Abstract
Background
Multiple sclerosis is an inflammatory demyelination process in the central nervous system (CNS) causing neurological disability and poor quality of life. Currently, Thai Food and Drug Administration (FDA)-approved disease-modifying therapy is costly, and most patients with multiple sclerosis are ineligible for treatment in Thailand as previous studies have challenged its cost-effectiveness. Off-label use of rituximab is inexpensive and highly effective in treating multiple sclerosis, but evidence of its cost-effectiveness in Thailand is yet to be collected.
Methods
This study aimed to evaluate the cost-utility and budget impact of rituximab for multiple sclerosis treatment compared with best supportive care, the standard practice in Thailand to treat the disease. A Markov model with a one-month cycle length and lifetime horizon was applied to compare the costs and outcomes of rituximab and best supportive care based on a societal perspective. Accordingly, incremental cost-effectiveness ratios were estimated. Probabilistic and one-way sensitivity analyses were conducted to investigate parameter uncertainty. In addition, the Markov model was used to assess the 5-year budget impact from the government perspective.
Results
A rituximab biosimilar demonstrated higher effectiveness and lower associated costs, compared to best supportive care, with the highest probability of being cost-effective (96%). The probability of relapse was the most sensitive parameter according to the one-way sensitivity analysis. The calculated budget impact of treating patients with multiple sclerosis in Thailand was 26,360,000 Thai baht (THB) or 844,255 United States dollars (USD) in the first fiscal year, and approximately 20,810,000–23,080,000 THB (666,608–739,388 USD) in the next four fiscal years.
Conclusion
In Thailand, a rituximab biosimilar would reduce the overall costs of multiple sclerosis treatment and should, therefore, be included in the National List of Essential Medicines.
Similar content being viewed by others
Background
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system that causes neurological disabilities, especially in young adults [1]. The incidence of MS is increasing worldwide, with two million cases reported in 2016 [2]. The prevalence and incidence of MS varies according to country and geographical areas [3]. The prevalence of MS is higher in Europe and the United States compared to that in Asia [4]. The estimated prevalence and incidence of MS in Thailand were 0.201 per 100,000 people and 0.073 per 100,000 person-years, respectively [5]. Chronic disease progression results in a significant increase in disability, leading to economic and social burdens [6]. Therefore, most patients who are unable to access MS treatment are affected by neurological disabilities, poor quality of life, and an additional socioeconomic burden [7].
Disease-modifying therapy (DMT), which includes injectable platform therapies, oral medication, or monoclonal antibodies, has been proven to prevent relapse and patient disabilities [8]. However, the cost associated with DMT is the most critical barrier preventing access to treatment [9]. The price of DMT approved by the Thai Food and Drug Administration (FDA) ranges from 39,544 to 61,714 Thai baht (THB) per month, which is equivalent to 1,041 − 1,625 United States dollars (USD), whereas the average Gross National Income (GNI) per capita of the Thai population is only 18,888 THB per month (497 USD). According to a recent survey by the Multiple Sclerosis International Federation (MSIF) [9], out of all the 102 countries where rituximab has been approved for the treatment of lymphoma and rheumatoid arthritis, 70 of them (including Thailand) have adopted it as off-label treatment for MS, because the benefits were considered to outweigh the need for regulatory approval [10]. Previous studies from several countries have suggested that rituximab is not only safe and highly effective in the control of MS progression [11,12,13,14,15,16,17,18,19,20], but it also has a lower cost than the currently approved DMT. The approximate annual cost of MS therapy in Thailand with the original rituximab is 100,000 THB (2,633 USD). However, a rituximab biosimilar is readily available in the country, and its efficacy has been shown to be similar to the original drug in the treatment of diffuse large B-cell lymphoma and severe rheumatoid arthritis [21, 22]. The current cost of therapy for the same period of time with the rituximab biosimilar is only 27,000 THB (711 USD).
Until recently, only a small minority of patients with MS in Thailand could get access to FDA-approved DMTs or to rituximab therapy, as neither of these treatments have been included in the National List of Essential Medicines (NLEM). This pharmaceutical reimbursement list is used by all Thai health insurance schemes, including the Civil Servant Medical Benefit Scheme (CSMBS) for government officers and their dependents (9%), the Social Security Scheme (SSS) for private employees (16%), and the Universal Coverage Scheme (UCS) for the general population (75%) [23]. The decision-making process to add a drug to the NLEM, especially one associated with high costs, requires the previous collection of efficacy, safety, cost-effectiveness, and budget impact information [24]. Although three cost-utility analyses of DMT in Thai patients with MS suggested that DMT would not be cost-effective at the willingness-to-pay (WTP) threshold estimated for the country of 160,000 THB (4,214 USD) per quality adjusted life year (QALY) gained [25,26,27], the cost-effectiveness of rituximab as an alternative has not yet been investigated. Therefore, the objective of this study was to evaluate the cost-utility of rituximab compared with best supportive care (BSC), the current standard practice for the treatment of patients with MS in Thailand. In addition, the budget impact for patients with MS if rituximab were to be included in the NLEM was estimated. The results of this study could inform decision-making on the potential inclusion of rituximab in the NLEM.
Methods
Study design
A Markov model was developed to evaluate the cost-utility of rituximab in the treatment of MS compared with BSC based on a societal perspective, as recommended by the guidelines for Health Technology Assessment (HTA) in Thailand [28]. Target population was the cohort of newly diagnosed relapsing-remitting multiple sclerosis (RRMS) patients above 18 years of age.
Interventions and comparator
Both rituximab and a rituximab biosimilar were included in the analysis as alternative treatment options. The dose of rituximab and the biosimilar drug was based on a standard fixed-dose regimen starting with an induction injection of 1000 mg at day 1 and day 14, repeated every six months until patients reached the Expanded Disability Status Scale (EDSS) score of 6.0. The difference between rituximab and biosimilar drugs in our study was the annual cost of the medication i.e., 100,000 THB (2,633 USD) versus 27,000 THB (711 USD), respectively.
Decision-analytic model
Model structure
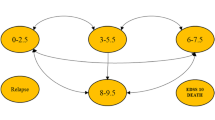
The Markov model used in this study was modified from a previously published cost-utility analysis of MS treatment in Thailand [26]. Face validity of the model was assessed through consultations with two clinicians with expertise in MS disease progression and two health economists with cost-effectiveness modeling expertise. These experts provided judgments on the appropriateness of the Markov model and input parameters, model structural uncertainty, computerized model, data sources, and model outcomes. Figure 1 shows the model structure that simulated MS patients’ clinical courses, and estimated costs and health outcomes over a lifetime horizon with a cycle length of one month. Five primary health states based on the EDSS were considered, including (i) patients without disability or mild disability (EDSS 0.0-2.5), (ii) patients with moderate disability or ambulatory limitation (EDSS 3.0-5.5), (iii) patients with severe disability who required walking aids or wheelchairs (EDSS 6.0-7.5), (iv) patients confined to bed (EDSS 8.0–9.5) and (v) deceased MS patients. Two additional health states related to relapse were defined, including (vi) patients without disability or mild disability (EDSS 0.0–2.5) and (vii) patients with moderate disability or ambulatory limitation (EDSS 3.0-5.5). The arrows in Fig. 1 denote permissible transitions between health states within the model.
Schematic Markov model for multiple sclerosis (MS) treatments. MS = multiple sclerosis, EDSS = Expanded Disability Status Scale, p_relapse = transition probability of progressing from EDSS 0.0–2.5 to relapse EDSS 0.0–2.5 (and vice-versa), or; transition probability of progressing from EDSS 3.0–5.5 to relapse EDSS 3.0–5.5 (and vice-versa); p_edss3, p_edss6, and p_edss 8 = transition probabilities of progressing from earlier EDSS health state to EDSS 3.0–5.5, to EDSS 6.0–7.5, and EDSS 8.0–9.5, respectively; p_death_edss0, p_death_edss3, or p_death_edss6; and p_edss10 = transition probabilities of progressing to death from EDSS or Relapse EDSS 0.0–2.5, from EDSS or Relapse EDSS 3.0–5.5, from EDSS 6.0–7.5, and from EDSS 8.0–9.5, respectively
Model assumptions
There are several assumptions in this model. First, it is assumed that all patients would experience all the EDSS health states. Second, patients would terminate treatment after reaching the EDSS 6.0-7.5 health state, which indicates progressive disease. According to current clinical practice guidelines in Thailand, patients who progress to a health state with an EDSS above 6.0 would receive BSC as standard treatment. Third, the same transition probability of relapse was applied to patients with EDSS 0.0-2.5 and EDSS 3.0-5.5. Fourth, the probability of relapse was considered diminished after patients with EDSS 6.0-7.5 and EDSS 8.0-9.5. Fifth, either rituximab or a biosimilar drug was considered to be first-line treatments, and both were assumed to have similar efficacy. However, the use of rituximab as a first-line agent may not be standard clinical practice in some settings as immunosuppressive agents such as azathioprine or methotrexate, which have lower associated costs, may be preferred in patients with MS [9]. Nevertheless, previous studies have shown that the clinical efficacy of immunosuppressive therapy for MS is lower compared to that of rituximab [29, 30]. Therefore, in this study, we excluded immunosuppressive agents. Sixth, patients could not transition to a health state associated with a lower EDSS, reflecting the chronic progression of MS over time. Seventh, the model did not specify the clinical point at which patients progressed to secondary progressive MS.
Model parameters
The input parameters used in the model were classified into four groups, as follows: (1) epidemiological data and transition probabilities, (2) effectiveness of treatment, (3) cost data, and (4) utility data. The parameter values are presented in Table 1.
Epidemiological data and transition probabilities
Based on our systematic review, the transition probabilities for MS progression and relapse were obtained from published studies conducted on a prospective longitudinal cohort of MS patients with long follow up period [31,32,33]. The transition probability for MS relapse was retrieved from a previous study in which MS patients were prospectively followed up for one to five years [31], and those of disease progression for MS patients receiving BSC were obtained from a cohort study that prospectively investigated time to progression classified by EDSS scores among 1,099 MS patients receiving care at University Hospital, London, Canada, between 1972 and 1984 [32]. In addition, the transition probabilities of death due to MS from EDSS 0.0–2.5 and EDSS 3.0–5.5 were obtained from a study in which 806 RRMS patients receiving treatment at the London Multiple Sclerosis Clinic in Canada were followed up for 28 years [33]. Data on the efficacy of rituximab to prevent relapse compared with placebo in RRMS were obtained from a single phase II randomized controlled trial in 2008 [34]. In addition, the probability of disability progression in rituximab-treated MS patients was retrieved from a phase III randomized controlled trial that represented the most recent study comparing rituximab with dimethyl fumarate [35]. The efficacy of rituximab to prevent relapse was not derived from this study because our specific objective was to compare the effect of rituximab therapy with that of no DMT. Finally, we assumed that the efficacy and safety of rituximab biosimilar were similar to those reported in the original clinical trials for rituximab, since no data involving the direct comparison of these parameters between rituximab and biosimilar drugs in the treatment of RRMS was available.
Cost data
Based on a societal perspective, both direct medical and non-medical costs corresponding to patients with MS in Thailand were included and obtained from a previously published cross-sectional study of patients with MS recruited between March 1, 2011 and September 30, 2014 [26]. Direct non-medical costs incurred by MS patients and their families included costs of transportation, food, accommodation, facility modification, required equipment, as well as formal and informal care [26]. Direct medical costs included all treatment costs except those of DMT. The cost of rituximab was obtained from the reference price database of the Division of National Drug Information (NDI), Ministry of Public Health [36], whereas the cost of biosimilar rituximab was retrieved from the hospital drug list of the Neurological Institute of Thailand [37]. The administrative cost of rituximab therapy was based on a standard fixed-dose regimen starting with induction rituximab 1000 mg at day1 and day 14, repeated every six months until patients reached an EDSS score of 6.0. Therefore, the cost of rituximab in the model was the average cost during a five-year administration period. Data on rituximab-related complications in patients with MS, including infusion-related reactions, severe and non-severe infections, and agranulocytosis, were obtained from a previously published meta-analysis [11]. In addition, the costs of each rituximab-related complication and rescue therapy were obtained from the Standard Cost List for Health Technology Assessment, which has been developed since 2014. The list contains a reference unit cost including direct medical, direct non-medical, and indirect costs and has been previously applied in cost analysis to better reflect costs in Thailand [38]. The consumer price index was applied to adjust all the costs to 2022 values, and the exchange rate of 37.97 THB to one USD was used.
Utility data
Following a systematic search, we obtained the utility data from a previous study by Siritho et al. [7], as this was the only cross-sectional study reporting the utility for Thai patients with MS classified by EDSS scores that we were able to identify. However, due to the fact that this study excluded Thai MS patients with relapse [7], we adopted the disutility due to MS relapse from a previously published study in the UK [39]. Health outcomes are presented in terms of QALYs, multiplication of life years (LYs), and utility scores.
Discount rate
Future costs and outcomes were adjusted to present values using a discount rate of 3%, as recommended by the guidelines for HTA in Thailand [28].
Results presentation
Total cost, LYs and QALYs associated to the three alternatives (BSC, rituximab and biosimilar drug) were estimated. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the incremental cost by the incremental QALYs associated with rituximab or the biosimilar drug in reference to BSC. Accordingly, the ICER was presented as cost (in THB) per QALY gained. The ICER results were compared with a WTP threshold of 160,000 THB (5,128 USD) per QALY gained, as recommended by the Subcommittee for the Development of the NLEM [28]. Data were analyzed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
Uncertainty analysis
Parameter uncertainty
One-way and probabilistic sensitivity analyses (PSA) were performed to address the sensitivity of the model to each of the input parameters. One-way sensitivity analysis was performed by varying each input parameter within its 95% CI, and a Tornado diagram was used to present the resulting range of ICER values. In addition, PSA was performed to simultaneously evaluate the uncertainty of all parameters using a 1,000 Monte Carlo simulation. Consequently, the cost-effectiveness acceptability curve (CEAC) was used to determine the probabilities of each alternative being cost-effective relative to a specific WTP threshold.
Budget impact analysis
A budget impact analysis from the government’s perspective was also conducted to assess the affordability of offering rituximab therapy to patients with MS. A Markov model-based approach was used to calculate the budget impact of replacing BSC with rituximab therapy in patients with MS from a governmental perspective during five consecutive fiscal years. The total budget was calculated by multiplying the number of patients by the direct medical costs per patient. We estimated the number of patients with MS in 2021 using the data on prevalence (0.201 per 100,000 individuals) and incidence (0.073 per 100,000 individuals) of MS in Thailand reported in 2012 [5], yielding approximately 572 patients with MS in 2021 and 51 new cases per year. The budget impact was estimated under the assumptions that no discounts were applied, the incidence rate of MS was stable, and the treatment coverage rate was 90%.
Results
Cost-utility analysis
Markov model simulated a scenario in which MS patients received BSC, rituximab, or a biosimilar drug for a period of 30 years. The estimated total costs, LYs, QALYs, and ICER are shown in Table 2. Rituximab and its biosimilar increased both LYs and QALYs gained by 0.34 and 1.79, respectively. However, rituximab increased the cost of treatment by 779,984 THB (20,542 USD), whereas in the case of the biosimilar drug the cost decreased by 237,469 THB (6,254 USD) compared to that of BSC. The ICER of rituximab was approximately 434,666 THB (11,447 USD) per QALY gained, and 2,265,882 THB (59,676 USD) per LY gained. On the other hand, the ICER of the rituximab biosimilar showed a dominant result indicating more effectiveness and a lower cost compared with BSC.
Uncertainty analysis
The one-way sensitivity analysis results for the rituximab biosimilar are presented in Fig. 2. The ICER was most sensitive to the probability of relapse, followed by the probability of progression from EDSS 0.0-2.5 to EDSS 3.0-5.5, direct non-medical costs for EDSS 6.0-7.5, the probability of progression from EDSS 3.0–5.5 to EDSS 6.0–7.5, direct non-medical cost for EDSS 6.0-7.5, the cost of rituximab therapy (a 20% change from the base case), and the efficacy of rituximab to prevent relapse.
Figure 3 shows the cost-effectiveness acceptability curves for all treatment options. The rituximab biosimilar had the highest probability of being cost-effective (70%) at a willingness-to-pay (WTP) threshold of 160,000 THB (4,214 USD), whereas that of BSC was approximately 30%.
Budget impact analysis
The budget impact from a governmental perspective over five years is shown in Fig. 4. The incremental budget was approximately 26,360,000 THB (844,255 USD), followed by 23,080,000 THB (739,388 USD), 22,400,000 THB (717,603 USD), 21,640,000 THB (693,223 USD), and 20,810,000 THB (666,608 USD) during five consecutive fiscal years, respectively.
Discussion
Regardless of the evidence indicating that DMT can prevent hospitalization and relapse as well as decrease disability in MS patients [8], most MS patients in Thailand are still unable to afford these expensive treatments, especially in low- or low-to- middle-income countries. In Thailand, DMT has not been included in the NLEM, as previous cost-effectiveness studies have suggested that it is not cost-effective. Currently, rituximab in both original and biosimilar forms has been approved for the treatment of lymphoma and rheumatoid arthritis but not MS in Thailand. Nevertheless, it has been used as an off-label treatment for patients with MS in clinical practice. Given that no cost-effectiveness information regarding the use of rituximab for MS treatment specific to Thailand has been reported, the drug has not been included in the NLEM, a pharmaceutical reimbursement list for all health insurance schemes in the country. Therefore, the Subcommittee for the Development of NLEM requested cost-effectiveness information on rituximab in order to consider whether it should be included into the NLEM. To the best of our knowledge, this study is the first to evaluate the cost-utility of rituximab compared to BSC in Thai patients with MS.
Our study suggests that the rituximab biosimilar would be dominant or cost-saving, indicating a lower cost but a higher effectiveness compared to BSC for MS treatment in Thailand. This study also demonstrated that the rituximab biosimilar has a high probability of being cost-effective compared with BSC, the current standard of treatment in the Thai healthcare system. The estimated budget impact of the rituximab biosimilar in patients with MS was approximately 25,000,000 THB per year during five consecutive fiscal years. In contrast, the original rituximab would not be cost-effective in the Thai context compared to BSC. Consequently, the results of this study could help inform policymakers on the potential advantages of including the rituximab biosimilar in the NLEM for MS treatment.
In support of this recommendation, it is worth mentioning that the use of rituximab in patients with MS is a common clinical practice in developed countries. For example, the efficacy of rituximab in reducing MS relapse has been supported by phase I and II clinical trials since 2008 [34, 40]. However, although phase III clinical trials on the effectiveness of this drug to manage patients with MS have not been performed, it has nevertheless been regularly prescribed to these patients during the past five years in several countries, including Sweden [18], the US [16, 20], Spain [17], Switzerland [15], France [12], Italy [13], Germany [41] and Lebanon [14]. Notably, based on the clinical guidelines of the Middle East and North Africa Committee (MENACTRIMS) Consensus, rituximab is recommended as an off-label treatment for patients with highly active MS disease. Moreover, it should be administered as an escalation treatment to patients with all levels of MS in special populations such as refugees or in countries where other proper MS treatments are not accessible [42]. In addition, according to the most recently updated guidelines from the American Academy of Neurology (AAN) published in 2018, rituximab is possibly more effective than placebo in reducing the risk of relapse at one year for patients with RRMS [43]. The efficacy of rituximab compared with the FDA-approved DMT, (i.e., dimethyl fumarate) has been confirmed through a phase III randomized controlled trial [35]. Therefore, this study can be considered as additional evidence on the advantages of including a rituximab biosimilar in the NLEM for the specific purpose of MS treatment. However, several considerations should be considered by physicians before prescribing rituximab or a biosimilar drug to MS patients, including side effects, disease activity, and off-label medication use in the treatment of MS.
Furthermore, the one-way sensitivity analysis indicated that the most sensitive parameter was the probability of relapse. The results indicate that treating patients with MS that experience frequent relapses may be a cost-saving intervention compared with BSC. In contrast, rituximab biosimilar would not be cost-effective in patients with few relapses. This could be explained by the fact that rituximab is particularly effective in patients with high disease activity. This is in line with the recommendations of several MS treatment guidelines [43, 44], as physicians tend to use DMT only in MS patients with active disease.
Some limitations of this study need to be addressed. First, the transitional probabilities of rituximab to prevent relapse and disability progression were obtained from only two randomized controlled studies, which were the best available evidences on the effect of rituximab in patients with MS to date [34, 35]. In 2008, a phase II double-blind randomized controlled trial [34] revealed the safety and efficacy of rituximab compared with placebo in patients with RRMS; more recently, a phase III randomized controlled trial from 2022 demonstrated that rituximab is safe and effective compared with dimethyl fumarate [35]. Second, we assumed that rituximab and its biosimilar had similar efficacy for MS treatment, since no head-to-head comparison between the two for the specific purpose of MS treatment is available in the literature. Nevertheless, the efficacy of the rituximab biosimilar in patients with diffuse large B-cell lymphoma [21] and severe rheumatoid arthritis [22] has been shown to be similar in pharmacokinetic studies. Third, due to the lack of data from Thailand, all transition probabilities for each health state were obtained from prospective longitudinal cohort studies published in other countries. Therefore, these parameters should be collected from local data in future studies. Fourth, as there is no data on disutility due to MS relapse available in Thailand, we obtained the relevant data from a UK study [39], which is the only available source to date. Thus, the utility of MS patients with relapse in Thailand should be the focus of future studies. Finally, it should be noted that as there is no clinical data of MS patients in Thailand, we could not validate the prediction of the model. However, we performed model validation via the face validity method through consultations with clinical experts and health economists on the appropriateness of model structure, parameters used, and data sources. Model validation should be performed in future studies. Moreover, we did not investigate how appropriate the cut-off scores were in the Markov model, which warrants further research.
In conclusion, this study demonstrated that, in the context of the Thailand healthcare system, treatment with a rituximab biosimilar was cost-saving and exhibited a high probability of being cost-effective when compared with the current practice. The estimated budget impact of treating patients with active RRMS were 26,360,000 THB (844,255 USD), followed by 23,080,000 THB (739,388 USD), 22,400,000 THB (717,603 USD), 21,640,000 THB (693,223 USD), and 20,810,000 THB (666,608 USD) during five consecutive fiscal years, respectively. This study may encourage policy decision makers to extend the indications of including rituximab biosimilar in the NLEM to treat patients with MS.
Data Availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BSC:
-
Best Supportive Care
- CEAC:
-
cost-effectiveness acceptability curve
- CNS:
-
Central Nervous System
- CSMBS:
-
Civil Servant Medical Benefit Scheme
- DMT:
-
Disease-modifying therapy
- EDSS:
-
Expanded Disability Status Scale
- FDA:
-
Food and Drug Administration
- HTA:
-
Health Technology Assessment
- LYs:
-
Life years
- MSIF:
-
Multiple Sclerosis International Federation
- NDI:
-
Division of National Drug Information
- NLEM:
-
National List of Essential Medicines
- QALY:
-
Quality-adjusted life years
- SSS:
-
Social Security Scheme
- THB:
-
Thai baht
- UCS:
-
Universal Coverage Scheme
- USD:
-
United States dollars
- WTP:
-
Willingness-to-pay
References
Howard J, Trevick S, Younger DS. Epidemiology of multiple sclerosis. Neurol Clin. 2016;34:919–39.
GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol. 2019;18:269–85.
Lane J, Ng HS, Poyser C, Lucas RM, Tremlett H. Multiple sclerosis incidence: a systematic review of change over time by geographical region. Mult Scler Relat Disord. 2022;63:103932.
Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013;13:128.
Prayoonwiwat N, Apiwattanakul M, Pasogpakdee P, Sirithon S, Chanatittarat C, Chaikledkaew U. Prevalence of idiopathic inflammatory demyelinating central nervous system disorder in Thailand. In:. In: Pan Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS) Taiwan In: Pan Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS). Taiwan; 2013.
García-Domínguez JM, Maurino J, Martínez-Ginés ML, Carmona O, Caminero AB, Medrano N, et al. Economic burden of multiple sclerosis in a population with low physical disability. BMC Public Health. 2019;19:609.
Siritho S, Thavorncharoensap M, Chanatittarat C, Pasogpakdee P, Apiwattanakul M, Prayoonwiwat N, et al. Health utilities of patients with multiple sclerosis and neuromyelitis optica spectrum disorders in Thailand. Mult Scler Relat Disord. 2018;24:151–6.
Ziemssen T, Derfuss T, de Stefano N, Giovannoni G, Palavra F, Tomic D, et al. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263:1053–65.
Laurson-Doube J, Rijke N, Helme A, Baneke P, Banwell B, Viswanathan S, et al. Ethical use of off-label disease-modifying therapies for multiple sclerosis. Mult Scler. 2021;27:1403–10.
Brancati S, Gozzo L, Longo L, Vitale DC, Drago F. Rituximab in multiple sclerosis: are we ready for regulatory approval? Front Immunol. 2021;12:661882.
Hu Y, Nie H, Yu HH, Qin C, Wu LJ, Tang ZP, et al. Efficacy and safety of rituximab for relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. Autoimmun Rev. 2019;18:542–8.
Durozard P, Maarouf A, Boutiere C, Ruet A, Brochet B, Vukusic S, et al. Efficacy of rituximab in refractory RRMS. Mult Scler. 2019;25:828–36.
D’Amico E, Zanghì A, Chisari CG, Fermo SL, Toscano S, Arena S, et al. Effectiveness and safety of rituximab in demyelinating diseases spectrum: an italian experience. Mult Scler Relat Disord. 2019;27:324–6.
Yamout BI, El-Ayoubi NK, Nicolas J, El Kouzi Y, Khoury SJ, Zeineddine MM. Safety and efficacy of rituximab in multiple sclerosis: a retrospective observational study. J Immunol Res. 2018;2018:9084759.
Scotti B, Disanto G, Sacco R, Guigli M, Zecca C, Gobbi C. Effectiveness and safety of rituximab in multiple sclerosis: an observational study from Southern Switzerland. PLoS ONE. 2018;13:e0197415.
Alldredge B, Jordan A, Imitola J, Racke MK. Safety and efficacy of rituximab: experience of a single multiple sclerosis center. Clin Neuropharmacol. 2018;41:56–9.
Alcalá C, Gascón F, Pérez-Miralles F, Gil-Perotín S, Navarré A, Boscá I, et al. Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol. 2018;265:1690–7.
Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, Fink K, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87:2074–81.
Berenguer-Ruiz L, Sempere AP, Gimenez-Martinez J, Gabaldon-Torres L, Tahoces L, Sanchez-Perez R, et al. Rescue therapy using rituximab for multiple sclerosis. Clin Neuropharmacol. 2016;39:178–81.
Barra ME, Soni D, Vo KH, Chitnis T, Stankiewicz JM. Experience with long-term rituximab use in a multiple sclerosis clinic. Mult Scler J Exp Transl Clin. 2016;2:2055217316672100.
Viswabandya A, Shah S, Mukhopadhyay A, Nagarkar RV, Batra SS, Lopez-Lazaro L, et al. Randomized, double-blind, pharmacokinetic equivalence trial comparing DRL-rituximab with MabThera in patients with diffuse large B-cell lymphoma. J Glob Oncol. 2019;5:1–13.
Haridas VM, Katta R, Nalawade A, Kharkar S, Zhdan V, Garmish O, et al. Pharmacokinetic similarity and comparative pharmacodynamics, safety, efficacy, and immunogenicity of DRL_RI versus reference rituximab in biologics-naïve patients with moderate-to-severe rheumatoid arthritis: a double-blind, randomized, three-arm study. BioDrugs. 2020;34:183–96.
Paek SC, Meemon N, Wan TTH. Thailand’s universal coverage scheme and its impact on health-seeking behavior. Springerplus. 2016;5:1952.
Tanvejsilp P, Taychakhoonavudh S, Chaikledkaew U, Chaiyakunapruk N, Ngorsuraches S. Revisiting roles of health technology assessment on drug policy in universal health coverage in Thailand: where are we? And what is next? Value Health Reg Issues. 2019;18:78–82.
Wongphan T. Cost-effectiveness of treatment of multiple sclerosis with fingolimod compared to other treatments. In: Thailand: The International Health Policy Program (IHPP); 2015.
Chanatittarat C, Chaikledkaew U, Prayoonwiwat N, Siritho S, Pasogpakdee P, Apiwattanakul M, et al. Economic burden of Thai patients with inflammatory demyelinating central nervous system disorders (IDCDs). Pharm Sci Asia. 2019;46:260–9.
Kingkaew P. Cost utility and budget impact analysis of disease modify therapy in relapsing-remitting multiple sclerosis. In:; 2020.
Working Group on the Guidelines for. Health Technology Assessment in Thailand. Guidelines for health technology assessment in Thailand. 2nd ed. Thailand: Nonthaburi; 2013.
Okuda DT. Chap. 23. Immunosuppressive treatments in multiple sclerosis. In: Goodin DS, editor. Handbook of clinical neurology, volume 122. Elsevier; 2014. pp. 503–11.
Casetta I, Iuliano G, Filippini G. Azathioprine for multiple sclerosis. Cochrane Database Syst Rev. 2007;2007:CD003982.
Weinshenker BG, Bass B, Rice GPA, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study: I. Clinical course and disability. Brain. 1989;112:133–46.
Goodkin DE, Hertsgaard D, Rudick RA. Exacerbation rates and adherence to disease type in a prospectively followed-up population with multiple sclerosis. Implications for clinical trials. Arch Neurol. 1989;46:1107–12.
Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133:1914–29.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88.
Svenningsson A, Frisell T, Burman J, Salzer J, Fink K, Hallberg S, et al. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing-remitting multiple sclerosis or clinically isolated syndrome in Sweden: a rater-blinded, phase 3, randomised controlled trial. Lancet Neurol. 2022;21:693–703.
Ministry of Public Health. Reference price of rituximab in 2019. https://ndi.fda.moph.go.th/drug_value/index/public/0/1080. Accessed Jan 28 2023.
Neurological Institute of Thailand. Hospital drug list; 2021.
Riewpaiboon A. Standard cost lists for health economic evaluation in Thailand. J Med Assoc Thai. 2014;97:127–34.
Hawton A, Green C. Health utilities for multiple sclerosis. Value Health. 2016;19:460–8.
Bar-Or A, Calabresi PAJ, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400.
Ellrichmann G, Bolz J, Peschke M, Duscha A, Hellwig K, Lee DH, et al. Peripheral CD19 + B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J Neurol. 2019;266:57–67.
Filippini G, Kruja J, Del Giovane C. Rituximab for people with multiple sclerosis. Cochrane Database Syst Rev. 2021;11:CD013874.
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:789–800.
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120.
Acknowledgements
The authors would like to thank Mr. Nattakrit Tongpoonsakdi for conducting a comprehensive language review of this manuscript before submitting to Elsevier language editing center.
Funding
This work is part of the training towards a PhD degree in Health Technology Assessment (HTA), for which a scholarship is provided by Mahidol University and the International Decision Support Initiative (iDSI) through a grant awarded to UC (OPP1087363). This study was conducted as part of the International Decision Support Initiative (www.idsihealth.org), which supports countries in obtaining the best value of money from health spending. The iDSI receives funding support from the Bill & Melinda Gates Foundation, the UK Department for International Development, and the Rockefeller Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.A. contributes to methodology, investigation, data curation, formal analysis, writing and revising the manuscript. U.C. is responsible for conceptualization, supervision, formal analysis, as well as writing and revising the manuscript. S.T., S.Y., M.A., A.T. are responsible for supervision and writing and revising the manuscript. All authors contribute the interpretation of findings and have approved the revised manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Institutional Review Board (IRB) of the Neurological Institute of Thailand (Approval number 63026). The need for informed consent for this study was waived by the Institutional Review Board (IRB) of the Neurological Institute of Thailand (Approval number 63026), as all data were obtained from published studies. All procedures performed in the study were in compliance with the international guidelines for human research protection such as Declaration of Helsinki, the Belmont Report.
Consent for publication
Not applicable. No individual participant data is present.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aungsumart, S., Turongkaravee, S., Youngkong, S. et al. Rituximab for the treatment of relapsing-remitting multiple sclerosis in Thailand: an economic evaluation and budget impact analysis. BMC Health Serv Res 23, 1096 (2023). https://doi.org/10.1186/s12913-023-10099-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-10099-1