Abstract
Purpose
The main aim of this study was to perform an integrative review on the effects of Erbium lasers irradiation on bacteria eradication and smear layer removal from dentin surfaces of tooth root canals.
Method
A bibliographic search was performed on PubMed using the following search terms: “ultrasonic” AND “Er:YAG” OR “Er,Cr:YSGG” AND “laser” AND “bacteria” OR “smear layer” OR “faecalis” OR “disinfection” AND “root canal” OR “endodontic”. Studies published in the English language within the last 12 years were selected regarding the objective of this study.
Results
Previous studies reported a percentage decrease of Enterococcus faecalis at around 99% using an association between Er:YAG or Er,Cr:YSGG laser at 0.5 W and 2.5% NaOCl. Er:YAG laser-assisted irrigation at 0.9 and 1 W showed similar outcomes when compared to ultrasonic activation but revealed slightly higher amount removal of remnant intraradicular debris. Er:YAG or Er,Cr:YSGG laser showed a higher smear layer removal and bacteria eradication compared to solely passive ultrasonic activation although other types of lasers were lesser effective than the ultrasonic activation. Er,Cr:YSGG laser at 0.25 to 1.25 W in association with NaOCl was as effective as ultrasonic activation on the eradication of Enterococcus faecalis and multispecies biofilms.
Conclusions
Er:YAG and Er,Cr:YSGG lasers revealed significant bacteria eradication and smear layer removal from tooth root canals. Additionally, energy, irradiance, and mode of laser-assisted irradiation can be improved to achieve optimum results, considering different remnant tooth structures and anatomic variables. The combination of ultrasonic irrigation and laser-assisted irradiation may provide full bacteria eradication and removal of the contaminated smear layer, avoiding further bacteria-infection issues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In traditional disinfection of tooth root canals, irrigating solutions demonstrate limited access to dentinal tubules, and bacteria can remain inside dentinal tubules after the instrumentation procedures [1]. Around 67% failed cases of root canal treatment revealed the presence of Enterococcus faecalis when compared to 18% of primary endodontic infections. Intraradicular biofilms were found in 74–80% teeth with apical periodontitis [2, 3]. Thus, the traditional disinfection methods of tooth root canals do not allow a proper removal of bacteria and contaminated smear layer concerning the apical third or constricted areas of the root canal [4]. An effective disinfection method must be applied for the success of the root canal treatment, which depends on the removal of bacteria, microbial products, instrumentation debris, and infected smear layer [3, 5, 6].
Recent technological improvements have allowed the use of laser-assisted approaches in endodontic treatment such as direct pulp protection, intraradicular shaping, or decontamination of the root canal dentin [7,8,9]. Laser-assisted irrigation (LAI) methods have been utilized and improved to enhance the removal of the smear layer and bacteria from the tooth root canals. Mid-infrared lasers like Erbium Chromium:Yttrium Scandium Gallium Garnet (Er,Cr:YSGG) and Erbium:Yttrium Aluminum Garnet (Er:YAG) are often used in LAI [10, 11]. Considering the wavelengths, both Er,Cr:YSGG (2780 nm) and Er:YAG (2940 nm) are well absorbed in water and in traditional root canal irrigation solutions such as sodium hypochlorite (NaOCl), ethylenediaminetetraacetic acid (EDTA), chlorhexidine (CHX), and citric acid [10,11,12,13]. Erbium lasers have been usually used in combination with NaOCl and/or EDTA solutions to improve the removal of the smear layer and bacteria [10, 11]. The Erbium-activated irrigation is based on the cavitation phenomena and acoustic streaming in intraradicular fluids related to the photomechanical effects of the lasers at low settings [14,15,16]. Considering the wavelength, Er,Cr:YSGG and Er:YAG lasers are entirely absorbed at the surface of the target tissues, resulting in minimum thermal propagation that becomes proper for tooth root canal treatment [1, 17, 18]. On the contrary, near-infrared lasers have shown their bactericidal effects mainly via photothermal effects without activation of traditional disinfection solutions [7, 19]. Therefore, the selection of laser and related parameters should be adjusted for adequate smear layer removal and bacteria eradication in combination with a disinfection solution [20,21,22].
Low thermal propagation in association with Erbium laser energy absorption can provide effective anti-bacterial effects without localized tissue damage [23, 24]. The strong absorption of the Erbium laser energy in water results in water vaporization and the formation of large elliptical vapor bubbles. High intraradicular pressure drives fluid out of the canal within the oscillation of the vapor bubbles. Then, a negative pressure occurs and pulls fluid back into the canal when the bubbles implode, inducing a secondary cavitation effect [16]. Such secondary cavitation induces high-speed fluid movement in and out of the canal. Collapsed shock waves and acoustic streaming are formed, leading to stresses on the tooth root canal surfaces [15]. Cavitation is mainly useful for the removal of the smear layer and debris due to the generated collapsing shear forces. Despite this, it might occur to a smaller extent that is not efficient to push irrigants into dentinal tubules. The efficiency of laser activation in bacterial eradication has often been assessed by counting the remaining Enterococcus faecalis, resistant bacteria usually found in persistent endodontic infections [25, 26]. However, several parameters have been reported in the literature, depending on the clinical situation. The use of radial firing tips (RFT) has been assessed on Er,Cr:YSGG laser irradiation, revealing promising results when compared to traditionally disinfected systems [27,28,29,30]. Thus, homogeneous laser irradiation occurs when using RFT, leading to the effective removal of the smear layer and bacteria eradication in the root canal [30].
Passive ultrasonic irrigation (PUI) is also reported as ultrasonic-assisted irrigation (UAI) since the ultrasonic non-cutting tip is used to activate the disinfection solution without instrumenting the tooth root canal [31,32,33]. Such a technique also involves a phenomenon of low-intensity acoustic streaming [23]. The photon-initiated photoacoustic streaming (PIPSTM) is based on the use of Er:YAG laser under subablation settings. That differs from the traditional laser-assisted approaches by activating the antimicrobial solutions in the endodontic system through an intense photoacoustic and photomechanical phenomenon, leading to faster streaming of fluids distant from the source [23, 34, 35]. A recent shock wave-enhanced emission photoacoustic streaming (SWEEPSTM) technology was developed to increase the disinfection efficiency of the PIPSTM method by positioning a laser fiber tip in the access cavity filled with irrigation fluid and then emitting a pulsed laser [36]. SWEEPSTM performs in a similar way to PIPSTM, although it has a distinct physical pathway since it sends pulse pairs into the liquid [23, 34, 35]. The SWEEPSTM irrigation phenomena amplify pressure waves when compared to the traditional PIPSTM irrigation which provides only a single Er:YAG pulse. The protocol for both PIPS and SWEEPSTM concepts has been changed over time that resulted in variable outcomes in previous studies. However, laser- and ultrasonic-assisted methods can increase the penetration of antimicrobial solutions into the dentinal tubules and narrow spaces in complex canal systems and restricted regions of the canal. Er:YAG laser irradiation through the PIPSTM method has been shown to induce a series of rapid and powerful photoacoustic shockwaves capable of forcefully propelling the irrigating solution throughout the entire root canal system [37,38,39].
The main aim of the present study was to carry out an integrative review of the effects of Erbium lasers irradiation on the eradication and smear layer removal from dentin surfaces of tooth root canals. It was hypothesized that laser-assisted irrigation with Er:YAG or Er,Cr:YSGG laser and passive ultrasonic activation can significantly improve the removal of bacteria, smear layer, and instrumentation debris.
Method
Information sources and search strategy
A bibliographic review was performed on PubMed (via the National Library of Medicine), considering such a database includes the major journals in the fields of dentistry and biomaterials. The following search terms were applied: “ultrasonic” AND “Er:YAG” OR “Er,Cr:YSGG” AND “laser” AND “bacteria” OR “smear layer” OR “faecalis” OR “disinfection” AND “root canal” OR “endodontic”. Also, a hand-search was performed on the reference lists of all primary sources and eligible studies of this integrative review for additional relevant publications. The inclusion criteria encompassed articles published in the English language from January 2011 until March 30th, 2023, reporting the effects of ultrasonic activation and Erbium lasers irradiation on the disinfection of tooth root canal dentin surfaces. The eligibility inclusion criteria used for article searches also involved in vitro cell culture assays, randomized controlled trials, animal assays, and prospective cohort studies. The exclusion criteria were the following: papers without an abstract, scarce data (i.e., laser parameters), case reports with a short follow-up period, short number of specimens, and articles assessing only other alternative disinfection methods. Studies based on publication date were not restricted during the search process. The present method was performed in accordance with the search strategy applied in previous studies on integrative or systematic reviews [17, 18, 40,41,42,43,44].
Study selection and data collection process
The selection of studies was conducted in three steps. At first, studies were primarily scanned for relevance by title, and the abstracts of those that were not excluded at this step were assessed. Two of the authors (JCMS and AB) independently analyzed the titles and abstracts of the retrieved potentially relevant articles meeting the inclusion criteria. The total number of articles was compiled for each combination of key terms, and therefore, the duplicates were removed using Mendeley reference manager (Elsevier). The second step comprised the evaluation of the abstracts and non-excluded articles according to the eligibility criteria on the abstract review. Selected articles were individually read and analyzed concerning the purpose of this study. At last, the eligible articles received a study nomenclature label, combining the first author’s name and the year of publication. The following variables were collected for this review: authors’ names, journal, publication year, purpose, study design, bacteria growth conditions and analyses, laser irradiation parameters, ultrasonic parameters, and disinfection solutions. The PICO question was adjusted to the issue where “P” was related to the specimens and “I” referred to the methods of analyses while “C” was related to comparison of findings and “O” to the main outcomes. The data of the reports were harvested directly into a specific data collection form to avoid multiple data recordings regarding multiple reports within the same study (e.g., reports with different set-ups). This evaluation was individually carried out by two researchers, followed by a joint discussion to select the relevant studies.
Results
The initial search in the available database yielded a total of 61 studies, although 11 duplicates were removed. The titles and abstracts were read seeking concordance with the inclusion criteria of the present study, and then 19 studies were discarded because they did not include significant information on Erbium lasers and ultrasonic activation approaches. The evaluation of titles and abstracts resulted in the selection of 31 potential studies of which three articles were excluded after full reading concerning the lack of available data. The results of the selection of articles are shown in Fig. 1.
Of the 28 studies included in this review, twenty-three (83%) studies evaluated the efficacy of Er:YAG laser and ultrasonic-activated irrigation [4, 20, 22, 24, 26, 33, 45,46,47,48,49,50,51,52,53,54,55,56,57,58,59], while five (17%) other studies assessed the effects of Er,Cr:YSGG laser irradiation [10, 11, 60,61,62,63]. Four (14.2%) studies investigated the effects of different agitation techniques on the penetration of antimicrobial solutions into dentinal tubules [24, 48, 64, 65]. Nine (32%) studies evaluated the antimicrobial effects of ultrasonic and laser-activated irrigation methods against Enterococcus faecalis [10, 11, 26, 33, 49, 52, 58, 60, 61], while four (14.2%) studies assessed the behavior of mixed-species biofilm under the abovementioned approaches [33, 53, 55, 62]. Seven (25%) studies investigated the effectiveness of ultrasonic and laser-assisted irrigation techniques on smear layer removal [4, 20,21,22, 45, 51, 58, 59, 63].
The main results described in Table 1 are reported as follows:
-
On Er:YAG laser irradiation, the eradication of Enterococcus faecalis ranged from 98.8 up to 99.99% using 2.5–6% NaOCl. That bacteria eradication ranged from 98.5 up to 99.997% for ultrasonic irrigation in combination with 2.5–6% NaOCl [26, 33, 49, 55]. The bacterial reduction reached 71.4% for ultrasonic irrigation and 90.2% for LAI using water irrigation solution, while 69.8–78.1% was recorded for ultrasonic irrigation and 91.9–97.6% under saline solution irrigation [26, 33, 56].
-
On the ultrasonic irrigation, the percentage of bacteria eradication ranged from 1.92 up to 69.3%, being the highest percentages recorded for 3%NaOCl-17%EDTA-3%NaOCl solutions followed by 6%NaOCl-18%Etidronic acid and 3%NaOCl-17%EDTA solutions [52]. The use of 6%NaOCl-18%Etidronic acid solutions provided the highest percentage of bacteria eradication, followed by 3%NaOCl-17%EDTA-3%NaOCl, 3%NaOCl-17%EDTA, and saline solutions.
-
On the Er,Cr:YSGG laser, the decrease of Enterococcus faecalis was recorded at around 99.96–99.99% in 2.5% NaOCl irrigation [10, 11]. Mean values of colony-forming unit (CFU/cm2) were recorded at 2.24 × 102 when Er,Cr:YSGG laser was combined with 0.5% NaOCl or 4% NaOCl and 15% EDTAC solutions, while 1.38 × 105 CFU/cm2 was measured for Er,Cr:YSGG laser irradiation free of disinfection solution, and 2.37 × 104 CFU/cm2 was recorded for ultrasound with 0.5% NaOCl or 4% NaOCl and 15% EDTAC solutions [60,61,62].
-
The smear layer removal with the use of a Er,Cr:YSGG laser was recorded at 1 mm from the apex. The percentage of samples that revealed dentinal tubules open and clean (no smear layer) was around 10% for passive ultrasonic irrigation, 100% for LAI (60 s activation and 30/.02 file), 30% for LAI (30 s activation, 30/.02 file), and 50% for LAI (60 s activation, 20/.02 file) [63]. Also, the removal of the smear layer was achieved when using ultrasonic irrigation [20,21,22, 45, 51].
-
Er:YAG laser irradiation combined with 5% NaOCl and 17% EDTA solutions showed penetration depth mean values of 961 μm into canal dentin using the LAI PIPSTM approach, while a 722 μm penetration depth was recorded for LAI SWEEPSTM. Passive ultrasonic reached a mean penetration depth of 823 μm in canal dentin [48].
Discussion
This integrative review showed that laser-assisted irrigation with Er:YAG or Er,Cr:YSGG lasers and ultrasonic activation can significantly improve the removal of bacteria, smear layer, and debris from tooth root canals. The findings reported by previous studies validate the hypothesis of this study. Such results indicate that a further combination between laser-assisted techniques and ultrasonic irrigation can increase the disinfection effects in tooth root canals even though such combination has not been previously clarified considering the variable parameters. In fact, many parameters can influence the success of the disinfection process such as canal instrumentation, canal anatomy, disinfection solutions, temperature, and laser parameters such as activation time, pulse rate, frequency, irradiance, and energy.
Disinfection by Erbium lasers and ultrasonic activation
In previous studies, Er:YAG laser systems with a wavelength of 2940 nm (Fig. 2) were set on a minimum power capacity ranging from 20 to 60 mJ energy and 10 to 50 Hz frequency, while the pulse rate was around 50 μs [4, 20, 22, 24, 26, 33, 45,46,47,48,49,50,51,52,53,54,55,56,57]. Er,Cr:YSSG lasers possess a wavelength of 2780 nm and have been set at low power, 10 to 35 Hz of frequency, 55 to 62.5 mJ of energy, and a pulse rate of 60 or 140 μs [10, 11, 60,61,62,63]. Er,Cr:YSGG laser activation of 2.5% NaOCl or 2% CHX solutions showed to be as effective as ultrasonic-assisted irrigation in percentage reduction of Enterococcus faecalis with the same disinfection solutions [10, 11]. The use of a low concentration of NaOCl (0.5%) in conjunction with laser activation revealed a similar bactericidal effect to that of solely 5% NaOCl solution. That allows the use of a less toxic concentration of NaOCl under Er,Cr:YSGG activation [60]. On the contrary, ultrasonic-assisted irrigation was not enough to enhance the antimicrobial efficacy within 0.5% NaOCl solutions [60, 61]. On multi-species biofilm, laser activation within 4% NaOCl and 15% EDTAC solutions showed similar results when compared to ultrasonic activation, either with 0.5 or 0.75 W of power settings [62]. Er,Cr:YSGG laser activation exhibited high effectiveness in bacteria eradication, considering the irradiance mode over a total activation period of 20 up to 90 s [10, 11, 60,61,62].
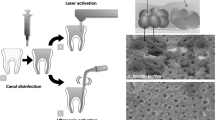
A Schematics of the root canal treatment followed by using Er:YAG laser irradiation and/or ultrasonic activation. SEM images of dentin tubules B covered with E. faecalis and C dentin tubules free of bacteria (adapted from Golob et al, 2017 [38]). (D, E) SEM images of dentin surfaces after smear layer removal (adapted from Peeters and Suardita, 2011 [63]). Reprint permission from the publisher
In a recent previous study, an infection model with Enterococcus faecalis was established in sixty-six extracted maxillary first molar root canals after instrumentation up to ProTaper Universal F2TM. Specimens were divided regarding the following disinfection methods: conventionally invasive access group (CIA), computer-guided minimally invasive access group (MIA), conventional irrigation (CI), passive ultrasonic agitation (PUI), and Er:YAG laser-activated irrigation (LA). Microbial samples were collected from tooth root canals by the paper tip method and cultured, and the colony-forming unit (CFU) values of each sample were calculated. Then, the root canals were enlarged to the size of F3, and dentin debris was collected from the F3 file. After dilution and culturing, the CFU values were calculated for each group. The disinfection effect of Er:YAG laser or ultrasonic-assisted computer-guided minimally invasive access was similar to conventionally invasive access, while Er:YAG laser showed higher bacteria eradication than ultrasonic activation [58].
Laser-assisted irrigation requires the positioning of the fiber tip at 5 mm from the working length and vertical movements while withdrawing the tip surrounded by disinfection solutions [24, 46, 51, 57]. When inserted into the canal, the tip must be centered, avoiding contact with the canal surfaces [46, 51]. Different studies have confirmed that the laser tip does not need to be positioned at the apex because the cavitation bubbles also allow cleaning in the apical region [45]. Thus, the spatial requirements (high taper and conicity) are less important for the action of the pulsed Er:YAG laser when compared to the PUI [57]. On the Er,Cr:YSGG laser, the endodontic tip was placed 2–5 mm into the canal or above the orifice of the pulp chamber with short up and down movements [11, 60, 62, 63]. As for the Er:YAG laser, extreme caution should be taken when activating irrigating solutions close to the apical constriction and preferably positioning the laser fiber at the entrance of the root canal to reduce the risk of extrusion [61]. Therefore, Erbium lasers are also appropriate in curved root canals, unlike PUI approaches. That could also be an advantage of Erbium-based LAI over solely ultrasonic activation, as the tip position is less affected by the complexity of the tooth root canals. Moreover, positioning the laser tip at the canal entrance may decrease the heating of the tooth structure or surrounding tissues such as the periodontal ligament and alveolar bone [61]. However, a significant bacterial reduction was reported on positioning the tip 6 mm from the working length instead at the canal entrance, as well as increasing the pulse energy from 20 up to 40 mJ. The effect of higher pulse energy can probably be explained by higher peak powers which yield larger primary cavitation bubbles at the fiber tip and hence larger liquid displacement and violent shock waves [33]. However, radial shock waves gather at the bottom part of the fiber tip in a conical-shape tip or PIPSTM due to inner reflections that result in a higher power density in comparison with a flat tip of similar diameter [15].
In fact, ultrasonic-assisted irrigation increases the flow of liquid and improves both the solvent and antibacterial capabilities and the removal effect of organic and inorganic debris from the tooth root canal surfaces [66]. The physical principle of passive ultrasonic irrigation (PUI) involves the formation of bubbles due to the pressure in the liquid under ultrasonic vibration. That becomes unstable, and then the bubbles collapse, causing an implosion comparable to a vacuum decompression. The collapse of the bubbles releases the impact energy responsible for the detergent effect. In the selected studies, various ultrasonic devices were used, such as the Newtron P5 XSTM, EMS piezoelectric ultrasonic unit, SybronEndoTM, EndoUltraTM, VDW.ULTRATM, or PerioScanTM. Such ultrasonic devices are commonly used in clinical activity and remain an affordable alternative to lasers. In determining the optimal ultrasonic irrigation effects, high frequencies ranging from 28 up to 40 kHz were assessed in the selected studies, of which 30 kHz was the most common assessed frequency. Most of the studies applied the device continuously for 60 s, although no consensus has been established. One previous study pointed out that PUI showed the lowest biofilm removal over the first 20 s [56]. In most studies, the ultrasonic power was not specified and could differ when compared to other devices, leading to some limitations in the correlation of parameters. The success of the PUI approach varies concerning the complexity of the tooth root canal anatomy. The tip of the ultrasonic file must be cautiously positioned to achieve adequate biofilm removal in the presence of an isthmus [56]. A high taper might allow a better disinfection solution flow into the canals and ensure a good oscillation of the ultrasonic tip. Thus, contact between the root canal surfaces and the ultrasonic endodontic file must be avoided since that decreases the amplitude and the irrigant streaming speed [61]. In the selected studies, the tip positioning of the ultrasonic device was usually located at 1 mm up to 4 mm from the working length. The absence of tip positioning in the apical third of the root can result in low bacteria eradication [60]. Those findings may emphasize the need for tip positioning at the apex to increase the bactericidal effect in the apical third. Therefore, the positioning of the tip can become limited in curved root canals, as tips must be placed at 1 to 2 mm from the working length [61]. In the selected studies, a decreasing pattern of disinfection solution penetration depth was noticed regarding the canal thirds. Passive ultrasonic irrigation was more efficient in the coronal third than in the middle and apical thirds and revealed a low penetration depth in the apical third. Those findings were reported with 5–5.25% NaOCl and 17% EDTA solutions [24, 48, 64, 65].
On photon-initiated photoacoustic streaming (PIPSTM), low pulse Er:YAG energies around 30 mJ have been used within a very short pulse length (50 μs), and a 20 Hz frequency, resulting in high peak powers and hence more efficient cavitation generation [20, 22, 24, 26, 33, 46,47,48,49,50, 52,53,54, 56, 57]. On the PIPSTM technique, the conical tip is usually placed at the entrance of the pulp chamber or a few millimeters into the canal and held stationary over laser activation [20, 22, 24, 26, 33, 46,47,48,49,50, 52,53,54, 56, 57]. Even though Er:YAG activation with the PIPSTM technique has shown potential outcomes, no significant differences between PIPSTM and PUI taking into account bacteria eradication were found in three of the selected studies [26, 33, 49]. On the contrary, PIPSTM showed a higher percentage of bacteria eradication when compared to PUI in several other previous studies [47, 52, 53, 56]. The antibacterial action of Er:YAG laser activation combined with 2.5–6% NaOCl solutions was highly effective [26, 33, 47, 49, 52, 53]. PIPSTM was even improved when a chelating agent was added [52]. Er:YAG laser removed a higher amount of apical biofilm and infected dentin tubules when compared to solely ultrasonic-assisted irrigation [55]. The bacteria eradication is illustrated in Fig. 2. Differences in disinfection efficiency between the activation types can be explained by the movement of NaOCl out of the canal with every pulse, which led the PIPSTM tip into a fluid-free canal after 4 s [46]. Those results indicate a stronger effect of LAI that is attributed to the extremely turbulent action of the disinfection solution induced by pulsed Erbium lasers [33]. In addition, the findings are in agreement with the fact that Erbium laser activation is known to enhance the release of Cl and O ions due to the heating effect [67]. Additionally, when the PIPSTM tips were positioned in the canal, some melting effect was noted when the tip was kept too long against the surfaces, but that did not negatively influence the scores. No damage to the models occurred when the laser tips were positioned at the entrance level; thus, care has to be taken when positioning the tip close to the apex for a long time [50]. Furthermore, a shock wave-enhanced emission photoacoustic streaming named SWEEPSTM consists of emitting pulse pairs into a liquid, leading to a higher amplification of the pressure waves when compared to the PIPSTM single irrigation [36]. That increases the disinfection efficiency of the photoacoustic streaming. Thus, SWEEPSTM consists of positioning a laser fiber tip in the access cavity filled with a disinfection solution that is quite similar to PIPSTM, although SWEEPSTM sends pulse pairs into the liquid [23, 34, 48, 68]. In a previous study, SWEEPSTM was associated with different concentrations of NaOCl solutions (0.5%, 1.0%, 2.0%, and 5.25%) for the disinfection of bovine root canals infected with E. faecalis. The colony-forming unit (CFU) for viable bacteria decreased after the tooth root canal disinfection procedure on SWEEPSTM in association with NaOCl low-concentration [36].
Smear layer removal
Regarding the previous studies, smear layer removal was more effective when 1–2.5% NaOCl and 17% EDTA solutions were combined during ultrasonic activation [45], although smear layer removal was more efficient in the coronal third, followed by the middle third [21, 51]. The effect of ultrasonic irrigation with 17% EDTA on smear layer removal applied to the coronal and apical thirds of the root canal was found to be depth-dependent [4]. The smear layer was removed from all regions of the root canal; however, the dentinal tubules were only open in the coronal and middle thirds [4, 63]. Ultrasonic-assisted irrigation was shown to be more efficient than laser-assisted activation when near-infrared lasers were used [20,21,22]. On the debris removal assessment with 2.5% NaOCl, ultrasonic irrigation performed similarly to near-infrared laser-assisted activation, although none of the tested methods was able to completely free the root canal systems of debris [46, 50, 54, 57].
In a previously selected study, the effectiveness of ER:YAG LAI was assessed on eighty-eight human maxillary molars with either a mesiobuccal or distobuccal canal. After instrumentation to a size of 25/0.02 taper, a ledge was produced at 2.2–0.7 mm short of the apex and checked using micro-CT. Specimens were divided into four groups: syringe irrigation (SI), ultrasonic-activated irrigation (UAI), agitation with the XP-endo finisher (XP), and LAI. The apical 1-mm region was examined using SEM to evaluate the remaining debris and smear layer. LAI showed significantly lower smear layer scores than SI and XP (P < 0.05), with an insignificant difference as compared with UAI. Also, LAI revealed significantly higher debris removal scores than SI (P < 0.05) when solely NaOCl solution was used [59]. Another previous study reported a higher smear layer removal when using SWEEPSTM or PIPSTM than that recorded for traditional disinfection methods with EDTA and NaOCl or ultrasonic irrigation [69]. Another previous study revealed a significantly higher removal of smear layer and debris by using SWEEPSTM when compared to the use of traditional syringe irrigation; however, the results were not significantly different when compared to passive ultrasonic Irrigation [70]. Thus, SWEEPSTM can enhance the effects of disinfection solutions such as NaOCl solutions [36].
The smear layer removal by Er:YAG laser activation appeared to be more effective in the coronal, middle, and then apical thirds [20, 45]. However, at subablative setting activation of 5.25% NaOCl with LAI showed poor results at 1, 3, and 5 mm from the apex. Laser activation was equal to or less effective than PUI, depending on the depth [51]. Laser activation with 2.5% NaOCl and 17% EDTA solutions was more effective than with solely NaOCl [45]. On the combination of EDTA and NaOCl solutions in the pulp chamber, laser activation removed more smear layers from both the middle and apical thirds of the tooth root canals than using PUI. On irradiation time, Er,Cr:YSGG laser activation with 17% EDTA revealed higher smear layer removal over 60 s than that for 30 s [63]. That indicates that the activation time of laser irradiation also affects the canal debridement, although other factors also have effects such as the tip, medium (solution or dry conditions), irradiance, and activation mode. Also, Erbium-based LAI for 30 or 60 s performed better than passive ultrasonic activation for 60 s. Laser-assisted irrigation in EDTA solution revealed effective removal of the smear layer and debris from the root canal apex [63]. Nevertheless, different Er,CR:YSGG laser units (i.e., Waterlaser MDTM and iPlusTM) have different pulse durations, and therefore, protocols have changed, that cannot be parallel-compared, despite comprising the same components and wavelength. On PIPSTM within NaOCl and EDTA solutions, activation was slightly more effective than ultrasonic-assisted irrigation on smear layer removal [20, 22]. PIPSTM provided less debris than solely laser- or ultrasonic-assisted techniques [46]. Er:YAG was more efficient in the middle third, than in the coronal and apical thirds, whereas the lowest smear layer removal was detected after using the PIPSTM technique at the coronal third [22]. Er:YAG and PIPSTM laser activation did not exhibit significant differences in smear layer removal in one previous study [20, 50]. The tip positioning did not alter the effectiveness of the smear layer removal by using laser activation within solely 17% EDTA, unlike with the use of ultrasonic activation [4]. On different combinations of disinfection solutions, Er:YAG laser activation performed similarly to ultrasonic activation with slightly less amount of remaining debris [20, 46, 50, 54, 57]. Considering the previous findings, near-infrared laser irradiation (i.e., Nd:YAG or Nd:YAP) was not capable of improving smear layer removal and canal debridement. In association with NaOCl and EDTA during 20 s to 80 s, near-infrared lasers did not succeed in efficiently removing the smear layer or debris in comparison to Er:YAG lasers or ultrasonic activation [20,21,22].
Laser-activation irrigation or PIPSTM within 17% EDTA and 5% NaOCl solutions for 30 or 90 s exhibited a significantly higher penetration area when compared to other groups [24, 48]. Er:YAG laser irradiation combined with 5% NaOCl and 17% EDTA solutions showed penetration depth mean values of 961 μm into canal dentin using the LAI PIPSTM approach, while a 722 μm penetration depth was recorded for LAI SWEEPSTM. Passive ultrasonic irrigation reached a mean penetration depth of 823 μm in canal dentin [48]. However, ultrasonic irrigation, LAI, and PIPSTM revealed decreasing penetration depth coronally to apically [24, 48]. Considering the minimally achievable penetration depths, PIPSTM achieved the deepest penetration in the middle and apical sections, with significant differences compared to all other groups [48]. The lower penetration depth of the antimicrobial solution can be caused by the following factors: higher smear layer remnants, the melting effect of laser on dentin tissues, and occlusion of dentinal tubules [64].
The efficacy of novel Er,Cr:YSGG laser radial firing tips (RFT) has been reported in the literature [27,28,29,30]. The laser irradiation with the RTP (140 μs pulse at 20 Hz and 37.5 or 62 mJ irradiance) has been compared with solely endodontic irrigation in 3% NaOCl solutions and interim calcium hydroxide paste in necrotic tooth root canal with chronic apical periodontitis. Anterior and premolar teeth were randomly assigned and radiographically assessed for a 6- or 12-month follow-up [27, 29]. Er,Cr:YSGG laser irradiation with RTP provided statistically significant decreases in periapical index scores regarding radiographic evaluation [27]. That approach has also been shown to be effective for the endodontic treatment of a transplanted multi-rooted tooth with apical periodontitis regarding a 3-year follow-up [28]. Thus, radial firing tips provide more homogeneous laser irradiation of root canal walls, as revealed by the removal of bacteria and the smear layer from tooth root canals in an in vitro study [30].
In fact, a variety of ultrasonic- and laser-assisted parameters are assessed in the studies. However, laser-activated irrigation is effective in a short time frame, and laser activation for 20 s is similarly effective as 3 ultrasonic activation sets [50]. Additionally, the selected studies differ considering the biofilm composition, incubation time, and bacteria culture methods. Also, some of the simulated in vitro methods can reveal different outcomes when compared to in vivo studies or clinical situations. Thus, no consensus currently exists on appropriate setups and incubation times in order to develop a representative biofilm model [33].
Conclusions
The present review reported significant findings on the in vitro effects of Erbium- and/or ultrasonic-assisted irrigation on the smear layer removal and eradication of biofilms from tooth root canals. The selected studies revealed that Er:YAG and Er,Cr:YSGG lasers were properly suited for laser-activated irrigation. Er:YAG laser within 2.5–6% NaOCl solutions achieved a percentage reduction of Enterococcus faecalis between 98.8 and 99.998%, while Er,Cr:YSGG lasers with 2.5% NaOCl solutions reached around 99.99% bacteria eradication. Thus, Er,Cr:YSGG is as effective as ultrasonic activation in the eradication of polymicrobial biofilm and Enterococcus faecalis. Additionally, Er,Cr:YSGG laser activation combined with 17% EDTA solutions showed better results in smear layer removal when compared to passive ultrasonic-assisted irrigation. PIPSTM also revealed better results when compared to ultrasonic-assisted irrigation in bacteria eradication from the tooth root canals. However, no differences were reported in the removal of debris between traditional laser-activated irrigation and PIPSTM. On different combinations of disinfection solutions, Er:YAG laser-activated irrigation performed similarly to ultrasonic activation with a slightly less amount of remaining debris, although Er:YAG revealed a higher removal of the smear layer when compared to ultrasonic-assisted irrigation. In fact, Erbium lasers are efficient at the entrance of the canal and over a shorter period of time when compared to ultrasonic-assisted irrigation. Laser-activated irrigation should be preferred over ultrasound in curved canal systems. Further studies should assess the efficiency of a protocol that associates the optimum laser and ultrasound parameters with intraradicular irrigation for specific tooth-related conditions. Then, limitations on low-smear layer removal could be prevented by optimizing laser and ultrasonic parameters.
References
Munoz HR, Camacho-Cuadra K (2012) In vivo efficacy of three different endodontic irrigation systems for irrigant delivery to working length of mesial canals of mandibular molars. J Endod 38:445–448. https://doi.org/10.1016/j.joen.2011.12.007
Sundqvist G, Figdor D, Persson S, Sjögren U (1998) Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:86–93. https://doi.org/10.1016/s1079-2104(98)90404-8
Ricucci D, Siqueira JFJ (2010) Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod 36:1277–1288. https://doi.org/10.1016/j.joen.2010.04.007
Sahar-Helft S, Sarp ASK, Stabholtz A et al (2015) Comparison of positive-pressure, passive ultrasonic, and laser-activated irrigations on smear-layer removal from the root canal surface. Photomed Laser Surg 33:129–135. https://doi.org/10.1089/pho.2014.3788
Siqueira JFJ (2002) Endodontic infections: concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 94:281–293. https://doi.org/10.1067/moe.2002.126163
Sakko M, Tjäderhane L, Rautemaa-Richardson R (2016) Microbiology of root canal infections. Prim Dent J 5:84–89. https://doi.org/10.1308/205016816819304231
Olivi G, De MR, DiVito E (2016) Lasers in endodontics: scientific background and clinical applications. Springer International Publishing
Kokuzawa C, Ebihara A, Watanabe S et al (2012) Shaping of the root canal using Er:YAG laser irradiation. Photomed Laser Surg 30:367–373. https://doi.org/10.1089/pho.2012.3226
Deng Y, Zhu X, Zheng D et al (2016) Laser use in direct pulp capping: a meta-analysis. J Am Dent Assoc 147:935–942. https://doi.org/10.1016/j.adaj.2016.07.011
Aydin SA, Taşdemir T, Buruk CK, Çelik D (2020) Efficacy of Erbium, chromium-doped yttrium, scandium, gallium and garnet laser-activated irrigation compared with passive ultrasonic irrigation, conventional irrigation, and photodynamic therapy against Enterococcus faecalis. J Contemp Dent Pract 21:11–16
Bago Jurič I, Plečko V, Anić I (2014) Antimicrobial efficacy of Er,Cr:YSGG laser-activated irrigation compared with passive ultrasonic irrigation and RinsEndo(®) against intracanal Enterococcus faecalis. Photomed Laser Surg 32:600–605. https://doi.org/10.1089/pho.2014.3767
Meire MA, Poelman D, De Moor RJ (2014) Optical properties of root canal irrigants in the 300-3,000-nm wavelength region. Lasers Med Sci 29:1557–1562. https://doi.org/10.1007/s10103-013-1307-4
Parker S (2007) Verifiable CPD paper: laser-tissue interaction. Br Dent J 202:73–81. https://doi.org/10.1038/bdj.2007.24
Blanken J, De Moor RJG, Meire M, Verdaasdonk R (2009) Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 1: a visualization study. Lasers Surg Med 41:514–519. https://doi.org/10.1002/lsm.20798
Matsumoto H, Yoshimine Y, Akamine A (2011) Visualization of irrigant flow and cavitation induced by Er:YAG laser within a root canal model. J Endod 37:839–843. https://doi.org/10.1016/j.joen.2011.02.035
de Groot SD, Verhaagen B, Versluis M et al (2009) Laser-activated irrigation within root canals: cleaning efficacy and flow visualization. Int Endod J 42:1077–1083. https://doi.org/10.1111/j.1365-2591.2009.01634.x
Febvey A, Silva F, Henriques B et al (2023) Root canal disinfection and maintenance of the remnant tooth tissues by using grape seed and cranberry extracts. Odontology 111:541–553. https://doi.org/10.1007/s10266-022-00766-w
Noronha Oliveira M, Schunemann WVH, Mathew MT et al (2018) Can degradation products released from dental implants affect peri-implant tissues? J Periodontal Res 53:1–11. https://doi.org/10.1111/jre.12479
Klinke T, Klimm W, Gutknecht N (1997) Antibacterial effects of Nd:YAG laser irradiation within root canal dentin. J Clin Laser Med Surg 15:29–31. https://doi.org/10.1089/clm.1997.15.29
Keles A, Kamalak A, Keskin C et al (2016) The efficacy of laser, ultrasound and self-adjustable file in removing smear layer debris from oval root canals following retreatment: a scanning electron microscopy study. Aust Endod J 42:104–111. https://doi.org/10.1111/aej.12145
da Costa Lima GA, Aguiar CM, Câmara AC et al (2015) Comparison of smear layer removal using the Nd:YAG laser, ultrasound, ProTaper Universal system, and CanalBrush methods: an in vitro study. J Endod 41:400–404. https://doi.org/10.1016/j.joen.2014.11.004
Akyuz Ekim SN, Erdemir A (2015) Comparison of different irrigation activation techniques on smear layer removal: an in vitro study. Microsc Res Tech 78:230–239. https://doi.org/10.1002/jemt.22466
Koch JD, Jaramillo DE, DiVito E, Peters OA (2016) Irrigant flow during photon-induced photoacoustic streaming (PIPS) using particle image velocimetry (PIV). Clin Oral Investig 20:381–386. https://doi.org/10.1007/s00784-015-1562-9
Akcay M, Arslan H, Mese M et al (2017) Effect of photon-initiated photoacoustic streaming, passive ultrasonic, and sonic irrigation techniques on dentinal tubule penetration of irrigation solution: a confocal microscopic study. Clin Oral Investig 21:2205–2212. https://doi.org/10.1007/s00784-016-2013-y
Stuart CH, Schwartz SA, Beeson TJ, Owatz CB (2006) Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod 32:93–98. https://doi.org/10.1016/j.joen.2005.10.049
Cheng X, Xiang D, He W et al (2017) Bactericidal effect of Er:YAG laser-activated sodium hypochlorite irrigation against biofilms of Enterococcus faecalis isolate from canal of root-filled teeth with periapical lesions. Photomed Laser Surg 35:386–392. https://doi.org/10.1089/pho.2017.4293
Martins MR, Carvalho MF, Vaz IP et al (2013) Efficacy of Er,Cr:YSGG laser with endodontical radial firing tips on the outcome of endodontic treatment: blind randomized controlled clinical trial with six-month evaluation. Lasers Med Sci 28:1049–1055. https://doi.org/10.1007/s10103-012-1172-6
Martins MR, Lima RC, Pina-Vaz I et al (2016) Endodontic treatment of an autogenous transplanted tooth using an Er,Cr:YSGG laser and radial firing tips: case report. Photomed Laser Surg 34:487–493. https://doi.org/10.1089/pho.2015.4061
Martins MR, Carvalho MF, Pina-Vaz I et al (2014) Outcome of Er,Cr:YSGG laser-assisted treatment of teeth with apical periodontitis: a blind randomized clinical trial. Photomed Laser Surg 32:3–9. https://doi.org/10.1089/pho.2013.3573
Schoop U, Barylyak A, Goharkhay K et al (2009) The impact of an erbium, chromium:yttrium-scandium-gallium-garnet laser with radial-firing tips on endodontic treatment. Lasers Med Sci 24:59–65. https://doi.org/10.1007/s10103-007-0520-4
van der Sluis LWM, Versluis M, Wu MK, Wesselink PR (2007) Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J 40:415–426. https://doi.org/10.1111/j.1365-2591.2007.01243.x
Jensen SA, Walker TL, Hutter JW, Nicoll BK (1999) Comparison of the cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canals. J Endod 25:735–738. https://doi.org/10.1016/S0099-2399(99)80120-4
De Meyer S, Meire MA, Coenye T, De Moor RJG (2017) Effect of laser-activated irrigation on biofilms in artificial root canals. Int Endod J 50:472–479. https://doi.org/10.1111/iej.12643
Olivi G, Divito E (2012) REVIEW photoacoustic endodontics using PIPSTM: experimental background and clinical protocol. Journal of the Laser and Health Academy 2012:22–25
DiVito E, Peters OA, Olivi G (2012) Effectiveness of the erbium:YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci 27:273–280. https://doi.org/10.1007/s10103-010-0858-x
Lei L, Wang F, Wang Y et al (2022) Laser activated irrigation with SWEEPS modality reduces concentration of sodium hypochlorite in root canal irrigation. Photodiagnosis Photodyn Ther 39:102873. https://doi.org/10.1016/j.pdpdt.2022.102873
Wang X, Cheng X, Liu B et al (2017) Effect of laser-activated irrigations on smear layer removal from the root canal wall. Photomed Laser Surg 35:688–694. https://doi.org/10.1089/pho.2017.4266
Golob BS, Olivi G, Vrabec M et al (2017) Efficacy of photon-induced photoacoustic streaming in the reduction of Enterococcus faecalis within the root canal: different settings and different sodium hypochlorite concentrations. J Endod 43:1730–1735. https://doi.org/10.1016/j.joen.2017.05.019
Kasić S, Knezović M, Beader N et al (2017) Efficacy of three different lasers on eradication of Enterococcus faecalis and Candida albicans biofilms in root canal system. Photomed Laser Surg 35:372–377. https://doi.org/10.1089/pho.2016.4258
Souza JCM, Sordi MB, Kanazawa M et al (2019) Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater 94:112–131. https://doi.org/10.1016/j.actbio.2019.05.045
Rodrigues YL, Mathew MT, Mercuri LG et al (2018) Biomechanical simulation of temporomandibular joint replacement (TMJR) devices: a scoping review of the finite element method. Int J Oral Maxillofac Surg 47:1032–1042. https://doi.org/10.1016/j.ijom.2018.02.005
Hajjar S, Melo-Ferraz A, Carvalho O et al (2022) An integrative review on the tooth root canal disinfection by combining laser-assisted approaches and antimicrobial solutions. Lasers Dent Sci 6:133–151. https://doi.org/10.1007/s41547-022-00163-0
Messous R, Henriques B, Bousbaa H et al (2021) Cytotoxic effects of submicron- and nano-scale titanium debris released from dental implants: an integrative review. Clin Oral Investig 25:1627–1640. https://doi.org/10.1007/s00784-021-03785-z
Martinez-Gonzalez M, Fidalgo-Pereira RC, Torres O et al (2023) Toxicity of resin-matrix cements in contact with fibroblast or mesenchymal cells. Odontology 111:310–327. https://doi.org/10.1007/s10266-022-00758-w
Ayranci LB, Arslan H, Akcay M et al (2016) Effectiveness of laser-assisted irrigation and passive ultrasonic irrigation techniques on smear layer removal in middle and apical thirds. Scanning 38:121–127. https://doi.org/10.1002/sca.21247
Deleu E, Meire MA, De Moor RJG (2015) Efficacy of laser-based irrigant activation methods in removing debris from simulated root canal irregularities. Lasers Med Sci 30:831–835. https://doi.org/10.1007/s10103-013-1442-y
Dönmez Özkan H, Metin K, Bakir ZB, Yiğit Özer S (2018) A novel protein testing model to assay the efficacy of multiple irrigation activation techniques for removal of ex vivo biomolecular film. Photomed Laser Surg 36:493–498. https://doi.org/10.1089/pho.2018.4452
Galler KM, Grubmüller V, Schlichting R et al (2019) Penetration depth of irrigants into root dentine after sonic, ultrasonic and photoacoustic activation. Int Endod J 52:1210–1217. https://doi.org/10.1111/iej.13108
Hage W, De Moor RJG, Hajj D et al (2019) Impact of different irrigant agitation methods on bacterial elimination from infected root canals. Dent J (Basel) 7(3):64. https://doi.org/10.3390/dj7030064
Kurzmann C, Meire MA, Lettner S et al (2020) The efficacy of ultrasonic and PIPS (photon-induced acoustic streaming) irrigation to remove artificially placed dentine debris plugs out of an artificial and natural root model. Lasers Med Sci 35:719–728. https://doi.org/10.1007/s10103-019-02912-3
Mancini M, Cerroni L, Iorio L et al (2018) FESEM evaluation of smear layer removal using different irrigant activation methods (EndoActivator, EndoVac, PUI and LAI). An in vitro study. Clin Oral Investig 22:993–999. https://doi.org/10.1007/s00784-017-2179-y
Neelakantan P, Cheng CQ, Mohanraj R et al (2015) Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro. Int Endod J 48:602–610. https://doi.org/10.1111/iej.12354
Ordinola-Zapata R, Bramante CM, Aprecio RM et al (2014) Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. Int Endod J 47:659–666. https://doi.org/10.1111/iej.12202
Passalidou S, Calberson F, De Bruyne M et al (2018) Debris removal from the mesial root canal system of mandibular molars with laser-activated irrigation. J Endod 44:1697–1701. https://doi.org/10.1016/j.joen.2018.06.007
Peters OA, Bardsley S, Fong J et al (2011) Disinfection of root canals with photon-initiated photoacoustic streaming. J Endod 37:1008–1012. https://doi.org/10.1016/j.joen.2011.03.016
Swimberghe RCD, De Clercq A, De Moor RJG, Meire MA (2019) Efficacy of sonically, ultrasonically and laser-activated irrigation in removing a biofilm-mimicking hydrogel from an isthmus model. Int Endod J 52:515–523. https://doi.org/10.1111/iej.13024
Verstraeten J, Jacquet W, De Moor RJG, Meire MA (2017) Hard tissue debris removal from the mesial root canal system of mandibular molars with ultrasonically and laser-activated irrigation: a micro-computed tomography study. Lasers Med Sci 32:1965–1970. https://doi.org/10.1007/s10103-017-2297-4
Shan X, Tian F, Li J et al (2022) Comparison of Er:YAG laser and ultrasonic in root canal disinfection under minimally invasive access cavity. Lasers Med Sci 37:3249–3258. https://doi.org/10.1007/s10103-022-03613-0
Aung NPS, Watanabe S, Okiji T (2021) Er:YAG laser-activated irrigation in comparison with different irrigation systems for cleaning the apical root canal area beyond ledge. Photobiomodul Photomed Laser Surg 39:759–765. https://doi.org/10.1089/photob.2021.0044
Betancourt P, Merlos A, Sierra JM et al (2019) Effectiveness of low concentration of sodium hypochlorite activated by Er,Cr:YSGG laser against Enterococcus faecalis biofilm. Lasers Med Sci 34:247–254. https://doi.org/10.1007/s10103-018-2578-6
Betancourt P, Merlos A, Sierra JM et al (2020) Er,Cr:YSGG laser-activated irrigation and passive ultrasonic irrigation: comparison of two strategies for root canal disinfection. Photobiomodul Photomed Laser Surg 38:91–97. https://doi.org/10.1089/photob.2019.4645
Race J, Zilm P, Ratnayake J et al (2019) Efficacy of laser and ultrasonic-activated irrigation on eradicating a mixed-species biofilm in human mesial roots. Aust Endod J 45:317–324. https://doi.org/10.1111/aej.12334
Peeters HH, Suardita K (2011) Efficacy of smear layer removal at the root tip by using ethylenediaminetetraacetic acid and erbium, chromium: yttrium, scandium, gallium garnet laser. J Endod 37:1585–1589. https://doi.org/10.1016/j.joen.2011.08.022
Ghorbanzadeh A, Aminsobhani M, Sohrabi K et al (2016) Penetration depth of sodium hypochlorite in dentinal tubules after conventional irrigation, passive ultrasonic agitation and Nd:YAG laser activated irrigation. J Lasers Med Sci 7:105–111. https://doi.org/10.15171/jlms.2016.18
Gu Y, Perinpanayagam H, Kum DJW et al (2017) Effect of different agitation techniques on the penetration of irrigant and sealer into dentinal tubules. Photomed Laser Surg 35:71–77. https://doi.org/10.1089/pho.2016.4125
Plotino G, Pameijer CH, Grande NM, Somma F (2007) Ultrasonics in endodontics: a review of the literature. J Endod 33:81–95. https://doi.org/10.1016/j.joen.2006.10.008
Macedo RG, Wesselink PR, Zaccheo F et al (2010) Reaction rate of NaOCl in contact with bovine dentine: effect of activation, exposure time, concentration and pH. Int Endod J 43:1108–1115. https://doi.org/10.1111/j.1365-2591.2010.01785.x
Guidotti R, Merigo E, Fornaini C et al (2014) Er:YAG 2,940-nm laser fiber in endodontic treatment: a help in removing smear layer. Lasers Med Sci 29:69–75. https://doi.org/10.1007/s10103-012-1217-x
Mancini M, Cerroni L, Palopoli P et al (2021) FESEM evaluation of smear layer removal from conservatively shaped canals: laser activated irrigation (PIPS and SWEEPS) compared to sonic and passive ultrasonic activation-an ex vivo study. BMC Oral Health 21:81. https://doi.org/10.1186/s12903-021-01427-0
Vatanpour M, Toursavadkouhi S, Sajjad S (2022) Comparison of three irrigation methods: SWEEPS, ultrasonic, and traditional irrigation, in smear layer and debris removal abilities in the root canal, beyond the fractured instrument. Photodiagnosis Photodyn Ther 37:102707. https://doi.org/10.1016/j.pdpdt.2021.102707
Acknowledgements
The authors acknowledge the Portuguese Foundation for Science and Technology (FCT) and the Division of Dental Biomaterials at the University of Zurich for the financial support. The financial support was provided by FCT (Portugal) within the subject of the following project: PTDC/EMEEME/ 4197/2021. Also, the authors acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) through the project CAPES- PRINT/88881.310728/2018-01.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The atuhors decalre there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blakimé, A., Henriques, B., Silva, F.S. et al. Smear layer removal and bacteria eradication from tooth root canals by Erbium lasers irradiation. Laser Dent Sci 7, 167–193 (2023). https://doi.org/10.1007/s41547-023-00194-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-023-00194-1