Abstract
Higher level of fluoride in underground water is a burning problem in many parts of the world. Continuous ingestion of fluoride containing water leads to fluorosis. In the present work, natural banana (Musa acuminate) peel (NBP), an unconventional adsorbent, has been explored for removal of fluoride from aqueous system. The NBP was characterized by using SEM, FTIR, and point of zero charge. In the batch study, maximum fluoride removal was achieved at the pH 4.0 (pHZPC 5.63), taking about 60 min to attain equilibrium. Kinetics data were fitted with a pseudo-second-order (R 2 = 0.99) and Bahangam models (R 2 = 1.00). The experimental data fitted well to a D-R adsorption isotherm (R 2 = 0.995). Thermodynamic data suggests that the adsorption of fluoride on NBP is an exothermic reaction. Therefore, the results suggest that natural banana peel dust could be a cost-effective, eco-friendly adsorbent for removal of fluoride from aqueous medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to rapid industrialization, huge quantity of pollutants discharges in to the environment. Fluorine is one such pollutant that threatens living organisms, in particular, humans [1]. Fluorine is the strongest electronegative element and belongs to group 17 in modern periodic table. Its atomic number and molecular weight is 9 and 19, respectively [2]. Very small quantity of fluorine is required for hardening of enamel and protection against dental caries. The excessive intake of fluoride leads to dental as well as skeletal fluorosis [3]. Apart from dental and skeletal fluorosis, fluoride can bind to the functional amino acid groups located around the active center of an enzyme to cause an in inhibitory effect which leads to decrease enzyme activity [4]. The main source of fluoride in the environment is entirely geogenic in nature [5]. However, recently, literature cited that it enters through anthropogenic activities such as industrial drains [1]. Very recently, Das et al. [6] and Maitra et al. [7] highlighted that fluoride can interfere in the plant system also. Due to its strong negative impact on both animal and plant kingdom, the World Health Organization (WHO) has set a stringent guideline value of fluoride in drinking water at 1.5 mg/L. [8]. The problem with high fluoride in drinking water is a major problem in several tropical countries like India [9]. The only possible remedy of this problem is defluoridation [10].
Many techniques are available to remove fluoride from contaminated water such as reverse osmosis, electrodialysis, Donan dialysis, nanofiltration, and ion exchange [11]. But very recently, attention of scientists has been focused to use very low-cost, easily available materials [12]. A good number of low cost materials have been tested such as sugarcane ash [13], Tea ash [14], coconut fiber dust [15], lemon leaf dust [16], egg shell dust [17], calcareous soil [18], and rice husk ash [19]. However, most of the adsorbents are suitable at lower concentration and showed excellent performance at an extreme pH value, such as activated coal showed good fluoride uptake capacity at pH less than 3 [20]. Therefore, it is a great challenge among the scientists to explore low-cost, easily available, and eco-friendly adsorbents which remove fluoride from contaminated water under neural pH [21].
Fruits peels have attracted considerable interest as potential candidates for removal of pollutants from aqueous solution due to their inexpensiveness, availability, environmental stability, and high surface area/sorption capacity [15]. Among the various fruits peel, banana peel is a major waste in many countries including India. Previous literature highlighted that banana peel extensively used for removal of heavy metals [22, 23]. But very handful of researcher tried to use banana peel as an adsorbent for removal of fluoride. From this viewpoint, present research has been formulated with the following objectives: (i) to prepare banana peel dust, (ii) to characterization of adsorbent through SEM, FTIR, and pHZPC, study; (iii) the experimental data will fitted in various isotherm, kinetic, and thermodynamics equations; (iv) comparative study with previously published adsorbents; and (v) application of banana peel dust for defluoridation from filed samples.
Materials and Methods
Preparation of Natural Banana Peel Dust
The raw waste banaba peels (Musa acuminate) were collected from nearby fruits stall of Burdwan town, West Bengal. After collection, peels were thoroughly washed with tap water followed by washing with de-ionized water and dried in hot air oven at 50 °C for 12 h. The dried peels were cut into small pieces and again dried in hot air oven for 24 h, maintaining 60 °C temperature. The dried banana peels were grinded in kitchen grinder to get the desired particle size (200 μm) using suitable the powder form of banana peel was stored in air light container (Table 1).
Point of Zero Charge (pHZPC)
Zero point charge of the NBP was determined by following the solid addition method [24]. Initially, a series of conical flasks were taken with 50 mL of 0.1 (M) KNO3 solutions in each flask along with 0.1 g NBP. The pH of the 0.1 (M) KNO3 solutions was adjusted by using 0.05 (N) HNO3 and 0.1 (N) KOH solution. After thoroughly shaking (24 h), the pH of the final solutions was recorded and a graph was constructed by pH vs ΔpH.
Adsorption Experiments
The entire adsorption experiment was carried out by varying the operating variables such as pH, initial adsorbate concentration (ppm), dose (g), adsorbent particle size, contact time (min), shaking speed, and temperature (K). Batch experiments were carried out in a 250-ml conical flask containing fluoride solution (2.0–20.0 ppm) with varying adsorbent dose (0.1–10 gL−1). The pH of the respective solutions was maintained by using 0.1(N) HCl and/or 0.1(N) NaOH. The contact time was (5–80 min) and temperature (300–333 K). After adsorption, the residual fluoride concentration was measured by ion selective electrode (Orion 4 star). The fluoride sample solution and fluoride standard solutions were diluted 1:1 with a TISAB solution [25]. The percentage of fluoride adsorption was calculated by using the following Eq. (1):
The adsorption capacity (mg/g) of the experimental adsorbent was calculated by using the following Eq. (2):
where C 0 and C e denoted the initial and equilibrium fluoride ion concentrations (mg/L), respectively. V is the volume of the solution in liters, and m is the mass of the adsorbent used (g).
Instrumentation
A number of instruments were used for characterization of adsorbent and estimation of fluoride from aqueous medium such as scanning electron microscop (SEM) (HITACHI-S-530, Scanning Electron Microscope and ELKO Engineering) at an accelerating voltage of 20.0 kV, Fourier transform infrared spectroscopy (FTIR) (BRUKER, Tensor 27), and ion selective electrode (Orion 4 star).
Results and Discussion
Zero Point Charge of NBP
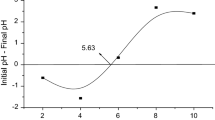
From Fig. 1, it is clear that the pHZPC of NBP is 5.63. That means, at this pH, the surface of NBP has no charge. However, below the pH (5.63), surface of NBP changes to positive and above the pH (5.63), the surface is negatively charged.
FTIR Analysis
FTIR is a valuable technique to identify the function groups present on the surface of the adsorbents. Present adsorbent exhibit various sharp peaks such as 3905–3258 cm−1 which is attributed to the alcoholic -OH stretching; 1631, 1269, and 1116–624 cm−1, respectively, assigned to –C = O stretching of carboxylic acid or ester or ether and N-H deformation of amines, respectively (Figure Supply 1). After adsorption, the shifting of -OH and the slight decrease in intensity indicate the involvement of OH in the fluoride adsorption (Figure not supplied). Almost similar observation was reported by Mondal et al. [14] for removal of fluoride by using low-cost waste tea ash as adsorbent.
SEM Analysis
Scanning electron micrograph has immense potentiality for understanding the surface morphology of the adsorbents. SEM photograph of NBP before and after loading of fluoride was presented in the Fig. 2a, b. From Fig. 2a, it is clearly revealed that the surface of NBP has numerous micro-rough, porous structures which might be the area of fluoride loading. However, after fluoride removal, the surface of NBP does not exhibit such rough areas as before (Fig.2b). Almost similar peel SEM structure of banana and orange has been demonstrated by previous researchers for removal of heavy metals from aqueous solution [26]. This observation clearly indicates that NBP has tremendous role towards the affinity of fluoride binding.
Effect of pH
pH of the solution has remarkable role in both metal and nonmetal adsorption process [26]. Figure 3 clearly revealed that F removal remarkable elevated with changing pH from 2.0 to 4.0. However, after pH 4, the removal of F adsorption sharply declines with increasing alkalinity of the fluoride solution. This phenomenon nicely interpreted with the help of ZPC of NBP. From the Fig. 1, it is clear that the ZPC of NBP is 5.63 and maximum F adsorption occurs at pH 4 which is below the pH zpc of NBP. That means the main driving force of F adsorption is purely electrostatic in nature [20] as per the Eqs. (4) and (3):
The removal of fluoride at lower pH is also reported by the earlier researchers [14, 18, 20]. Almost similar observation was reported by yadav et al. [27] in their earlier work where they argued that under acidic condition, the surface of the adsorbent changes to positive change which facilitate the fluoride adsorption through anion exchange.
Effect of Initial Concentration
The effect of initial concentration of fluoride is depicted in the Fig. 4. From the Fig. 4, it is clear that fluoride removal increased with increasing influent concentration from 2.0 to 20.0 mg/L. and maximum fluoride uptake of 0.96 mg/g was recorded at 30.08 mg/L. This could be attributed to the possible higher interaction between F ions and the binding sites on the NBP surface and saturation of all binding sites with F ions. Therefor, it was inferred that higher concentration of F in the solution is responsible for the increase in the percentage of F removal until the saturation of the NBP biomass is atained. Almost similar uptake pattern of fluoride was reported by Koilraj and Kannan [1].
Effects of Adsorbent Dose
The fluoride removal increases with the amount of NBP used (Fig. 5). From the Fig. 5, it is clear that, when the adsorbent amount increased from 0.1 to 0.15 g/100 ml, the fluoride removal increased from 89 to 96.7%, respectively. This gradual increment is due to the enhancement of the surface area which promoted the sorption of fluoride [12]. On the other hand, the adsorption capacity (qe mg/g) value decreased for a fixed influent (fluoride) concentration (16 mg/L) with the increase of adsorbent dose. This finding nicely fitted with the increase of adsorbent dose for a fixed solute load, and surface sites heterogeneity of the adsorbent [28]. According to surface site heterogeneity model, the surface is composed of sites with a spectrum of binding energies at the high adsorbent dose, the availability of higher energy sites decreases with a large fraction of lower energy sites occupied, resulting in a low qe value. However, at lower adsorbent dose, all types of sites entirely exposed and the adsorption on the surface is saturated faster showing a higher qe value [29, 30].
Effect of Contact Time
Figure 6 shows fluoride removal at different contact times using NBP as biosorbent. With increasing contact time, fluoride removal increased rapidly. However, after reaching maximum value (94%), removal gradually approached a more or less constant value indicating attainment of equilibrium. The adsorption nature of fluoride on NBP may be considered to be occurring in three distinct phases. In the first phase, rapid adsorption occurs. This may be the results of instantaneous sorption of fluoride on the surface of NBP. In the second phase, sorption of fluoride decrease. This is perhaps due to migration of fluoride from boundary layer to the interior pore [12]. In the third phase, the entire adsorption process attained equilibrium at 60 min due to non-availability of active sites. Almost similar observation reported by Nasr et al. [12] and Fan et al. [31]. As after 60 min there is no further improvement in fluoride adsorption, an equilibrium time of 60 min was chosen for all subsequent experiments.
Effect of Temperature
Temperature is a crucial parameter in adsorption process. The effect of temperature on fluoride removal was studied in the solution temperature range from 300 to 333 K and it is presented in Fig. 7. It reveals that fluoride removal decreased with the increase in temperature. This result suggests higher temperature did not favor adsorption of F ions. The removal efficiency of fluoride at higher temperature can be attributed by the fact that the molecules get excitation at higher temperature and ultimately breakdown the attractive force between surface atom of the adsorbent and fluoride [32].
Effect of Co-ions on Fluoride Uptake
In general, field samples contain many anions besides the anion of interest. Therefore, the study of fluoride uptake in presence of other co-ions is highly desirable with respect to environmental contamination point of view. Present study was conducted with the anions such as carbonate, nitrate, sulfate, chloride, and phosphate and the results are presented in Fig. 8. The concentration of fluoride was taken as 30 mg/L along with 50 mg/L concentration other co-ions. Results revealed that fluoride uptake strongly influenced by carbonate followed by phosphate and sulfate. The strong interference of fluoride uptake by carbonate is due to the preferential binding of carbonate on the surface of adsorbent [33]. The interference of phosphate is also reported by earlier researcher [34]. The relative interference of fluoride uptake by co-ions is in the order of CO3 2− > PO4 3− > SO4 2− > NO3 − > Cl−.
Isotherm Study on Fluoride Adsorption
The entire biosorption of fluoride on NBP was assessed by using widely used four isotherms namely Langmuir, Freundlich, and D-R, and Temkin. Results revealed that among the four isotherms, Freundlich isotherm [35] nicely fitted with high correlation coefficient, suggesting multilayer adsorption of fluoride on NBP. The dimensionless separation factors (RL) suggested that the fluoride adsorption is favorable as the values of RL less than 1 and greater than zero for all studied initial concentration of fluoride [36]. All the isotherms constant along with the regression coefficients (R L ) are provided in the Table 2. From Table 2, it is clear that the maximum adsorption capacity was recorded as 16.14 mg/g from D-R equation and the value is higher than Langmuir isotherm [37]. KL (L/g) is the adsorption constant related to the affinity of binding sites (L/g) and lower values of KL (0.13) indicate that the particles radius of adsorbent was small towards adsorption [38]. Moreover, the adsorption energy (E) was also calculated from D-R equation and it clearly revealed that fluoride adsorption onto the NBP is absolutely physisorption in nature (as the value of E < 8). Table 2 also provides the valuable information regarding heat of adsorption (BT) and Temking isotherm binding constant, KT (L/min). Results indicate that the value of BT < 8, therefore, interaction between F and NBP is purely physisorption in nature.
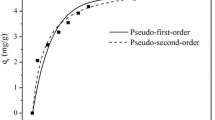
Kinetic Study in Adsorption of Fluoride
The kinetics of fluoride adsorption by NBP was assessed by the use of pseudo-first-order, pseudo-second-order, intraparticles diffusion and Bahangam kinetic models. The adsorption kinetics not only describes the adsorption mechanism of adsorbates on the adsorbents but also describes the adsorption rate which controls the contact time of adsorbates at the solid-liquid interface [39]. The linearized forms of all the four kinetics models along with different constant terms and regression coefficient values are presented in Table 3. From the Table 3, it is clear that biosorption kinetics nicely fitted with Bahangam equation and pseudo-second-order kinetics. However, pseudo-first-order and intraparticles models showed moderate agreement with the studied results. The kinetics of adsorbate-adsorbent interaction depends on many factors such as nature of adsorbate and adsorbent, pH of the aqueous medium, temperature of the adsorption reaction, average time of contact between adsorbate and adsorbent and mass transport process [40]. The data were further used to investigate the slow step occurring in the present adsorption system. The applicability of Bahangam’s equation was given by Aharoni and Ungarish [41]. As such loglog[C0/(C0-qtm)] was plotted against log t (Figure not shown) and the constant values presented in Table 3. The plot was found liner indicating that kinetics confirmed to Bahangam’s equation and therefore the adsorption of fluoride on NBP was pore diffusion control [42].
Thermodynamic Parameters
The entire biosorption of fluoride by NBP were calculated by the following Eqs. (5–6):
where ∆G 0, ∆S 0, and ∆H 0are the thermodynamics parameters free energy, entropy and enthalpy, respectively, and other two constants are K (is the distribution co-efficient (\( K=\frac{q_e}{C_e} \))) and R ( is the universal gas constant). The Van’t Hoff plot of lnKc against 1/T (plot not shown) was used to calculate the free energy (∆G 0), entropy (∆S 0), and enthalpy (∆H 0) by varying temperature from 313 to 343 K, and the calculated value is depicted in Table 4. The results suggest that the F adsorption is not favorable at higher temperature. Moreover, the negative value of enthalpy change indicated that fluoride biosorption on NBP was exothermic [43]. The positive entropy change during adsorption was due to gain of translational degree of freedom [44]. The negative free energy change with increasing temperature suggests the feasibility of the process and its spontaneous nature [45].
Comparison with Other Adsorbents
In order to compare the adsorption capacity of the studied adsorbent with previously reported adsorbents, a comprehensive table has been provided (Table 5). From the Table 5, it is clear that the adsorbents such as Cynodon dactylon-based activated carbon, lemon leaf dust (LLD-1, LLD-2 and LLD-3), and coconut fiber dust (CFD-1, CFD-2 and CFD-3) showed moderate to high fluoride adsorption capacity ranging from 4.617 to 38.46 mg/g. However, the other adsorbents such as used tea leaves, treated banana peel, treated coffee husk, activated rice husk, Eichhornia crassipes, lichen, and activated silica gel showed low fluoride adsorption capacity (ranges from 0.395 to 0.81). The present adsorbent showed the adsorption capacity 1.212 mg/g and it is better than activated bagasse carbon, activated alumina, eggshell dust, and fungal biomass (Table 5).
Biosorption of Fluoride from Filed Samples
In order to check the efficacy of tested adsorbent, batch studies were conducted to evaluate the adsorption performance of the filed samples. The samples were collected from three fluoride affected villages of Birbhum district, West Bengal, India. Initially physic-chemical parameters along with fluoride level were measured and depicted in Table 6. The entire batch adsorption was performed with the best optimized conditions of the banana peel (dose 0.15 g/100 ml; pH 4.0; contact time 60 min; temperature 302 K). The studied adsorbent removed fluoride as 83.65, 78.83, and 98.80% for the water samples collected from the villages of Alampur, Saldha, and Nasipur, respectively.
Conclusions
Research was carried out to explore banana peels as a high capacity, economically viable, and low-cost adsorbent for removal of fluoride. Adsorbent characterization has been done by pHZPC, SEM, and FT-IR studies. The pH played pivotal on the sorbate adsorption capacity onto the natural banana peel biosorbent. The decrease in the solution pH led to a significant increase in the adsorption capacities of all parameters on the banana peel biosorbent with maximum adsorption capacity. Batch study results indicate that best fluoride removal condition is 10 mg/L initial fluoride concentration, 0.15 g100 ml adsorbent dose, pH 6.0, and contact time 60.0 min. Isotherm of the batch study best fitted with D-R isotherm model with high R 2 (0.995) values. The kinetics of the batch study clearly demonstrated that fluoride adsorption on banana peel dust is nicely fitted with Bahangam and pseudo second-order kinetic models, and thermodynamic study suggests that the defluridation with NBP is an exothermic process. The relative interference of fluoride uptake by co-ions is in the order of CO3 2− > PO4 3− > SO4 2− > NO3 − > Cl−. Finally, the studied adsorbent showed excellent performance towards removal of fluoride from field samples. Therefore, the use of banana peel could be a cost-effective adsorbent and may provide an alternative method for removal of fluoride from contaminated water.
References
Koilraj P, Kannan S (2013) Aqueous fluoride removal using ZnCr layered double hyroxides and their polymeric composites: batch and column studies. Chem Eng J 234:406–415
Bhaumik R, Mondal NK (2014) Adsorption of fluoride from aqueous solution by a new low-cost adsorbent: thermally and chemically activated coconut fibre dust. Clean Techn Environ Policy DOI. doi:10.1007/s10098-015-0937-6
Mondal NK, Das B, Bhaumik R, Bour T, Roy P (2012) Calcareous soil as a promising adsorbent to remove fluoride from aqueous solution: equilibrium, kinetic and thermodynamic study. J Modern Chem Technol 3(3):1–21
Trivedi MH, Verma RJ, Chinoy NJ et al (2007) Effect of high fluoride water on intelligence of school children in India. Fluoride 40:178–183
Peckham S, Lowery D, Spencer S (2015) Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. J Epidemiol Commu Health 0:1–6. doi:10.1136/jech-2014-204971
Das C, Dey U, Chakraborty D, Datta JK, Mondal NK (2015) Fluoride toxicity effects in potato plant (Solanum tuberosum l.) grown in contaminated soils. Octa J EnvironRes 3(2):136–143
Maitra A, Datta JK, Mondal NK (2013) Amelioration of fluoride toxicity with the use of indigenous inputs. J Stress Physiol Biochem 9(3):207–219
WHO (2011) Guideline for drinking-water quality, 4th edn. WorldHealth Organization, Geneva
Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Matter 137(1):456–463
Kameda T, Oba J, Yoshioka T (2015) Recyclable Mg–Al layered double hydroxides for fluoride removal: kinetic and equilibrium studies. J Hazard Mater 300:475–482
Kabay N, Arar O, Samatya S, Yüksel U, Yüksel M (2008) Separation of fluoride from aqueous solution by electrodialysis: effect of process parameters and other ionic species. J Hazard Mater 153:107–113
Nasar AB, Walha K, Charcosset C, Amar RB (2011) Removal of fluoride ions using cuttle fish bones. J Fluoride Chem 132:57–62
Mondal NK, Bhaumik R, Roy P, Das B, Datta JK (2013) Investigation on fixed bed column performance of fluoride adsorption by sugarcane charcoal. J Environ Biol 34:1059–1064
Mondal NK, Bhaumik R, Bour T, Das B, Roy P, Datta JK (2012) Studies on defluoridation of water by tea ash: an unconventional biosorbent. Chem Sci Tranction 1(2):239–256
Bhaumik R, Mondal NK (2015) Adsorption of fluoride from aqueous solution by a new low-cost adsorbent: thermally and chemically activated coconut fiber dust. Clean Techn Environ Policy. doi:10.1007/s10098-015-0937-6
Bhaumik R, Mondal NK, Chattoraj S, Datta JK (2013) Application of response surface methodology for optimization of fluoride removal mechanism by newly developed biomaterial. Am J Anal Chem 4:404–419
Bhaumik R, Mondal NK, Das B, Roy P, Pal KC, Das C, Banerjee A, Datta JK (2012) Eggshell powder as an adsorbent for removal of fluoride from aqueous solution: equilibrium, kinetic and thermodynamic studies. E J Chemistry 29(3):1457–1480
Mondal NK, Das B, Bhaumik R, Roy P (2012c) Calcareous soil as a promising adsorbent to remove fluoride from aqueous solution: equilibrium, kinetic and thermodynamic study. J Mod Chem Chemi Technol 3(3):1–12
Mondal NK, Bhaumik R, Banerjee A, Datta JK, Baur T (2012) A comparative study on the batch performance of fluoride adsorption by activated silica gel and activated rice husk ash. Inter J Environ Sci 2(3):1643–1661
Tripathy SS, Bersillon JL, Gopal K (2006) Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep Purif Technol 50:310–317
Kumar E, Bhatnagar A, Ji M, Jung W, Lee SH, Kim SJ, Lee G, Song H, Choi JY, Yang JS, Jeon BH (2009) Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Res 43:490–498
Abbasi Z, Alikarami M, Nezhad ER, Moradi F, Moradi V (2013) Adsorptive removal of Co2+ and Ni2+ by peels of banana from aqueous solution. Univer J Chem 1(3):90–95
Memon JR, Memon SQ, Bhanger MI, Memon GZ, El-Turki A, Allen GC (2008) Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal. Colloids Surf B: Biointerfaces 66:260–265
Mondal MK (2010) Removal of Pb(II) from aqueous solution by adsorption using activated tea waste. Korean J Chem Eng 27(1):144–151
Kennedy JH (1990) Analytical chemistry principles, 2nd edn. W.B. Saunders, New York
Ghosh SB, Bhaumik R, Mondal NK (2016) Optimization study of adsorption parameters for removal of fluoride using aluminium-impregnated potato plant ash by response surface methodology. Clean Techn Environ Policy 18:1069–1083
Yadav AK, Abbassi R, Gupta A, Dadashzadeh M (2013) Removal of fluoride from aqueous solution and groundwater by wheat straw, sawdust and activated bagasse carbon of sugarcane. Ecol Eng 52:211–218
Chen N, Feng C, Li M (2014) Fluoride removal on Fe-Al-impregnated granular ceramic adsorbent from aqueous solution. Clean Techn Environ Policy 16:609–617
Daifullah AAM, Yakout SM, Elreefy SA (2007) Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from stem pyrolysis of rice straw. J Hazard Mater 147:633–643
Das N, Pattanaik P, Das R (2005) Defluoridation of drinking water using activated titanium rich bauxite. J Colloid Interface Sci 292:1–10
Fan X, Parker DJ, Smith MD (2003) Adsorption kinetics of fluoride on low cost materials. Water Res 37:4929–4937
Sujana MG, Anand S (2010) Fluoride removal studies from contaminated ground water by using bauxite. Desalination 267(2–3):222–227
Miyata S (1983) Anion-exchange properties of hydrotalcite-like compounds, clay. Clay Miner 31:305–311
Mondal NK, Bhaumik R, Datta JK (2015) Removal of fluoride by aluminium impregnated coconut fiber from synthetic fluoride solution and natural water. Alexandria Eng J. doi:10.1016/j.aej.2015.08.006
Freundlich H (1906) Over the adsorption in solution. J Phy Chem 57(A):385
Chen N, Zhang Z, Feng C, Li M, Zhu D, Chen R, Sugiura N (2010) An excellent fluoride sorption behaviour of ceramic adsorbent. J Hazard Mater 183:460–465
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica, and platinum. J Amer Chem Society 40:1361–1403
Anwar J, Shafique U, Zaman W, Salman M, Dar A, Anwar S (2010) Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Biores Technol 101:1752–1755
Ho YS, Porter JF, McKay G (2002) Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel and lead single component systems. Water Air Soil Pollut 141:1–33
Achak M, Hafidi A, Ouazzani N, Sayadic S, Mandi L (2009) Low cost biosorbent banana peel for the removal of phenolic compounds from olive mill wastewater: kinetic and equilibrium studies. J Hazard Mater 166:117–125
Aharoni C, Ungarish M (1977) Kinetics of activated chemisorptions. Part 2. Theoretical models. J Chem Soc Faraday Trans 73:456–464
Gupta VK, Ali I, Saini VK (2007) Defluoridation of wastewater using waste carbon slurry. Water Res 41:3307–3316
Sujana MG, Anand S (2011) Fluoride removal studies from contaminated ground water by using bauxite. Desalination 267:222–227
Chen SY, Shen W, Yu F, Wang HP (2009) Kinetic and thermodynamic studies of adsorption of Cu2+ and Pb2+ onto amidoximated bacterial cellulose. Polym Bull 63:283
Chen L, He S, He B-Y, Wang T-J, C-Li S, Zhang C, Jin Y (2012) Synthesis of iron-doped titanium oxide nanoadsorbent and its adsorption characteristics for fluoride in drinking water. Ind Eng Chem Res 51:13150–13156
Methodis PA, Selvapathy P (2005) Studies on removal of fluoride from water by used tea leaves carbon. In: 3rd international conference on plants and Environmental Pollution, Lucknow, India
Alagumuthu G, Veeraputhiran V, Venkataraman R (2011) Fluoride adsorption using Cynodon dactylon-based activated carbon. Hem Ind 65:23–35
Ramanaiah SV, Venkatamohan S, Rajkumar B, Sarma PN (2006) Monitoring of fluoride concentration in groundwater of Prakasham District in India: correlation with PhysicoChemical parameters. J Environ Sci Engg 48:129–134
Getachew T, Hussen A, Rao VM (2015) Defluoridation of water by activated carbon prepared from banana (Musa paradisiaca) peel and coffee (Coffea arabica) husk. Int J Environ Sci Technol 12:1857–1866
Mondal NK, Kundu M, Das K, Bhaumik R, Datta JK (2013a) Biosorption of fluoride from aqueous phase onto aspergillus and its calcium-impregnated biomass and evaluation of adsorption kinetics. Fluoride 46(4):239–245
Ganvir V, Das K (2011) Removal of fluoride from drinking water using aluminum hydroxide coated rice husk ash. J Hazard Mater 185:1287–1294. doi:10.1016/j.jhazmat.2010.10.044
Alagumuthu G, Rajan M (2010a) Kinetic and equilibrium studies on fluoride removal by zirconium (IV)-impregnated ground nutshell carbon. Hem Ind 64(4):295–304
Sinha S, Pandey KP, Mohan D, Singh KP (2003) Removal of fluoride from aqueous solutions by Eichhornia crassipes biomass and its carbonizsed form, Ind. Eng Chem Res 42:6911–6918
Mondal NK, Kundu M (2016) Biosorption of fluoride from aqueous solution using lichen and its Ca-pretreated biomass. Water Conserv Sci Eng. doi:10.1007/s41101-016-0009-8
Mondal NK, Bhaumik R, Datta JK (2016) Fluoride adsorption by calcium carbonate, activated alumina and activated sugarcane ash. Environ Process. doi:10.1007/s40710-0169-1083
Acknowledgements
The authors are grateful to Dr. Rajarshi Ghosh, Assistant Professor, Department of Chemistry, Burdwan University, Burdwan, West Bengal, India for recording FTIR data and they also extend their gratitude to Dr. Srikanta Chakraborty, Incharge of SEM, USIC, University of Burdwan, West Bengal, India for SEM study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
.
Figure S1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Mondal, N.K. Natural Banana (Musa acuminate) Peel: an Unconventional Adsorbent for Removal of Fluoride from Aqueous Solution through Batch Study. Water Conserv Sci Eng 1, 223–232 (2017). https://doi.org/10.1007/s41101-016-0015-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-016-0015-x