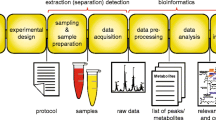
Abstract
Lipidomics is a subfield of metabolic phenotyping that focuses on high-throughput profiling and quantification of lipids. Essential roles of lipidomics in translational and clinical research have emerged, especially over the past decade. Most lipidomic pipelines have been developed using mass spectrometry (MS)-based methods. Because of the complexity of the data, generally, computational demands are much higher in untargeted lipidomic studies. In the current paper, we primarily discussed the recent advances in untargeted liquid chromatography–mass spectrometry-based lipidomics, covering various facets from analytical strategies to functional interpretations. The current practice of tandem MS-based lipid annotation in untargeted lipidomics studies was demonstrated. Notably, we highlighted the essential characteristics of machine learning models, together with a data partitioning strategy, to facilitate appropriate modeling and validation in metabolic phenotyping studies. Critical aspects of data sharing were briefly mentioned. Finally, certain recommendations were suggested toward more standardized and sustainable lipidomics analysis strategies as independent platforms, and as members of the omics family.



Similar content being viewed by others
References
van Meer G, de Kroon AIPM. Lipid map of the mammalian cell. J Cell Sci. 2011;124(1):5. https://doi.org/10.1242/jcs.071233.
O'Donnell VB, Ekroos K, Liebisch G, Wakelam M. Lipidomics: current state of the art in a fast moving field. WIREs Syst Biol Med. 2020;12(1):e1466. https://doi.org/10.1002/wsbm.1466.
Holčapek M, Liebisch G, Ekroos K. Lipidomic analysis. Anal Chem. 2018;90(7):4249–57. https://doi.org/10.1021/acs.analchem.7b05395.
Yang K, Han X. Lipidomics: Techniques, applications, and outcomes related to biomedical sciences. Trends Biochem Sci. 2016;41(11):954–69. https://doi.org/10.1016/j.tibs.2016.08.010.
Nguyen A, Rudge SA, Zhang Q, Wakelam MJO. Using lipidomics analysis to determine signalling and metabolic changes in cells. Curr Opin Biotechnol. 2017;43:96–103. https://doi.org/10.1016/j.copbio.2016.10.003.
Lv J, Zhang L, Yan F, Wang X. Clinical lipidomics: a new way to diagnose human diseases. Clin Transl Med. 2018;7(1):12. https://doi.org/10.1186/s40169-018-0190-9.
Zhang L, Han X, Wang X. Is the clinical lipidomics a potential goldmine? Cell Biol Toxicol. 2018;34(6):421–3. https://doi.org/10.1007/s10565-018-9441-1.
Kohlwein SD. Opinion articles on lipidomics—a critical assessment of the state-of-the-art. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):729–30. https://doi.org/10.1016/j.bbalip.2017.05.009.
Cajka T, Fiehn O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal Chem. 2014;61:192–206. https://doi.org/10.1016/j.trac.2014.04.017.
Stevens VL, Hoover E, Wang Y, Zanetti KA. Pre-analytical factors that affect metabolite stability in human urine, plasma, and serum: a review. Metabolites. 2019;9(8):156. https://doi.org/10.3390/metabo9080156.
Bowden JA, Ulmer CZ, Jones CM, Koelmel JP, Yost RA. NIST lipidomics workflow questionnaire: an assessment of community-wide methodologies and perspectives. Metabolomics. 2018;14(5):53. https://doi.org/10.1007/s11306-018-1340-1.
Liebisch G, Ahrends R, Arita M, Arita M, Bowden JA, Ejsing CS, Griffiths WJ, Holčapek M, Köfeler H, Mitchell TW, Wenk MR, Ekroos K. Lipidomics needs more standardization. Nat Metab. 2019;1(8):745–7. https://doi.org/10.1038/s42255-019-0094-z.
Vuckovic D. Improving metabolome coverage and data quality: advancing metabolomics and lipidomics for biomarker discovery. Chem Commun. 2018;54(50):6728–49. https://doi.org/10.1039/C8CC02592D.
Vale G, Martin SA, Mitsche MA, Thompson BM, Eckert KM, McDonald JG. Three-phase liquid extraction: a simple and fast method for lipidomic workflows. J Lipid Res. 2019;60(3):694–706. https://doi.org/10.1194/jlr.D090795.
Sostare J, Di Guida R, Kirwan J, Chalal K, Palmer E, Dunn WB, Viant MR. Comparison of modified Matyash method to conventional solvent systems for polar metabolite and lipid extractions. Anal Chim Acta. 2018;1037:301–15. https://doi.org/10.1016/j.aca.2018.03.019.
Löfgren L, Forsberg G-B, Ståhlman M. The BUME method: a new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci Rep. 2016;6(1):27688. https://doi.org/10.1038/srep27688.
Danne-Rasche N, Coman C, Ahrends R. Nano-LC/NSI MS refines lipidomics by enhancing lipid coverage, measurement sensitivity, and linear dynamic range. Anal Chem. 2018;90(13):8093–101. https://doi.org/10.1021/acs.analchem.8b01275.
Vasilopoulou CG, Sulek K, Brunner A-D, Meitei NS, Schweiger-Hufnagel U, Meyer SW, Barsch A, Mann M, Meier F. Trapped ion mobility spectrometry and PASEF enable in-depth lipidomics from minimal sample amounts. Nat Commun. 2020;11(1):331. https://doi.org/10.1038/s41467-019-14044-x.
Hutchins PD, Russell JD, Coon JJ. Accelerating lipidomic method development through in silico simulation. Anal Chem. 2019;91(15):9698–706. https://doi.org/10.1021/acs.analchem.9b01234.
Xuan Q, Zheng F, Yu D, Ouyang Y, Zhao X, Hu C, Xu G. Rapid lipidomic profiling based on ultra-high performance liquid chromatography–mass spectrometry and its application in diabetic retinopathy. Anal Bioanal Chem. 2020. https://doi.org/10.1007/s00216-020-02632-6.
Surowiec I, Johansson E, Stenlund H, Rantapää-Dahlqvist S, Bergström S, Normark J, Trygg J. Quantification of run order effect on chromatography–mass spectrometry profiling data. J Chromatogr A. 2018;1568:229–34. https://doi.org/10.1016/j.chroma.2018.07.019.
Lam SM, Tian H, Shui G. Lipidomics, en route to accurate quantitation. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):752–61. https://doi.org/10.1016/j.bbalip.2017.02.008.
Cajka T, Smilowitz JT, Fiehn O. Validating quantitative untargeted lipidomics across nine liquid chromatography–high-resolution mass spectrometry platforms. Anal Chem. 2017;89(22):12360–8. https://doi.org/10.1021/acs.analchem.7b03404.
Barupal DK, Fan S, Wancewicz B, Cajka T, Sa M, Showalter MR, Baillie R, Tenenbaum JD, Louie G, Alzheimer’s Disease Neuroimaging Initiative, Alzheimer’s Disease Metabolomics Consortium, Kaddurah-Daouk R, Fiehn O. Generation and quality control of lipidomics data for the alzheimer’s disease neuroimaging initiative cohort. Sci Data. 2018;5(1):180263. https://doi.org/10.1038/sdata.2018.263.
Kirwan JA, Weber RJM, Broadhurst DI, Viant MR. Direct infusion mass spectrometry metabolomics dataset: a benchmark for data processing and quality control. Sci Data. 2014;1(1):140012. https://doi.org/10.1038/sdata.2014.12.
Lange M, Fedorova M. Evaluation of lipid quantification accuracy using HILIC and RPLC MS on the example of NIST® SRM® 1950 metabolites in human plasma. Anal Bioanal Chem. 2020. https://doi.org/10.1007/s00216-020-02576-x.
Tague ED, Woodall BM, Harp JR, Farmer AT, Fozo EM, Campagna SR. Expanding lipidomics coverage: effective ultra performance liquid chromatography-high resolution mass spectrometer methods for detection and quantitation of cardiolipin, phosphatidylglycerol, and lysyl-phosphatidylglycerol. Metabolomics. 2019;15(4):53. https://doi.org/10.1007/s11306-019-1512-7.
Züllig T, Trötzmüller M, Köfeler HC. Lipidomics from sample preparation to data analysis: a primer. Anal Bioanal Chem. 2020;412(10):2191–209. https://doi.org/10.1007/s00216-019-02241-y.
Tsugawa H, Ikeda K, Arita M. The importance of bioinformatics for connecting data-driven lipidomics and biological insights. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):762–5. https://doi.org/10.1016/j.bbalip.2017.05.006.
Kind T, Liu K-H, Lee DY, DeFelice B, Meissen JK, Fiehn O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat Methods. 2013;10(8):755–8. https://doi.org/10.1038/nmeth.2551.
Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35(suppl_2):W606–W612612. https://doi.org/10.1093/nar/gkm324.
Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, VanderGheynst J, Fiehn O, Arita M. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods. 2015;12(6):523–6. https://doi.org/10.1038/nmeth.3393.
Koelmel JP, Kroeger NM, Ulmer CZ, Bowden JA, Patterson RE, Cochran JA, Beecher WWC, Garrett TJ, Yost RA. LipidMatch: an automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinform. 2017;18(1):331. https://doi.org/10.1186/s12859-017-1744-3.
Alcoriza-Balaguer MI, García-Cañaveras JC, López A, Conde I, Juan O, Carretero J, Lahoz A. LipidMS: an R package for lipid annotation in untargeted liquid chromatography-data independent acquisition-mass spectrometry lipidomics. Anal Chem. 2019;91(1):836–45. https://doi.org/10.1021/acs.analchem.8b03409.
Hutchins PD, Russell JD, Coon JJ. LipiDex: an integrated software package for high-confidence lipid identification. Cell Syst. 2018;6(5):621–5.e5. https://doi.org/10.1016/j.cels.2018.03.011.
Koelmel PJ, Li X, Stow MS, Sartain JM, Murali A, Kemperman R, Tsugawa H, Takahashi M, Vasiliou V, Bowden JA, Yost RA, Garrett TJ, Kitagawa N. Lipid annotator: towards accurate annotation in non-targeted liquid chromatography high-resolution tandem mass spectrometry (LC-HRMS/MS) lipidomics using a rapid and user-friendly software. Metabolites. 2020;10:3. https://doi.org/10.3390/metabo10030101.
Hartler J, Triebl A, Ziegl A, Trötzmüller M, Rechberger GN, Zeleznik OA, Zierler KA, Torta F, Cazenave-Gassiot A, Wenk MR, Fauland A, Wheelock CE, Armando AM, Quehenberger O, Zhang Q, Wakelam MJO, Haemmerle G, Spener F, Köfeler HC, Thallinger GG. Deciphering lipid structures based on platform-independent decision rules. Nat Methods. 2017;14(12):1171–4. https://doi.org/10.1038/nmeth.4470.
Fernandez-Lopez M, Gil-de-la-Fuente A, Godzien J, Ruperez FJ, Barbas C, Otero A. LAS: a lipid annotation service capable of explaining the annotations it generates. Comput Struct Biotechnol J. 2019;17:1113–22. https://doi.org/10.1016/j.csbj.2019.07.016.
Liebisch G, Vizcaíno JA, Köfeler H, Trötzmüller M, Griffiths WJ, Schmitz G, Spener F, Wakelam MJO. Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res. 2013;54(6):1523–30. https://doi.org/10.1194/jlr.M033506.
Pauling JK, Hermansson M, Hartler J, Christiansen K, Gallego SF, Peng B, Ahrends R, Ejsing CS. Proposal for a common nomenclature for fragment ions in mass spectra of lipids. PLoS ONE. 2017;12(11):e0188394. https://doi.org/10.1371/journal.pone.0188394.
Koelmel JP, Ulmer CZ, Jones CM, Yost RA, Bowden JA. Common cases of improper lipid annotation using high-resolution tandem mass spectrometry data and corresponding limitations in biological interpretation. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):766–70. https://doi.org/10.1016/j.bbalip.2017.02.016.
Cheng X, Jiang X, Tam KY, Li G, Zheng J, Zhang H. Sphingolipidomic analysis of C. elegans reveals development- and environment-dependent metabolic features. Int J Biol Sci. 2019;15(13):2897–910. https://doi.org/10.7150/ijbs.30499.
Kang YP, Yoon J-H, Long NP, Koo G-B, Noh H-J, Oh S-J, Lee SB, Kim HM, Hong JY, Lee WJ, Lee SJ, Hong S-S, Kwon SW, Kim Y-S. Spheroid-induced epithelial-mesenchymal transition provokes global alterations of breast cancer lipidome: a multi-layered omics analysis. Front Oncol. 2019;9:145. https://doi.org/10.3389/fonc.2019.00145.
Lee D-K, Long NP, Jung J, Kim TJ, Na E, Kang YP, Kwon SW, Jang J. Integrative lipidomic and transcriptomic analysis of X-linked adrenoleukodystrophy reveals distinct lipidome signatures between adrenomyeloneuropathy and childhood cerebral adrenoleukodystrophy. Biochem Biophys Res Commun. 2019;508(2):563–9. https://doi.org/10.1016/j.bbrc.2018.11.123.
Ni Z, Fedorova M. LipidLynxX: lipid annotations converter for large scale lipidomics and epilipidomics datasets. bioRxiv. 2020. https://doi.org/10.1101/2020.04.09.033894.
Xu L, Wang X, Jiao Y, Liu X. Assessment of potential false positives via orbitrap-based untargeted lipidomics from rat tissues. Talanta. 2018;178:287–93. https://doi.org/10.1016/j.talanta.2017.09.046.
Gathungu RM, Larrea P, Sniatynski MJ, Marur VR, Bowden JA, Koelmel JP, Starke-Reed P, Hubbard VS, Kristal BS. Optimization of electrospray ionization source parameters for lipidomics to reduce misannotation of in-source fragments as precursor ions. Anal Chem. 2018;90(22):13523–32. https://doi.org/10.1021/acs.analchem.8b03436.
Criscuolo A, Zeller M, Fedorova M. Evaluation of lipid in-source fragmentation on different orbitrap-based mass spectrometers. J Am Soc Mass Spectrom. 2020;31(2):463–6. https://doi.org/10.1021/jasms.9b00061.
Forest A, Ruiz M, Bouchard B, Boucher G, Gingras O, Daneault C, Frayne IR, Rhainds D, The iGenoMed Consortium, The NIDDK IBD Genetics Consortium, Tardif J-C, Rioux JD, Rosiers CD. Comprehensive and reproducible untargeted lipidomic workflow using LC-QTOF validated for human plasma analysis. J Proteome Res. 2018;17(11):3657–70. https://doi.org/10.1021/acs.jproteome.8b00270.
Feng Y, Chen B, Yu Q, Li L. Identification of double bond position isomers in unsaturated lipids by m-CPBA epoxidation and mass spectrometry fragmentation. Anal Chem. 2019;91(3):1791–5. https://doi.org/10.1021/acs.analchem.8b04905.
Rampler E, Criscuolo A, Zeller M, El Abiead Y, Schoeny H, Hermann G, Sokol E, Cook K, Peake DA, Delanghe B, Koellensperger G. A novel lipidomics workflow for improved human plasma identification and quantification using RPLC-MSn methods and isotope dilution strategies. Anal Chem. 2018;90(11):6494–501. https://doi.org/10.1021/acs.analchem.7b05382.
Zhang W, Shang B, Ouyang Z, Xia Y. Enhanced phospholipid isomer analysis by online photochemical derivatization and RPLC-MS. Anal Chem. 2020. https://doi.org/10.1021/acs.analchem.0c00690.
Xuan Q, Hu C, Yu D, Wang L, Zhou Y, Zhao X, Li Q, Hou X, Xu G. Development of a high coverage pseudotargeted lipidomics method based on ultra-high performance liquid chromatography–mass spectrometry. Anal Chem. 2018;90(12):7608–16. https://doi.org/10.1021/acs.analchem.8b01331.
Cajka T, Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal Chem. 2016;88(1):524–45. https://doi.org/10.1021/acs.analchem.5b04491.
Blaženović I, Kind T, Ji J, Fiehn O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites. 2018;8(2):31.
Zhou Z, Tu J, Xiong X, Shen X, Zhu ZJ. LipidCCS: prediction of collision cross-section values for lipids with high precision to support ion mobility–mass spectrometry-based lipidomics. Anal Chem. 2017;89(17):9559–666. https://doi.org/10.1021/acs.analchem.7b02625.
González-Riano C, Dudzik D, Garcia A, Gil-de-la-Fuente A, Gradillas A, Godzien J, López-Gonzálvez A, Rey-Stolle F, Rojo D, Ruperez FJ, Saiz J, Barbas C. Recent developments along the analytical process for metabolomics workflows. Anal Chem. 2020;92(1):203–26. https://doi.org/10.1021/acs.analchem.9b04553.
Di Guida R, Engel J, Allwood JW, Weber RJM, Jones MR, Sommer U, Viant MR, Dunn WB. Non-targeted UHPLC-MS metabolomic data processing methods: a comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabolomics. 2016;12(5):93. https://doi.org/10.1007/s11306-016-1030-9.
Goh WWB, Wang W, Wong L. Why batch effects matter in omics data, and how to avoid them. Trends Biotechnol. 2017;35(6):498–507. https://doi.org/10.1016/j.tibtech.2017.02.012.
Li B, Tang J, Yang Q, Li S, Cui X, Li Y, Chen Y, Xue W, Li X, Zhu F. NOREVA: normalization and evaluation of MS-based metabolomics data. Nucleic Acids Res. 2017;45(W1):W162–W170170. https://doi.org/10.1093/nar/gkx449.
Hastie T, Tibshirani R, Friedman J. Overview of supervised learning. The elements of statistical learning: data mining, inference, and prediction. New York: Springer; 2009. p. 9–41.
Posma JM. Chapter 9—multivariate statistical methods for metabolic phenotyping. In: Lindon JC, Nicholson JK, Holmes E, editors. The handbook of metabolic phenotyping. Oxford: Elsevier; 2019. p. 261–308.
Mendez KM, Broadhurst DI, Reinke SN. The application of artificial neural networks in metabolomics: a historical perspective. Metabolomics. 2019;15(11):142. https://doi.org/10.1007/s11306-019-1608-0.
Long NP, Yoon SJ, Anh NH, Nghi TD, Lim DK, Hong YJ, Hong S-S, Kwon SW. A systematic review on metabolomics-based diagnostic biomarker discovery and validation in pancreatic cancer. Metabolomics. 2018;14(8):109. https://doi.org/10.1007/s11306-018-1404-2.
Zhao X, Niu L, Clerici C, Russo R, Byrd M, Setchell KDR. Data analysis of MS-based clinical lipidomics studies with crossover design: a tutorial mini-review of statistical methods. Clin Mass Spectrom. 2019;13:5–17. https://doi.org/10.1016/j.clinms.2019.05.002.
Smilde AK, Westerhuis JA, Hoefsloot HCJ, Bijlsma S, Rubingh CM, Vis DJ, Jellema RH, Pijl H, Roelfsema F, Van Der Greef J. Dynamic metabolomic data analysis: a tutorial review. Metabolomics. 2010;6(1):3–17. https://doi.org/10.1007/s11306-009-0191-1.
Beirnaert C, Peeters L, Meysman P, Bittremieux W, Foubert K, Custers D, Van der Auwera A, Cuykx M, Pieters L, Covaci A, Laukens K. Using expert driven machine learning to enhance dynamic metabolomics data analysis. Metabolites. 2019;9(3):54.
Playdon MC, Joshi AD, Tabung FK, Cheng S, Henglin M, Kim A, Lin T, van Roekel EH, Huang J, Krumsiek J, Wang Y, Mathé E, Temprosa M, Moore S, Chawes B, Eliassen AH, Gsur A, Gunter MJ, Harada S, Langenberg C, Oresic M, Perng W, Seow WJ, Zeleznik QA. Metabolomics analytics workflow for epidemiological research: perspectives from the consortium of metabolomics studies (COMETS). Metabolites. 2019;9(7):145. https://doi.org/10.3390/metabo9070145.
Acevedo A, Durán C, Ciucci S, Gerl M, Cannistraci CV. LIPEA: lipid pathway enrichment analysis. bioRxiv. 2018. https://doi.org/10.1101/274969.
Molenaar MR, Jeucken A, Wassenaar TA, van de Lest CHA, Brouwers JF, Helms JB. LION/web: a web-based ontology enrichment tool for lipidomic data analysis. GigaScience. 2019;8:6. https://doi.org/10.1093/gigascience/giz061.
Kuo TC, Tseng YJ. LipidPedia: a comprehensive lipid knowledgebase. Bioinformatics. 2018;34(17):2982–7. https://doi.org/10.1093/bioinformatics/bty213.
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Gonzalez-Beltran A, Gray AJG, Groth P, Goble C, Grethe JS, Heringa J, ’t Hoen PAC, Hooft R, Kuhn T, Kok R, Kok J, Lusher SJ, Martone ME, Mons A, Packer AL, Persson B, Rocca-Serra P, Roos M, van Schaik R, Sansone S-A, Schultes E, Sengstag T, Slater T, Strawn G, Swertz MA, Thompson M, van der Lei J, van Mulligen E, Velterop J, Waagmeester A, Wittenburg P, Wolstencroft K, Zhao J, Mons B. The FAIR guiding principles for scientific data management and stewardship. Scientific Data. 2016;3(1):160018. doi:10.1038/sdata.2016.18
Conesa A, Beck S. Making multi-omics data accessible to researchers. Sci Data. 2019;6(1):251. https://doi.org/10.1038/s41597-019-0258-4.
Rocca-Serra P, Sansone SA. Experiment design driven FAIRification of omics data matrices, an exemplar. Sci Data. 2019;6(1):271. https://doi.org/10.1038/s41597-019-0286-0.
Piwowar HA, Vision TJ. Data reuse and the open data citation advantage. PeerJ. 2013;1:e175. https://doi.org/10.7717/peerj.175.
Liebisch G, Ekroos K, Hermansson M, Ejsing CS. Reporting of lipidomics data should be standardized. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):747–51. https://doi.org/10.1016/j.bbalip.2017.02.013.
Sud M, Fahy E, Cotter D, Azam K, Vadivelu I, Burant C, Edison A, Fiehn O, Higashi R, Nair KS, Sumner S, Subramaniam S. Metabolomics workbench: an international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 2015;44(D1):D463–D470470. https://doi.org/10.1093/nar/gkv1042.
Haug K, Salek RM, Conesa P, Hastings J, de Matos P, Rijnbeek M, Mahendraker T, Williams M, Neumann S, Rocca-Serra P, Maguire E, González-Beltrán A, Sansone S-A, Griffin JL, Steinbeck C. MetaboLights—an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2012;41(D1):D781–D786786. https://doi.org/10.1093/nar/gks1004.
Ara T, Enomoto M, Arita M, Ikeda C, Kera K, Yamada M, Nishioka T, Ikeda T, Nihei Y, Shibata D, Kanaya S, Sakurai N. Metabolonote: a Wiki-based database for managing hierarchical metadata of metabolome analyses. Front Bioeng Biotechnol. 2015;3:38. https://doi.org/10.3389/fbioe.2015.00038.
Stephenson DJ, Hoeferlin LA, Chalfant CE. Lipidomics in translational research and the clinical significance of lipid-based biomarkers. Transl Res. 2017;189:13–29. https://doi.org/10.1016/j.trsl.2017.06.006.
Hyötyläinen T, Orešič M. Analytical lipidomics in metabolic and clinical research. Trends Endocrinol Metab. 2015;26(12):671–3. https://doi.org/10.1016/j.tem.2015.08.006.
German JB, Hammock BD, Watkins SM. Metabolomics: building on a century of biochemistry to guide human health. Metabolomics. 2005;1(1):3–9. https://doi.org/10.1007/s11306-005-1102-8.
Broadhurst D, Goodacre R, Reinke SN, Kuligowski J, Wilson ID, Lewis MR, Dunn WB. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics. 2018;14(6):72. https://doi.org/10.1007/s11306-018-1367-3.
Beger RD, Dunn WB, Bandukwala A, Bethan B, Broadhurst D, Clish CB, Dasari S, Derr L, Evans A, Fischer S, Flynn T, Hartung T, Herrington D, Higashi R, Hsu P-C, Jones C, Kachman M, Karuso H, Kruppa G, Lippa K, Maruvada P, Mosley J, Ntai I, O’Donovan C, Playdon M, Raftery D, Shaughnessy D, Souza A, Spaeder T, Spalholz B, Tayyari F, Ubhi B, Verma M, Walk T, Wilson I, Witkin K, Bearden DW, Zanetti KA. Towards quality assurance and quality control in untargeted metabolomics studies. Metabolomics. 2019;15(1):4. https://doi.org/10.1007/s11306-018-1460-7.
Viant MR, Ebbels TMD, Beger RD, Ekman DR, Epps DJT, Kamp H, Leonards PE, Loizou GD, MacRae JI, Van Ravenzwaay B, Rocca-Serra P, Salek RM, Walk T, Weber RJM. Use cases, best practice and reporting standards for metabolomics in regulatory toxicology. Nat Commun. 2019;10(1):3041. https://doi.org/10.1038/s41467-019-10900-y.
Jonasdottir HS, Brouwers H, Toes REM, Ioan-Facsinay A, Giera M. Effects of anticoagulants and storage conditions on clinical oxylipid levels in human plasma. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(12):1511–22. https://doi.org/10.1016/j.bbalip.2018.10.003.
Sampson JN, Boca SM, Shu XO, Stolzenberg-Solomon RZ, Matthews CE, Hsing AW, Tan YT, Ji BT, Chow WH, Cai Q, Liu DK, Yang G, Xiang YB, Zheng W, Sinha R, Cross AJ, Moore SC. Metabolomics in epidemiology: sources of variability in metabolite measurements and implications. Cancer Epidemiol Biomark Prev. 2013;22(4):631. https://doi.org/10.1158/1055-9965.EPI-12-1109.
Trivedi DK, Hollywood KA, Goodacre R. Metabolomics for the masses: the future of metabolomics in a personalized world. New Horiz Transl Med. 2017;3(6):294–305. https://doi.org/10.1016/j.nhtm.2017.06.001.
Siskos AP, Jain P, Römisch-Margl W, Bennett M, Achaintre D, Asad Y, Marney L, Richardson L, Koulman A, Griffin JL, Raynaud F, Scalbert A, Adamski J, Prehn C, Keun HC. Interlaboratory reproducibility of a targeted metabolomics platform for analysis of human serum and plasma. Anal Chem. 2017;89(1):656–65. https://doi.org/10.1021/acs.analchem.6b02930.
Thompson JW, Adams KJ, Adamski J, Asad Y, Borts D, Bowden JA, Byram G, Dang V, Dunn WB, Fernandez F, Fiehn O, Gaul DA, Hühmer AFR, Kalli A, Koal T, Koeniger S, Mandal R, Meier F, Naser FJ, O’Neil D, Pal A, Patti GJ, Pham-Tuan H, Prehn C, Raynaud FI, Shen T, Southam AD, St. John-Williams L, Sulek K, Vasilopoulou CG, Viant M, Winder CL, Wishart D, Zhang L, Zheng J, Moseley MA. International ring trial of a high resolution targeted metabolomics and lipidomics platform for serum and plasma analysis. Anal Chem. 2019;91(22):14407–16. https://doi.org/10.1021/acs.analchem.9b02908.
Marchand CR, Farshidfar F, Rattner J, Bathe OF. A framework for development of useful metabolomic biomarkers and their effective knowledge translation. Metabolites. 2018;8(4):59. https://doi.org/10.3390/metabo8040059.
Ala-Korpela M, Davey SG. Metabolic profiling–multitude of technologies with great research potential, but (when) will translation emerge? Int J Epidemiol. 2016;45(5):1311–8. https://doi.org/10.1093/ije/dyw305.
Murphy RC, Axelsen PH. Mass spectrometric analysis of long-chain lipids. Mass Spectrom Rev. 2011;30(4):579–99. https://doi.org/10.1002/mas.20284.
Bowden JA, Heckert A, Ulmer CZ, Jones CM, Koelmel JP, Abdullah L, Ahonen L, Alnouti Y, Armando AM, Asara JM, Bamba T, Barr JR, Bergquist J, Borchers CH, Brandsma J, Breitkopf SB, Cajka T, Cazenave-Gassiot A, Checa A, Cinel MA, Colas RA, Cremers S, Dennis EA, Evans JE, Fauland A, Fiehn O, Gardner MS, Garrett TJ, Gotlinger KH, Han J, Huang Y, Neo AH, Hyötyläinen T, Izumi Y, Jiang H, Jiang H, Jiang J, Kachman M, Kiyonami R, Klavins K, Klose C, Köfeler HC, Kolmert J, Koal T, Koster G, Kuklenyik Z, Kurland IJ, Leadley M, Lin K, Maddipati KR, McDougall D, Meikle PJ, Mellett NA, Monnin C, Moseley MA, Nandakumar R, Oresic M, Patterson R, Peake D, Pierce JS, Post M, Postle AD, Pugh R, Qiu Y, Quehenberger O, Ramrup P, Rees J, Rembiesa B, Reynaud D, Roth MR, Susanne Sales S, Schuhmann K, Schwartzman ML, Serhan CN, Shevchenko A, Somerville SE, St John-Williams L, Surma MA, Takeda H, Thakare R, Thompson JW, Torta F, Triebl A, Trötzmüller M, Ubhayasekera SJK, Vuckovic D, Weir JM, Welti R, Wenk MR, Wheelock CE, Yao L, Yuan M, Zhao XH, Zhou S. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using standard reference material, 1950 metabolites in frozen human plasma. J Lipid Res. 2020. https://doi.org/10.1194/jlr.M079012.
Kopczynski D, Coman C, Zahedi RP, Lorenz K, Sickmann A, Ahrends R. Multi-OMICS: a critical technical perspective on integrative lipidomics approaches. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):808–11. https://doi.org/10.1016/j.bbalip.2017.02.003.
Azad RK, Shulaev V. Metabolomics technology and bioinformatics for precision medicine. Brief Bioinform. 2018;20(6):1957–71. https://doi.org/10.1093/bib/bbx170.
Long NP, Nghi TD, Kang YP, Anh NH, Kim HM, Park SK, Kwon SW. Toward a standardized strategy of clinical metabolomics for the advancement of precision medicine. Metabolites. 2020;10(2):51. https://doi.org/10.3390/metabo10020051.
Gross RW. The evolution of lipidomics through space and time. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(8):731–9. https://doi.org/10.1016/j.bbalip.2017.04.006.
Cuperlovic-Culf M. Machine learning methods for analysis of metabolic data and metabolic pathway modeling. Metabolites. 2018;8(1):4. https://doi.org/10.3390/metabo8010004.
Yuan ZX, Majchrzak-Hong S, Keyes GS, Iadarola MJ, Mannes AJ, Ramsden CE. Lipidomic profiling of targeted oxylipins with ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2018;410(23):6009–29. https://doi.org/10.1007/s00216-018-1222-4.
Dasilva G, Muñoz S, Lois S, Medina I. Non-targeted LC-MS/MS assay for screening over 100 lipid mediators from ARA, EPA, and DHA in biological samples based on mass spectral fragmentations. Molecules. 2019;24(12):2276. https://doi.org/10.3390/molecules24122276.
Burla B, Muralidharan S, Wenk MR, Torta F. Sphingolipid analysis in clinical research. In: Giera M, editor. Clinical metabolomics: methods and protocols. New York: Springer York; 2018. p. 135–162.
Narayanaswamy P, Shinde S, Sulc R, Kraut R, Staples G, Thiam CH, Grimm R, Sellergren B, Torta F, Wenk MR. Lipidomic “deep profiling”: an enhanced workflow to reveal new molecular species of signaling lipids. Anal Chem. 2014;86(6):3043–7. https://doi.org/10.1021/ac4039652.
Wozny K, Lehmann WD, Wozny M, Akbulut BS, Brügger B. A method for the quantitative determination of glycerophospholipid regioisomers by UPLC-ESI-MS/MS. Anal Bioanal Chem. 2019;411(4):915–24. https://doi.org/10.1007/s00216-018-1517-5.
Prinville V, Ohlund L, Sleno L. Targeted analysis of 46 bile acids to study the effect of acetaminophen in rat by LC-MS/MS. Metabolites. 2020;10(1):26. https://doi.org/10.3390/metabo10010026.
Kaabia Z, Laparre J, Cesbron N, Le Bizec B, Dervilly-Pinel G. Comprehensive steroid profiling by liquid chromatography coupled to high resolution mass spectrometry. J Steroid Biochem Mol Biol. 2018;183:106–15. https://doi.org/10.1016/j.jsbmb.2018.06.003.
Pham HT, Arnhard K, Asad YJ, Deng L, Felder TK, St John-Williams L, Kaever V, Leadley M, Mitro N, Muccio S, Prehn C, Rauh M, Rolle-Kampczyk U, Thompson JW, Uhl O, Ulaszewska M, Vogeser M, Wishart DS, Koal T. Inter-laboratory robustness of next-generation bile acid study in mice and humans: international ring trial involving 12 laboratories. J Appl Lab Med. 2019;1(2):129–42. https://doi.org/10.1373/jalm.2016.020537.
John C, Werner P, Worthmann A, Wegner K, Tödter K, Scheja L, Rohn S, Heeren J, Fischer M. A liquid chromatography-tandem mass spectrometry-based method for the simultaneous determination of hydroxy sterols and bile acids. J Chromatogr A. 2014;1371:184–95. https://doi.org/10.1016/j.chroma.2014.10.064.
Gao F, McDaniel J, Chen EY, Rockwell HE, Nguyen C, Lynes MD, Tseng YH, Sarangarajan R, Narain NR, Kiebish MA. Adapted MS/MSALL shotgun lipidomics approach for analysis of cardiolipin molecular species. Lipids. 2018;53(1):133–42. https://doi.org/10.1002/lipd.12004.
Zhang Q, Xu H, Liu R, Gao P, Yang X, Jin W, Zhang Y, Bi K, Li Q. A novel strategy for targeted lipidomics based on LC-tandem-MS parameters prediction, quantification, and multiple statistical data mining: evaluation of lysophosphatidylcholines as potential cancer biomarkers. Anal Chem. 2019;91(5):3389–96. https://doi.org/10.1021/acs.analchem.8b04715.
Reinicke M, Dorow J, Bischof K, Leyh J, Bechmann I, Ceglarek U. Tissue pretreatment for LC–MS/MS analysis of PUFA and eicosanoid distribution in mouse brain and liver. Anal Bioanal Chem. 2019. https://doi.org/10.1007/s00216-019-02170-w.
Meierhofer D. Acylcarnitine profiling by low-resolution LC-MS. PLoS ONE. 2019;14(8):e0221342. https://doi.org/10.1371/journal.pone.0221342.
Acknowledgements
We thank Tran Diem Nghi for excellent technical support and manuscript revision. Figures were created using some materials provided by Biorender illustration. This work was supported by BK21 Plus Program of Korea in 2020.
Funding
This work was supported by the Bio-Synergy Research Project of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M3A9C4048796).
Author information
Authors and Affiliations
Contributions
SWK and JL supervised the overall project. NPL mainly contributed to this work. SP contributed to the data treatment and modeling section. NHA and SJK participated in the data processing and preparation of tables and illustrations. SWK, HMK, and SJY contributed to the sections of analytical aspects and the section of perspectives. JL gave a consultation in the data treatment and modeling section. All authors read, critically revised, and accepted the final content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
About this article
Cite this article
Long, N.P., Park, S., Anh, N.H. et al. Advances in Liquid Chromatography–Mass Spectrometry-Based Lipidomics: A Look Ahead. J. Anal. Test. 4, 183–197 (2020). https://doi.org/10.1007/s41664-020-00135-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-020-00135-y