Abstract
Introduction
A subcutaneous (SC) formulation of natalizumab has been recently authorised for multiple sclerosis patients. This study aimed to assess the implications of the new SC formulation, and to compare the annual treatment costs of SC versus intravenous (IV) natalizumab therapy from both the Spanish healthcare system (direct health cost) and the patient (indirect cost) perspectives.
Methods
A patient care pathway map and a cost-minimisation analysis were developed to estimate SC and IV natalizumab annual costs over a 2-year time horizon. Considering the patient care pathway and according to natalizumab experience (IV) or estimation (SC), a national expert panel involving neurologists, pharmacists, and nurses provided information/data regarding resource consumption for drug and patient preparation, administration, and documentation. One hour of observation was applied to the first six (SC) or 12 (IV) doses, and 5 min for successive doses. The Day hospital (infusion suite) facilities at a reference hospital were considered for IV administrations and the first six SC injections. For successive SC injections, either a reference hospital or regional hospital in a consulting room was considered. Productivity time associated with travel (56 min to reference hospital, 24 min to regional hospital) and waiting time pre- and post-treatment (SC 15 min, IV 25 min) were assessed for patients and caregivers (accompanying 20% of SC and 35% of IV administrations). National salaries for healthcare professionals were used for cost estimation (€, year 2021).
Results
At years 1 and 2, total time and cost savings (excluding drug acquisition cost) per patient, driven by saving on administration and patient and caregiver productivity for SC at a reference hospital versus IV at a reference hospital, were 116 h (a reduction of 54.6%) and €3682.82 (a reduction of 66.2%). In the case of natalizumab SC at a regional hospital, the total time and cost saving were 129 h (a reduction of 60.6%) and €3883.47 (a reduction of 69.8%).
Conclusions
Besides the potential benefits of convenient administration and improving work–life balance, as suggested by the expert panel, natalizumab SC was associated with cost savings for the healthcare system by avoiding drug preparation, reducing administration time, and freeing up infusion suite capacity. Additional cost savings could be derived with regional hospital administration of natalizumab SC by reducing productivity loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The availability of the new formulation of subcutaneous (SC) natalizumab increases the therapeutic options for relapsing-remitting multiple sclerosis patients and professionals. This new option offers the potential benefits of convenient administration and improvement of work–life balance for patients. |
The new administration option of natalizumab SC was associated with cost savings for the healthcare system by reducing the total treatment time and freeing up infusion suite capacity, which allows increased hospital efficiency. |
Additional cost savings could be derived with administration of natalizumab SC at a regional hospital by reducing work productivity loss of patients and caregivers. |
1 Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, and neurodegenerative disease of the central nervous system resulting in persistent neurological damage [1, 2]. The relapsing-remitting multiple sclerosis (RRMS) phenotype, characterised by relapses followed by either complete recovery periods or partial recovery periods without disease progression, is identified according to the clinical and radiological progression of the disease [3]. Manifestations such as rapid deterioration of neurological function, evidence of uncontrolled inflammatory activity, or accumulation of lesions on magnetic resonance imaging (MRI) are defined as highly active RRMS [2, 4]. Spain is considered to be a medium-high risk region, with around 55,000 people with MS. The prevalence is 80–180 cases, and the incidence is 4.2 new cases per 100,000 inhabitants [5, 6]. The most prevalent MS phenotype is RRMS (around 85% of MS patients) [7], and 4.0–23.1% of them manifest highly active RRMS [8].
Natalizumab (Tysabri®) is a high-efficacy disease-modifying therapy (DMT) indicated in adults with highly active RRMS, including patients with highly active disease despite previous treatment with DMT and patients with rapidly evolving severe RRMS clinically defined by two or more disabling relapses in 1 year associated with brain MRI activity (one or more gadolinium-enhancing lesion or an increase in T2 lesion compared to a previous MRI) [9]. Natalizumab intravenous (IV) infusion has proved to be an effective, well-tolerated treatment for patients with highly active RRMS, supported by clinical trials and prescription data on approximately 251,119 people worldwide showing over 984,009 patient-years of experience. Based on studies (DELIVER [10] and REFINE [11]) and considering the analyses of pharmacokinetics and pharmacodynamics, a new subcutaneous (SC) pharmaceutical formulation of natalizumab has recently been approved in Europe (April 2021) [12]. Natalizumab SC offers a new route of administration to meet individual patient needs that is considered highly comparable to the IV regimen, with a safety profile similar to IV natalizumab [9].
According to the Summary of Product Characteristics (SmPC), the main differences between the two routes are the administration times and observation times after administration [9]. Observation by a healthcare professional is required during the administration of natalizumab. IV administration of natalizumab requires a 1 h infusion, whereas the SC natalizumab therapy involves the injection of two consecutive pre-filled syringes, one after the other without significant delay (expected to take less than 5 min) [9]. A post-dose observation period is required after completing administration of natalizumab. This observation is established as 1 h for the first 12 doses in the case of IV infusions and for the first six doses in the case of SC injections. The reduction or elimination of this post-dose observation period can be applied for the consecutive natalizumab doses, according to clinical judgement, if the patient has not experienced any reaction to the previous infusions or injections [9].
MS usually begins in young adulthood (between 20 and 40 years of age) and, depending on the severity of the disease, leads to a decrease in work and activity, which has an impact on the economic burden on the patient and society [13,14,15]. The economic burden for the National Health Service (NHS) (direct costs) is estimated at €4989 per patient relapse (€, 2017) [16]. Natalizumab is the first high-efficacy MS therapy that offers two possible routes of administration, providing patients and physicians the flexibility to choose the option that best fits their individual needs [17]. Additionally, the introduction of natalizumab SC injection would have advantages related to resource savings (health direct cost and indirect cost) as time is reduced by avoiding drug preparation, reducing administration time, and freeing up infusion suite capacity.
Based on a revised, consensus-based patient care pathway, this study aimed to assess the cost implications for MS patients and the Spanish healthcare system associated with the implementation of natalizumab SC versus the IV route of administration.
2 Materials and Methods
For the assessment of the cost implications between the two alternatives, a cost-minimisation analysis model was designed in Microsoft Excel. The process of selecting the inputs to this model was carried out in two phases; the first one was the patient care pathway mapping for resource identification and quantification, and the second one was the development of the cost-minimisation analysis.
2.1 Care Pathway Mapping
A multidisciplinary panel of eight national experts in MS involving neurologists, pharmacists, and nurses called the Tysabri® in Subcutaneous Administration (TASC) working group was set up by the study team on 6 May 2021, to identify the key points in the transition from IV to SC administration of natalizumab. The TASC group is not an official organisation at a national level; even so, the members of the TASC group were selected for their global vision in the treatment of MS with natalizumab. All of them had previous experience in the management of natalizumab SC in terms of clinical trials, as well as experience in using IV natalizumab in their hospitals. In addition, they represented different types of hospitals in Spain and different geographies, to ensure the generalisability of the care pathway.
In the context of this analysis, reference hospitals are characterised by a higher complexity of the care function and thus have a larger service catchment area. In contrast, regional hospitals have basic specialities and tend to be less distant from patients, especially in regions with large geographical areas. The TASC group defined the patient care pathway of natalizumab treatment from patient selection, drug administration, and monitoring (follow-up) until the discontinuation of treatment for both administration routes carried out in a hospital setting, identifying the critical points, common or exclusive to SC and/or IV administration, in naïve and switch (from IV to SC) patients. Additionally, given the proximity of regional hospitals, a specific care pathway was described for patients eligible for referral to the regional hospital with natalizumab SC therapy (continuing the follow-up and monitoring of the patient at the reference hospital). Previous publications based on MS care units [18], the Spanish consensus of natalizumab use [19], and regional MS guidelines [20] were utilised to design the patient care pathway flow. Two meetings of the group were conducted, coordinated by an independent external moderator. The objectives of the first meeting were to establish the main contributions of the use of natalizumab SC in the management of MS and to identify critical points in the switch from IV to SC administration. The second, a consensus meeting, validated the patient pathway mapping and their recommendations to facilitate the transition to the SC route of administration in the hospital setting.
The TASC group also provided information about the resource consumption on the patient care pathway, based on their experience with IV natalizumab or an estimation for SC natalizumab administration. Moreover, other parameters such as patient productivity loss and caregiver productivity loss were explored. For this purpose, a structured questionnaire was designed and shared with the members of the TASC group for each expert to complete individually, providing quantitative value responses. Subsequently, a consensus meeting was held, with the participation of the entire TASC group, where the individual responses were presented in the form of propositions (mean, mode, and range). All parameters were discussed in a consensus process, where experts validated them and made decisions by agreement, working together to find mutually representative and acceptable values. This information was used to perform an economic evaluation. No patient-level data were collected in this study.
2.2 Cost-Minimisation Analysis
A simple cost-analysis decision model was developed to compare the annual costs of natalizumab IV infusion and natalizumab SC injection therapy in a reference and/or regional hospital, as appropriate. The analysis used the Spanish NHS and societal perspective, including direct health costs and the work productivity loss of the patients and caregivers. The time horizon of the model was 2 years because the SmPC mentioned that continued therapy after 2 years should be reassessed [9]. Despite the time horizon being over 1 year, no discount rate was applied, because the 2-year horizon was considered a short-term horizon not requiring any time adjustment. In fact, the time horizon was decided to be extended to 2 years in order to be able to assure that all costs relevant to the decision problem were accounted for. The appraised population is defined as new patients appropriate for receipt of natalizumab IV or SC therapy in the hospital setting.
To select the model inputs, a structured questionnaire (including care pathway mapping and other parameters related to patient outcomes) was developed and individually completed by each member of the TASC group according to their experience in their routine clinical practice. All selected input parameters from the TASC group were further discussed and validated in a consensus meeting before being included in the model.
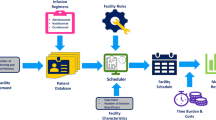
The model considered the administration process cost and the work productivity loss cost of patients and caregivers. The administration process included the healthcare professionals’ time taken up by medication and patient preparation, SC or IV administration, observation, and documentation. The work productivity loss considered the administration process time at the hospital, waiting times in the hospital before and after the administration process, and accessibility, defined by the travel time to the regional or reference hospital (Fig. 1). For work productivity loss, the time data were provided by the TASC group as an estimate within the administrative process of the hospitals, so these data were not provided directly by the patients.
2.2.1 Health Resources Considered in the Model
To facilitate the identification of resources used during the administration process, the different tasks were split into two groups: pre-administration tasks (regimen verification, preparation time, patient accommodation, prescription validation, dispensing order, and documentation and registration) and administration/observation tasks (taking of vital signs and cannulation of peripheral line, patient observation during the treatment of infusion/injection, post-dose observation, taking vital signs, cleaning and removal of peripheral line, and final documentation and registration of patient information) (Table 1).
Pre-administration tasks comprised the dedicated time professionals spent before the dose administration. An average healthcare professional “dedication time” was calculated considering weighted participation of the different professionals (neurologists, nurses, pharmacists, and technical pharmacists) in the different tasks (Table 1).
Administration/observation tasks comprised the procedures involving the administration of doses in the infusion suite facilities at a Day hospital or in the consulting room performed by the nurse staff. In line with natalizumab SmPC, administration every 4 weeks was considered for both IV and SC routes. The total doses in the 2-year period were split into first doses (six doses for natalizumab SC and 12 doses for natalizumab IV) and consecutive doses (from dose 7 onwards for natalizumab SC and from 13 onwards for natalizumab IV). The model assumed that all IV infusion doses and first SC injection doses would be performed in infusion suite facilities at a reference hospital. The consecutive SC injection doses (dose 7 onwards) would be performed in a consulting room, with two options: at a reference hospital or at a regional hospital.
In relation to work productivity loss, this estimation included the total time spent inside the hospital and the time spent travelling (round trip) to the hospital. For the first part, the TASC group provided an estimation of the waiting times before (entry) and after (exit) treatment at the hospital. The entry time was set at 15 min for the IV route and 10 min for the SC route, and the exit time was set at 10 and 5 min for the IV and SC routes, respectively. Likewise, 20% and 35% of patients treated with natalizumab SC and IV, respectively, were considered to require the assistance of a caregiver. For the second part, the accessibility of a reference hospital was estimated to require an average travel time (round trip) of 56 min, while for a regional hospital, this time was 24 min, based on the published data [21].
2.2.2 Costs
In this analysis, an equivalent drug acquisition cost was assumed for SC (two pre-filled syringes of 150 mg natalizumab SC) and IV (one vial of 300 mg natalizumab) therapies, so this category was not included in the total cost.
In keeping with the perspective of the analysis, direct health costs and indirect costs were considered in the total cost. The unitary costs for the dedication time of the healthcare professionals were derived from national salaries [22]. The lost workday cost for patients and caregivers was represented by the national average wage [23]. The fees published in official regional bulletins of a national health database were used for the hospital facilities and consulting room costs [24] (Table 2). All costs included in the model are expressed in euros valued for the year 2021 (€, 2021).
Three scenarios were assessed. The first one assumed the administration of all natalizumab IV doses happened at infusion suite facilities (natalizumab IV in the reference hospital scenario). The second one assumed the administration of the first six doses of SC therapy happened at infusion suite facilities and the consecutive doses in a consulting room of a reference hospital (natalizumab SC in the reference hospital scenario). The third one assumed the administration of the first six doses of SC therapy happened in the infusion suite facilities at a reference hospital and the consecutive doses in a consulting room at a regional hospital (natalizumab SC in the regional hospital scenario).
3 Results
3.1 Care Pathway Mapping
The TASC group’s assessment of the care pathway for patients receiving natalizumab reported that the new SC route of administration will not involve major changes to the current care pathway. Once the management of MS patients was defined, potential changes in the patient pathway with SC administration were linked to the administration facilities (infusion suite or consulting room), the information and/or informed consent to be provided to the patient, and the administration and post-dose observation times. The new SC route did not imply changes in the treatment prescription validation circuit and subsequent dispensing of the treatment in the hospital pharmacy. The current management of MS patients and the potential variations to the patient pathway associated with SC administration are presented in the Appendix, Figure 1 (see the electronic supplementary material). The key points to be reinforced or considered in the transition from IV to SC natalizumab were also identified (Table 3).
A referral of natalizumab SC to regional hospitals was considered to improve the balance between the patient's work and life and other daily activities. Dispensing and administration would be at a regional hospital and the follow-up and control maintained at a reference hospital. The referral would be desirable after the first 6 months of natalizumab SC treatment at the reference hospital. However, a generalised referral strategy cannot be planned; hence, it would be analysed on a case-by-case basis and continuous training of the professionals of the regional hospitals would be essential, preferably provided by multidisciplinary teams of the reference centre. The TASC group proposed a referral scheme for natalizumab SC administration in the regional hospital considering patient follow-up and monitoring would be provided by the reference hospital (Appendix, Figure 2, see the electronic supplementary material).
Other advantages of changing from IV to SC considered by the TASC group were:
-
Positive emotional impact: Patients associate the IV route with more severe treatments.
-
Convenience: Venous access and catheter-associated infection risk are avoided.
-
Less time spent at hospital: The time needed would change from 1 h of administration followed by 1 h of observation (IV) to less than 5 min of administration and only 1 h of observation for the first six doses (SC) [25].
-
Improved work–life balance and adherence to treatment: These improvements might be especially impactful in patients who have not reported the disease at work.
-
Less saturation of the infusion suite facilities at the Day hospital, due to a greater flexibility in schedule appointments.
3.2 Cost-Minimisation Analysis
The resultant total cost per patient was €3213.54, €1210.00, and €1139.54 at year 1 and €2349.81, €670.93, and €540.34 at year 2 for the IV at a reference hospital scenario, the SC at a reference hospital scenario, and the SC at a regional hospital scenario, respectively. The disaggregated cost comparison of natalizumab SC and IV is presented in Table 4. At year 1, the health direct cost per patient regarding the Spanish NHS and the indirect cost were €1980.13 and €1233.41 for IV at a reference hospital, €612.84 and €597.16 for SC at a reference hospital, and €612.84 and €526.70 for SC at a regional hospital, respectively. Equally, at year 2, the health direct cost and indirect cost per patient were €1348.95 and €1000.86 for IV at a reference hospital, €177.13 and €493.80 for SC at a reference hospital, and €177.13 and €363.21 for SC at a regional hospital, respectively.
Natalizumab SC administered at a reference hospital would reduce overall costs by 62.3% (− €2003.54 per patient at year 1) and 71.4% (− €1678.88 per patient at year 2) versus natalizumab IV. With regional hospital administration, natalizumab SC reductions would be 64.5% (− €2074.00 per patient at year 1) and 77.0% (− €1809.47 per patient at year 2) (Fig. 2).
Total time per patient was 120, 54, and 50 h at year 1 and 94, 42, and 34 h at year 2 for IV at a reference hospital, SC at a reference hospital, and SC at a regional hospital, respectively. The disaggregated time comparison of natalizumab SC and IV is presented in Table 5.
Natalizumab SC administered at a reference hospital would reduce overall time by 54.4% (− 65 h per patient at year 1) and 54.7% (− 51 h per patient at year 2) versus natalizumab IV. With regional hospital administration (from dose 7 onwards), natalizumab SC reductions would be 58.2% (− 70 h per patient at year 1) and 63.6% (− 60 h per patient at year 2) (Fig. 3).
At years 1 and 2, total time and cost saving (excluding drug acquisition cost) per patient, driven by saving on administration and patient and caregiver productivity for natalizumab SC at a reference hospital versus natalizumab IV at a reference hospital, were 116 h (a reduction of 54.6%) and €3682.42 (a reduction of 66.2%). In the case of natalizumab SC at a regional hospital, savings were 129 h (a reduction of 60.6%) and €3883.47 (a reduction of 69.8%).
4 Discussion
The objective of the study was to assess the implications associated with the new SC route versus IV natalizumab therapy for MS patients and the Spanish NHS.
First, the TASC group concluded that the availability of natalizumab SC would increase the therapeutic options for RRMS patients and healthcare professionals. The main benefits of natalizumab SC therapy for the patient manifested by TASC group were the prevention of venous access problems due to the less invasive route of administration, increased patient comfort, higher compatibility between professional life and treatment, improved quality of life, and reduced intervention time (especially from the sixth month onwards). On the other hand, the most challenging aspects would be that hospital and healthcare professionals would have to readjust protocols for the transition. The TASC group recommended starting the transition from natalizumab IV to natalizumab SC in centres with experience in managing MS patients (MS care unit), mainly because clinical and radiological monitoring and surveillance require neurologists and radiologists with MS experience to identify potential serious adverse events. Furthermore, the TASC group mentioned that the prospect of referral from the reference hospital to the regional hospital after administration of six doses of natalizumab SC would show benefits for patients and for the NHS.
Second, the objective of the cost analysis was to estimate the economic benefit of an alternative route of administration in patients who are candidates for natalizumab treatment, in terms of healthcare direct costs (payer costs) and indirect costs (work productivity loss). IV infusion requires a longer administration process time than SC administration and the availability of an infusion suite facility at the Day hospital at the reference hospital. In comparison, natalizumab SC offers more hospital areas as possibilities for treatment, such as consulting rooms, available at regional hospitals not just the reference hospital. Moreover, a reference hospital is not always easily accessible to all patients with RRMS, especially in rural areas and large healthcare areas [26, 27].
The cost analyses demonstrated that the administration of natalizumab SC injections at a reference or regional hospital (the latter is often close to the patient's home) constitutes an advantage over natalizumab IV infusion at a reference hospital. The overall cost savings per patient showed a reduction of 66.2% (€3682.42) at a reference hospital and 69.8% (€3883.47) in the case of utilising a regional hospital over the first 2 years of treatment. This reduction was due to the shorter time spent on administration, which is reduced by 54.6% (116 h) and 60.6% (129 h) for reference and regional hospital scenarios, respectively. The main cost reduction of natalizumab SC was due to the reduction in administration observation times from the sixth month onwards. Therefore, additional cost savings in subsequent years (after the first 2 years) of up to 77% are potentially expected. This cost reduction can be improved by opting for consultation administration, which will free up hours in the Day hospital, improving hospital capacity, and thus improve care for other patients. These results are in line with other economic evaluations such as a cost-minimisation analysis from a socio-economic perspective on the introduction of natalizumab SC injection performed in Sweden (presented at congress), which showed savings associated with SC natalizumab estimated at 83.2% in administration costs and reductions of 47% in time in comparison with natalizumab IV [25]. In this evaluation, the cost categories included were acquisition, administration, adverse events, and social cost [25].
The benefits of the transition from IV to SC administration have also been assessed for other medications in other therapeutic areas. Evidence in oncology drugs indicated health direct cost savings using SC instead of IV administration, as well as reduced hospital time, flexibility, and fewer side effects at the injection site, such as infection by infusion catheters in the hospital setting [28, 29]. One study assessing the trastuzumab IV to SC transition [30], showed a mean relative reduction of 50% (27.2 min for IV and 13.2 min for SC) in healthcare professional dedication time, resulting from avoiding IV catheter installation and removal, line flushing, and drug reconstitution [30]. Also, the SC administration resulted in a fivefold reduction in chair time and a fourfold reduction in patient treatment room time, resulting in 24 h of free time over the full course of treatment (18 cycles) [30]. The total direct costs were €29,431.75 and €28,452.12 (€, 2016) for IV and SC, respectively, resulting in a saving of €979.60 (€, 2016) over a full course of treatment [30]. Another study of the transition from IV rituximab (20% of patients) to the SC route, estimated a saving to the payer of $153,000 (€, 2021), increasing provider time capacity by 270 h and freeing up patient time by 470 h [31].
Furthermore, administration at the nearest regional hospital to the patient will reduce the labour costs associated with patients and their caregivers. Regions should have similar levels of accessibility, but in large regions, accessibility to higher level services (reference hospital) depends on the spatial distribution of the population [21].
One limitation of this model is the assumption of an equal acquisition cost between the IV and the SC form. Likewise, data on the patient consequences of the transition from the IV to SC form (patient satisfaction, treatment adherence, discontinuation, health-related quality of life, adverse events) were not included; the TASC recommended analysing these items with a specific methodology. Along this line, the ongoing NOVA long-term study (NCT03689972) is looking at patient preference for IV or SC [32]. Particularly, there is evidence of less adherence in patients with high-level MS severity in comparison to patients with low MS severity [33], and the natalizumab SC administration could improve this gap [33]. Furthermore, it is expected that patient preferences would be favourable for SC administration as it has already occurred in the transitions of other pathologies. Usually patients prefer SC administration over IV because of convenience [34,35,36,37] or quality-of-life improvement [38]. For example, SC rituximab scored higher than IV in terms of patient satisfaction with treatment (87.5 vs 75.0%) and impact on activities of daily living/adherence to treatment (both 83.3 vs 58.3%) [39]. Additionally, a systematic review showed that the preference for IV infusions is due to the lower frequency of administration. As the frequency between IV and SC natalizumab is similar, more optimal results are expected for the SC presentation of natalizumab [40]. Another limitation was that the percentage of active patients was not considered. The study by Rath et al. [37] identified the proportion of patients employed and the working time used for treatment administration. In this case, an exploration in the Spanish setting is needed. A limitation related to the TASC group is the lack of patient participation, which could have provided data closer to patients’ reality in terms of measuring the lost work productivity, possibly underestimated because only the care time of the health professional and an average travel time to the hospital were considered. Similarly, although initial impressions suggest that the new formulation will improve quality of life and satisfaction, it is felt that these issues need to be explored directly with patients. Further study of these aspects is required.
Obtaining economic model inputs from expert panels is a common practice in economic evaluation to quantify uncertain situations. In this study, the experience of the TASC group is relevant because the SC presentation was not commercially available before the cost analysis. This is the main strength of our study, which allowed estimation of the cost difference of a drug that will be available in a short period of time, including the experience of an expert panel on MS patient management. However, the results generated in the model should be ratified in subsequent studies with observationally generated data.
5 Conclusion
The transition from IV to SC could show potential benefits for the patient in terms of more convenient administration, nearby accessibility, shorter intervention time, and improved work–life balance. From the hospital and neurologist perspective, lowering time requirements could reduce costs and free up healthcare professional staff, while maintaining adequate control and patient adherence.
In terms of costs, natalizumab SC was associated with cost savings for the healthcare system by avoiding drug preparation, reducing administration process time, and freeing up hospital capacity. Additional cost savings could be derived with regional hospital administration of natalizumab SC by reducing productivity loss.
Change history
28 April 2023
The ESM has been exchanged.
References
Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–80. https://doi.org/10.1056/NEJMra1401483.
García Merino A, Ramón Ara Callizo J, Fernández Fernández O, Landete Pascual L, Moral Torres E, Rodríguez-Antigüedad Zarrantz A. Consensus statement on the treatment of multiple sclerosis by the Spanish Society of Neurology in 2016. Neurol Barc Spain. 2017;32:113–9. https://doi.org/10.1016/j.nrl.2016.02.026.
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–86. https://doi.org/10.1212/WNL.0000000000000560.
Arrambide G, Iacobaeus E, Amato MP, Derfuss T, Vukusic S, Hemmer B, et al. Aggressive multiple sclerosis (2): treatment. Mult Scler J. 2020;26:1045–63. https://doi.org/10.1177/1352458520924595.
Perez-Carmona N, Fernandez-Jover E, Sempere AP. Epidemiology of multiple sclerosis in Spain. Rev Neurol. 2019;69:32–8. https://doi.org/10.33588/rn.6901.2018477.
Multiple Sclerosis International Federation. Number of people with MS. Atlas of MS. n.d. https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms. Accessed 15 Feb 2022.
Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. 2014;27:271–8. https://doi.org/10.1097/WCO.0000000000000094.
Ellenberger D, Flachenecker P, Fneish F, Frahm N, Hellwig K, Paul F, et al. Aggressive multiple sclerosis: a matter of measurement and timing. Brain. 2020;143:e97. https://doi.org/10.1093/brain/awaa306.
European Medicine Agency (EMA). EPAR. Summary of Product Characteristics (SmPC) Tysabri® (natalizumab). n.d. https://www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf.
Plavina T, Fox EJ, Lucas N, Muralidharan KK, Mikol D. A randomized trial evaluating various administration routes of natalizumab in multiple sclerosis. J Clin Pharmacol. 2016;56:1254–62. https://doi.org/10.1002/jcph.707.
Trojano M, Ramió-Torrentà L, Grimaldi LM, Lubetzki C, Schippling S, Evans KC, et al. A randomized study of natalizumab dosing regimens for relapsing-remitting multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 2021. https://doi.org/10.1177/13524585211003020.
European Medicine Agency (EMA). Assessment report Tysabri (EPAR) n.d. https://www.ema.europa.eu/en/documents/variation-report/tysabri-h-c-603-x-0116-epar-assessment-report-extension_en.pdf.
Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler Houndmills Basingstoke Engl. 2017;23:1123–36. https://doi.org/10.1177/1352458517694432.
Kobelt G, Berg J, Lindgren P, Fredrikson S, Jönsson B. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2006;77:918–26. https://doi.org/10.1136/jnnp.2006.090365.
Casado M, Echave M, Ruiz L, Oyagüez I. Evaluación económica de intervenciones sanitarias en esclerosis multiple. Aplicación e interpretación a partir de estudios publicados de natalizumab. Rev Esp Econ Salud. 2018;13(142):53.
Casado V, Bonaventura I, Brieva L, Martínez-Yélamos S, Martín G, Presas-Rodriguez S, et al. Direct costs of relapses in patients with relapsing-remitting multiple sclerosis. Neurol Perspect. 2021;1:160–9. https://doi.org/10.1016/j.neurop.2021.05.003.
López PA, Alonso R, Silva B, Carnero Contentti E. Natalizumab subcutaneous injection for the treatment of relapsing multiple sclerosis patients: a new delivery route. Mult Scler Relat Disord. 2021;55: 103179. https://doi.org/10.1016/j.msard.2021.103179.
Soelberg Sorensen P, Giovannoni G, Montalban X, Thalheim C, Zaratin P, Comi G. The multiple sclerosis care unit. Mult Scler Houndmills Basingstoke Engl. 2019;25:627–36. https://doi.org/10.1177/1352458518807082.
Fernández O, García-Merino JA, Arroyo R, Álvarez-Cermeño JC, Izquierdo G, Saiz A, et al. Spanish consensus on the use of natalizumab (Tysabri®)-2013. Neurol Barc Spain. 2015;30:302–14. https://doi.org/10.1016/j.nrl.2013.10.004.
Servicio Canario de Salud. Guía de actuación en pacientes con esclerosis múltiple. Gobierno de Canarias. Plan de Calidad para el Sistema Nacional de Salud. Ministerio de Sanidad Servicios Sociales e Igualdad; 2015.
Kompil M, Jacobs-Crisioni C, Dijkstra L, Lavalle C. Mapping accessibility to generic services in Europe: a market-potential based approach. Sustain Cities Soc. 2019;47: 101372. https://doi.org/10.1016/j.scs.2018.11.047.
Boletín oficial de la Comunidad de Madrid. Orden 4 February 2021. [Consejería de Hacienda y Función Pública, por la que se dictan Instrucciones para la Gestión de las Nóminas del Personal de la Comunidad de Madrid para 2021]. n.d. https://www.bocm.es/boletin/CM_Orden_BOCM/2021/02/12/BOCM-20210212-7.PDF.
España - Salario Medio 2020. datosmacro.com n.d. https://datosmacro.expansion.com/mercado-laboral/salario-medio/espana. Accessed 23 Nov 2021.
eSalud. Oblikue eHealth. Database of economic information of the sanitary sector. Available from: n.d. http://www.oblikue.com.
Gianinazzi M, Soderbarg K, Jomaa K, Ralph L, Davidson N, Fink K. A cost-minimalization analysis shows significant resource savings associated with the introduction of natalizumab by subcutaneous injection in Sweden, from a socio-economic perspective [Poster]. Presented at the 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 13–15 October 2021, Vienna, Austria. n.d.
Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs Clin Immunother Biopharm Gene Ther. 2018;32:425–40. https://doi.org/10.1007/s40259-018-0295-0.
Stoner KL, Harder H, Fallowfield LJ, Jenkins VA. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient. 2014. https://doi.org/10.1007/s40271-014-0075-y.
North RT, Harvey VJ, Cox LC, Ryan SN. Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study. Clin Outcomes Res CEOR. 2015;7:423–30. https://doi.org/10.2147/CEOR.S85599.
Olsen J, Jensen KF, Olesen DS, Knoop A. Costs of subcutaneous and intravenous administration of trastuzumab for patients with HER2-positive breast cancer. J Comp Eff Res. 2018;7:411–9. https://doi.org/10.2217/cer-2017-0048.
López-Vivanco G, Salvador J, Diez R, López D, De Salas-Cansado M, Navarro B, et al. Cost minimization analysis of treatment with intravenous or subcutaneous trastuzumab in patients with HER2-positive breast cancer in Spain. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2017;19:1454–61. https://doi.org/10.1007/s12094-017-1684-4.
Harvey MJ, Zhong Y, Morris E, Beverage JN, Epstein RS, Chawla AJ. Assessing the transition from intravenous to subcutaneous delivery of rituximab: benefits for payers, health care professionals, and patients with lymphoma. PLoS ONE. 2022;17: e0261336. https://doi.org/10.1371/journal.pone.0261336.
Foley JF, Defer G, Ryerson LZ, Cohen JA, Arnold DL, Butzkueven H, et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol. 2022;21:608–19. https://doi.org/10.1016/S1474-4422(22)00143-0.
Burkhard A, Toliver J, Rascati K. Association between multiple sclerosis disease severity and adherence to disease-modifying therapies. J Manag Care Spec Pharm. 2021;27:915–23. https://doi.org/10.18553/jmcp.2021.27.7.915.
Falanga M, Canzona A, Mazzoni D. Preference for subcutaneous injection or intravenous infusion of biological therapy among Italian patients with SLE. J Patient Exp. 2019;6:41–5. https://doi.org/10.1177/2374373518770811.
Santus P, Ferrando M, Baiardini I, Radovanovic D, Fattori A, Braido F. Patients beliefs on intravenous and subcutaneous routes of administration of biologics for severe asthma treatment: a cross-sectional observational survey study. World Allergy Organ J. 2019;12: 100030. https://doi.org/10.1016/j.waojou.2019.100030.
Pivot X, Gligorov J, Müller V, Barrett-Lee P, Verma S, Knoop A, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–70. https://doi.org/10.1016/S1470-2045(13)70383-8.
Rath L, Campagna MP, Stankovich J, Ellis J, Jokubaitis V, McCarthy D, et al. Patient preferences for time and location of infusible therapies in multiple sclerosis and neuroimmunologic disorders. Int J MS Care. 2021;23:114–8. https://doi.org/10.7224/1537-2073.2020-075.
Syrios J, Pappa E, Volakakis N, Grivas A, Alafis J, Manioudaki S, et al. Real-world data on health-related quality of life assessment in patients with breast cancer receiving subcutaneous trastuzumab. Breast Cancer Basic Clin Res. 2018;12:1178223418758031. https://doi.org/10.1177/1178223418758031.
Lugtenburg P, Avivi I, Berenschot H, Ilhan O, Marolleau JP, Nagler A, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102:1913–22. https://doi.org/10.3324/haematol.2017.173583.
Overton PM, Shalet N, Somers F, Allen JA. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer Adherence. 2021;15:811–34. https://doi.org/10.2147/PPA.S303279.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
This analysis was supported by Biogen Spain S.L.U.
Conflict of interest
Ana María Alonso Torres has received compensation for serving on advisory boards for Biogen Spain S.L.U., Bristol Myers Squibb (BMS), Janssen, Novartis, Roche, and Sanofi; speaker honoraria from Almirall, Biogen Spain S.L.U., BMS, Janssen, Merck, Novartis, Roche, and Sanofi. Ángel Guillermo Arévalo Bernabé has received compensation for serving on advisory boards for Biogen Spain S.L.U. and Merck. Noelia Becerril Ríos has received compensation for serving on advisory boards and speaker fees from Almirall, Bayer, Biogen Spain S.L.U., BMS, Janssen, Merck, Novartis, and Sanofi. María Fuensanta Hellín Gil has received compensation for serving on advisory boards and a speaker event for Biogen Spain S.L.U. José Manuel Martínez Sesmero has received compensation for serving on advisory boards for Biogen Spain S.L.U., Merck, Roche, and Teva Pharmaceutical. Virginia Meca Lallana has received compensation for serving on scientific advisory boards and has received speaker honoraria from Almirall, Biogen Spain S.L.U., BMS, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Terumo, and Teva Pharmaceutical. Lluís Ramió-Torrentá has received compensation for serving on advisory boards and speaker honoraria from Almirall, Biogen Spain S.L.U., BMS, Merck, Novartis, Roche, Sanofi, and Teva Pharmaceutical. Alfredo Rodriguez-Antigüedad Zarranz has received compensation for serving on advisory boards or speaker honoraria from Biogen, BMS, Janssen, Merck‐Serono, Novartis, Roche, and Sanofi. Laura Gómez Maldonado and Inés Triana Junco are employees of Biogen Spain S.L.U. and hold shares or stocks as part of their remuneration. Manuel Gómez-Barrera, Nataly Espinoza Cámac, and Itziar Oyagüez work for Pharmacoeconomics & Outcomes Research Iberia (PORIB), an independent research organisation, which received funding pursuant to a contract with Biogen Spain S.L.U.
Ethics approval
Not applicable. No patient data were used.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The questionnaire used for collection and validation of data used in the present analysis, and the model that support the findings of this study are available on reasonable request from the corresponding author.
Code availability
Not applicable.
Author contributions
All authors contributed to the parameters included in the study. AMAT, AGAB, NBR, MFHG, JMMS, VML, LlRT, and ARA conceived, MGB and IO designed, and MGB conducted (collected/analysed data) the study. The first draft of the manuscript was written by MGB, NEC, and IO and all authors commented on later versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alonso Torres, A.M., Arévalo Bernabé, A.G., Becerril Ríos, N. et al. Cost-Analysis of Subcutaneous vs Intravenous Administration of Natalizumab Based on Patient Care Pathway in Multiple Sclerosis in Spain. PharmacoEconomics Open 7, 431–441 (2023). https://doi.org/10.1007/s41669-023-00394-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00394-2