(II) level of certified reference material platinum ore (SARM 7B). The proposed method was successfully applied for Pd(II) determination in various environmental water samples such as river, lake, sea and tap water, and spent auto catalyst.







Similar content being viewed by others
References
Anthemidis AN, Themelis DG, Stratis JA (2001) Stopped-flow injection liquid-liquid extraction spectrophotometric determination of palladium in airborne particulate matter and automobile catalysts. Talanta 54:37–43. doi:10.1016/S0039-9140(00)00620-2
Antonio F, Cajamarca S, Zanetti M, Caroline M, Douglas P, Dragunski C, Rocker C (2016) Investigation on the performance of chemically modified aquatic macrophytes-salvinia molesta for the micro-solid phase preconcentration of Cd (II) on-line coupled to FAAS. Bull Environ Contam Toxicol 97:863–869. doi:10.1007/s00128-016-1923-3
Avino P, Santoro E, Sarto F, Violante V, Rosada A (2011) Neutron activation analysis for investigating purity grade of copper, nickel and palladium thin films used in cold fusion experiments. J Radıoanal Nucl Chem 290:427–436. doi:10.1007/s10967-011-1296-3
Booth SG, Chang SY, Uehara A, La Fontaine C, Cibin G, Schroeder SLM, Dryfe RAW (2017) In situ XAFS Study of palladium electrodeposition at the liquid/liquid interface. Electrochim Acta 235:251–261. doi:10.1016/j.electacta.2017.03.059
Bosch Ojeda C, Sánchez Rojas F, Manuel Cano Pavón J (2007) On-line preconcentration of palladium(II) using a microcolumn packed with a chelating resin, and its subsequent determination by graphite furnace atomic absorption spectrometry. Microchim Acta 158:103–110. doi:10.1007/s00604-006-0700-0
Bruzzoniti MC, Mucchino C, Tarasco E, Sarzanini C (2003) On-line preconcentration, ion chromatographic separation and spectrophotometric determination of palladium at trace level. J Chromatogr A 1007:93–100. doi:10.1016/S0021-9673(03)00931-2
Fan Z, Shen J, Li R, Li S (2012) Synthesis and adsorption behavior of surface Cu(II) Ion-imprinted Poly(allylamine)-Silica gel material. Polym-Plast Technol 51:1289–1295. doi:10.1080/03602559.2012.700543
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem 57:385–470
Fujiwara K, Ramesh A, Maki T, Hasegawa H, Ueda K (2007) Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto L-lysine modified crosslinked chitosan resin. J Hazard Mater 146:39–50. doi:10.1016/j.jhazmat.2006.11.049
Ghaedi M, Montazerozohori M, Nazari E, Nejabat R (2013) Functionalization of multiwalled carbon nanotubes for the solid-phase extraction of silver, cadmium, palladium, zinc, manganese and copper by flame atomic absorption spectrometry. Hum Exp Toxıcol 32:687–697. doi:10.1177/0960327112467039
Ghasemi J, Asadpour S (2007) Thermodynamics’ study of the adsorption process of methylene blue on activated carbon at different ionic strengths. J Chem Thermodyn 39:967–971. doi:10.1016/j.jct.2006.10.018
Godlewska-Żyłkiewicz B, Leśniewska B, Wilczewska AZ (2012) Evaluation of ion imprinted polymers for the solid phase extraction and electrothermal atomic absorption spectrometric determination of palladium in environmental samples. Int J Envıron Anal Chem 7319:1–16. doi:10.1080/03067319.2012.656096
Golshaei R, Shemirani F, Davudabadi Farahani M (2015) Combination of solid phase extraction based on nano alumina and liquid-liquid extraction for selective determination of palladium in food samples. J Anal Chem 70:310–315. doi:10.1134/S1061934815030089
Gurung M, Babu B, Morisada S, Kawakita H, Ohto K (2013) N-aminoguanidine modified persimmon tannin: a new sustainable material for selective adsorption, preconcentration and recovery of precious metals from acidic chloride solution. Bioresource Technol 129:108–117. doi:10.1016/j.biortech.2012.11.012
Hassanien MM (2009) FAAS determination of palladium after its selective recovery by silica modified with hydrazone derivative. Microchim Acta 167:81–89. doi:10.1007/s00604-009-0219-2
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. J Environ Sci Health Part B 76:183–191. doi:10.1205/095758298529326
Iglesias M, Anticó E, Salvadó V (2003) On-line determination of trace levels of palladium by flame atomic absorption spectrometry. Talanta 59:651–657. doi:10.1016/S0039-9140(02)00616-1
Imamoglu M, Aydin AO, Dundar MS (2005) Determination of gold, palladium and copper by flame atomic absorption spectrometry after preconcentration on silica gel modified with 3-(2-aminoethylamino) propyl group. Cent Eur J Chem 3:252–262. doi:10.2478/BF02475994
Jamali MR, Assadi Y, Shemirani F, Salavati-Niasari M (2007) Application of thiophene-2-carbaldehyde-modified mesoporous silica as a new sorbent for separation and preconcentration of palladium prior to inductively coupled plasma atomic emission spectrometric determination. Talanta 71:1524–1529. doi:10.1016/j.talanta.2006.07.034
Jia X, Gong D, Wang J, Huang F, Duan T, Zhang X (2016) Arsenic speciation in environmental waters by a new specific phosphine modified polymer microsphere preconcentration and HPLC–ICP-MS determination. Talanta 160:437–443. doi:10.1016/j.talanta.2016.07.050
Karaçetin G, Sivrikaya S, Imamoglu M (2014) Adsorption of methylene blue from aqueous solutions by activated carbon prepared from hazelnut husk using zinc chloride. J Anal Appl Pyrol 110:270–276. doi:10.1016/j.jaap.2014.09.006
Karadaş C, Kara D (2013) On-line preconcentration and determination of trace elements in waters and reference cereal materials by flow injection–FAAS using newly synthesized 8-hydroxy-2-quinoline carboxaldehyde functionalized Amberlite XAD-4. J Food Compos Anal 32:90–98. doi:10.1016/j.jfca.2013.07.003
Karadaş C, Turhan O, Kara D (2013) Synthesis and application of a new functionalized resin for use in an on-line, solid phase extraction system for the determination of trace elements in waters and reference cereal materials by flame atomic absorption spectrometry. Food Chem 141:655–661. doi:10.1016/j.foodchem.2013.03.042
Kondo K, Kanazawa Y, Matsumoto M (2015) Adsorption of noble metals using silica gel modified with surfactant molecular assembly containing an extractant. Separat Sci Technol 50:1453–1460. doi:10.1080/01496395.2014.976877
Kovalev IA, Bogacheva LV, Tsysin GI, Formanovsky AA, Zolotov YA (2000) FIA-FAAS system including on-line solid phase extraction for the determination of palladium, platinum and rhodium in alloys and ores. Talanta 52:39–50. doi:10.1016/S0039-9140(00)00314-3
Krishna MVB, Ranjit M, Chandrasekaran K, Venkateswarlu G, Karunasagar D (2009) On-line preconcentration and recovery of palladium from waters using polyaniline (PANI) loaded in mini-column and determination by ICP-MS; elimination of spectral interferences. Talanta 79:1454–1463. doi:10.1016/j.talanta.2009.06.008
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K Sven Vetensk Handl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Leśniewska BA, Godlewska I, Godlewska—Żyłkiewicz B (2005) The study of applicability of dithiocarbamate-coated fullerene C60 for preconcentration of palladium for graphite furnace atomic absorption spectrometric determination in environmental samples. Spectrochim Acta B 60:377–384. doi:10.1016/j.sab.2004.11.013
Limbeck A, Rudolph E, Hann S, Koellensperger G, Stingeder G, Rendl J (2004) Flow injection on-line pre-concentration of platinum coupled with electrothermal atomic absorption spectrometry. J Anal Atom Spectrom 19:1474. doi:10.1039/B407546N
Liu P, Pu Q, Su Z (2000) Synthesis of silica gel immobilized thiourea and its application to the on-line preconcentration and separation of silver, gold and palladium. Analyst 125:147–150. doi:10.1039/A906074J
Liu L, Liu S, Zhang Q, Li C, Bao C, Liu X, Xiao P (2013) Adsorption of Au(III), Pd(II), and Pt(IV) from aqueous solution onto graphene oxide. J Chem Eng Data 58:209–216. doi:10.1021/je300551c
Messerschmidt J, von Bohlen A, Alt F, Klockenkamper R (2000) Separation and enrichment of palladium and gold in biological and environmental samples, adapted to the determination by total reflection X-ray fluorescence. Analyst 125:397–399. doi:10.1039/b000471p
Mladenova E, Dakova I, Karadjova I, Karadjov M (2012) Column solid phase extraction and determination of ultra-trace Au, Pd and Pt in environmental and geological samples. Microchem J 101:59–64. doi:10.1016/j.microc.2011.10.007
Moazezi N, Moosavian MA (2016) Removal of rubidium ions by polyaniline nanocomposites modified with cobalt-Prussian blue analogues. J Environ Chem Eng 4:2440–2449. doi:10.1016/j.jece.2016.04.018
Muzikar M, Fontàs C, Hidalgo M, Havel J, Salvadó V (2006) A preconcentration system using polyamine metalfix-chelamine resin for the on-line determination of palladium(II) and platinum(IV) by inductively coupled plasma optical emission spectrometry. Talanta 70:1081–1086. doi:10.1016/j.talanta.2006.02.021
Nakajima J, Ohno M, Chikama K, Seki T, Oguma K (2009) Determination of traces of palladium in stream sediment and auto catalyst by FI-ICP-OES using on-line separation and preconcentration with QuadraSil TA. Talanta 79:1050–1054. doi:10.1016/j.talanta.2009.02.035
Niemelä M, Pitkäaho S, Ojala S, Keiski RL, Perämäki P (2012) Microwave-assisted aqua regia digestion for determining platinum, palladium, rhodium and lead in catalyst materials. Microchem J 101:75–79. doi:10.1016/j.microc.2011.11.001
Radi S, Toubi Y, Bacquet M, Degoutin S, Cazier F (2013) 1-(Pyridin-2-yl) Imine functionalized silica gel: synthesis, characterization, and preliminary use in metal ion extraction. Separat Sci Technol 48:1349–1355. doi:10.1080/01496395.2012.726309
Ramakul P, Yanachawakul Y, Leepipatpiboon N, Sunsandee N (2012) Biosorption of palladium (II) and platinum (IV) from aqueous solution using tannin from Indian almond (Terminalia catappa L.) leaf biomass: kinetic and equilibrium studies. Chem Eng J 193:102–111. doi:10.1016/j.cej.2012.04.035
Rojas FS, Ojeda CB, Pavón JMC (2006) Automated on-line separation preconcentration system for palladium determination by graphite furnace atomic absorption spectrometry and its application to palladium determination in environmental and food samples. Talanta 70(5):979–983. doi:10.1016/j.talanta.2006.05.048
Rossi E, Errea MI, de Cortalezzi MMF, Stripeikis J (2017) Selective determination of Cr(VI) by on-line solid phase extraction FI-SPE-FAAS using an ion exchanger resin as sorbent: an improvement treatment of the analytical signal. Microchem J 130:88–92. doi:10.1016/j.microc.2016.08.004
Saçmacı Ş, Şahan S, Saçmacı M, Şahin U, Ülgen A, Kartal Ş (2013) On-line determination of palladium by flame atomic absorption spectrometry coupled with a separation/preconcentration minicolumn containing a new sorbent. Intern J Environ Anal Chem 93:1223–1235. doi:10.1080/03067319.2012.727809
Saitoh T, Suzuki S, Hiraide M (2005) Solid phase extraction of some precious metals from hydrochloric acid to polystyrene-divinylbenzene porous resin impregnated with polyoxyethylene-type nonionic surfactant. J Chromatogr A 1097:179–182. doi:10.1016/j.chroma.2005.10.002
Sayın M, Can M, Imamoglu M, Arslan M (2015) 1,3,5-Triazine-pentaethylenehexamine polymer for the adsorption of palladium (II) from chloride-containing solutions. React Funct Polym 88:31–38. doi:10.1016/j.reactfunctpolym.2015.02.003
Schoeman E, Bradshaw SM, Akdogan GA, Snyders CAJJ (2017) Extraction of platinum and palladium from a heap leach cyanide solution using strong base anion exchange resins. Int J Miner Process 162:27–35. doi:10.1016/j.minpro.2017.02.017
Sharma RK, Pandey A, Gulati S, Adholeya A (2012) An optimized procedure for preconcentration, determination and on-line recovery of palladium using highly selective diphenyldiketone-monothiosemicarbazone modified silica gel. J Hazard Mater 209–210:285–292. doi:10.1016/j.jhazmat.2012.01.022
Sivrikaya S, Altundag H, Zengin M, Imamoglu M (2011) Separation, preconcentration, and recovery of Pd(II) Ions using newly modified silica gel with Bis(3-Aminopropyl)Amine. Separat Sci Technol 46:2032–2040. doi:10.1080/01496395.2011.572111
Sivrikaya S, Imamoglu M, Kara D (2014) On-line solid phase extraction of nickel, copper, and cadmium using a newly synthesized polyamine silica gel-loaded mini-column for flame atomic absorption spectrometric determination. Atomic Spectrosc 35:168–176
Sivrikaya S, Imamoglu M, Yıldız SZ, Kara D (2016) Novel functionalized silica gel for on-line preconcentration of Cadmium (II), Copper (II), and Cobalt (II) with determination by flame atomic absorption spectrometry. Anal Lett 49:943–957. doi:10.1080/00032719.2015.1067812
Soylak M, Tuzen M (2008) Coprecipitation of gold(III), palladium(II) and lead(II) for their flame atomic absorption spectrometric determinations. J Hazard Mater 152:656–661. doi:10.1016/j.jhazmat.2007.07.027
Tarley CRT, Corazza MZ, de Oliveira FM, Somera BF, Nascentes CC, Segatelli MG (2017a) On-line micro-solid phase preconcentration of Cd2+ coupled to TS-FF-AAS using a novel ion-selective bifunctional hybrid imprinted adsorbent. Microchem J 131:57–69. doi:10.1016/j.microc.2016.11.013
Tarley T, Diniz KM, Cajamarca FA (2017b) Study on the performance of micro-flow injection preconcentration method on-line coupled to thermospray flame furnace AAS using MWCNTs wrapped with polyvinylpyridine nanocomposites as adsorbent. RSC Adv 7:19296–19304. doi:10.1039/C7RA01220A
Tavakkoli N, Habibollahi S, Keshavarzianpour M (2014) Solid phase extraction of trace amounts of palladium in environmental water samples on multi-walled carbon nanotubes as a new sorbent: comparison with activated carbon. Desalin Water Treat 52:350–356. doi:10.1080/19443994.2013.792143
Tavakoli L, Yamini Y, Ebrahimzadeh H, Nezhadali A, Shariati S, Nourmohammadian F (2008) Development of cloud point extraction for simultaneous extraction and determination of gold and palladium using ICP-OES. J Hazard Mater 152:737–743. doi:10.1016/j.jhazmat.2007.07.039
Velmurugan M, Thirumalraj B, Chen SM, Al-Hemaid FMA, Ajmal Ali M, Elshikh MS (2017) Development of electrochemical sensor for the determination of palladium ions (Pd2+) using flexible screen printed un-modified carbon electrode. J Colloid Interf Sci 485:123–128. doi:10.1016/j.jcis.2016.08.073
Wang H, Li C, Bao C, Liu L, Liu X (2011) Adsorption and determination of Pd (II) and Pt(IV) onto 3′-Nitro-4- amino azobenzene modified chitosan. J Chem Eng Data 56:4203–4207. doi:10.1021/je2007154
Wang Q, Gao W, Liu Y, Yuan J, Xu Z, Zeng Q, Schröder M (2014) Simultaneous adsorption of Cu(II) and SO42- ions by a novel silica gel functionalized with a ditopic zwitterionic Schiff base ligand. Chem Eng J 250:55–65. doi:10.1016/j.cej.2014.03.106
Wu XZ, Liu P, Pu QS, Sun QY, Su ZX (2004) Preparation of dendrimer-like polyamidoamine immobilized silica gel and its application to online preconcentration and separation palladium prior to FAAS determination. Talanta 62:918–923. doi:10.1016/j.talanta.2003.10.011
Ye J, Liu SX, Tian M, Li W, Hu B, Zhou W, Jia Q (2014) Preparation and characterization of magnetic nanoparticles for the on-line determination of gold, palladium, and platinum in mine samples based on flow injection micro-column preconcentration coupled with graphite furnace atomic absorption spectrometry. Talanta 118:231–237. doi:10.1016/j.talanta.2013.10.018
Zalupski PR, Mcdowell R, Dutech G, Zalupski PR, Mcdowell R, Dutech G (2014) The adsorption of gold, palladium, and platinum from acidic chloride solutions on mesoporous carbons. Solvent Extr Ion Exch 32:737–748. doi:10.1080/07366299.2014.951278
Zhang S, Pu Q, Liu P, Sun Q, Su Z (2002) Synthesis of amidinothioureido-silica gel and its application to flame atomic absorption spectrometric determination of silver, gold and palladium with on-line preconcentration and separation. Anal Chim Acta 452:223–230. doi:10.1016/S0003-2670(01)01476-3
Zheng H, Zhang D, Wang WY, Fan YQ, Li J, Han HP (2007) Highly selective determination of palladium(II) after preconcentration using Pd(II)-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Microchim Acta 157:7–11. doi:10.1007/s00604-006-0649-z
Zhou L, Xu J, Liang X, Liu Z (2010) Adsorption of platinum (IV) and palladium (II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J Hazard Mater 182:518–524. doi:10.1016/j.jhazmat.2010.06.062
Zhou F, Qian L, Li G, Zhu K, Peng J, Li C (2014a) On-line flow injection preconcentration of palladium in environmental waters using a microcolumn packed with silica nanoparticles modified by γ-aminopropyltriethoxisilane and determination by flame atomic absorption spectrometry. Anal Lett 47:543–555. doi:10.1080/00032719.2013.845834
Zhou SY, Song N, Liu SX, Chen DX, Jia Q, Yang YW (2014b) Separation and preconcentration of gold and palladium ions with a carboxylated pillar[5]arene derived sorbent prior to their determination by flow injection FAAS. Microchim Acta 181:1551–1556. doi:10.1007/s00604-014-1229-2
Zolfonoun E, Yousefi SR (2016) Simultaneous determination of rare earth elements by ICP OES after on-line enrichment using multi-walled carbon nanotubes coated cellulose acetate membrane. J Braz Chem Soc 27:2348–2353. doi:10.5935/0103-5053.20160131
Acknowledgements
This work was supported by the Sakarya University Research Fund with Project Number 2017-50-01-027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivrikaya, S., Karslı, B. & Imamoglu, M. On-line Preconcentration of Pd(II) Using Polyamine Silica Gel Filled Mini Column for Flame Atomic Absorption Spectrometric Determination. Int J Environ Res 11, 579–590 (2017). https://doi.org/10.1007/s41742-017-0051-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-017-0051-1