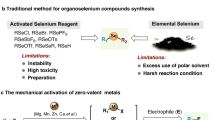
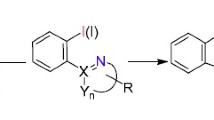
Abstract
Organoselenium compounds have been the subject of extensive research since the discovery of the biologically active compound ebselen. Ebselen has recently been found to show activity against the main protease of the virus responsible for COVID-19. Other organoselenium compounds are also well-known for their diverse biological activities, with such compounds exhibiting interesting physical properties relevant to the fields of electronics, materials, and polymer chemistry. In addition, the incorporation of selenium into various organic molecules has garnered significant attention due to the potential of selenium to enhance the biological activity of these molecules, particularly in conjunction with bioactive heterocycles. Iodine and iodine-based reagents play a prominent role in the synthesis of organoselenium compounds, being valued for their cost-effectiveness, non-toxicity, and ease of handling. These reagents efficiently selenylate a broad range of organic substrates, encompassing alkenes, alkynes, and cyclic, aromatic, and heterocyclic molecules. They serve as catalysts, additives, inducers, and oxidizing agents, facilitating the introduction of different functional groups at alternate positions in the molecules, thereby allowing for regioselective and stereoselective approaches. Specific iodine reagents and their combinations can be tailored to follow the desired reaction pathways. Here, we present a comprehensive review of the progress in the selenylation of organic molecules using iodine reagents over the past decade, with a focus on reaction patterns, solvent effects, heating, microwave, and ultrasonic conditions. Detailed discussions on mechanistic aspects, such as electrophilic, nucleophilic, radical, electrochemical, and ring expansion reactions via selenylation, multiselenylation, and difunctionalization, are included. The review also highlights the formation of various cyclic, heterocyclic, and heteroarenes resulting from the in situ generation of selenium intermediates, encompassing cyclic ketones, cyclic ethers, cyclic lactones, selenophenes, chromones, pyrazolines, pyrrolidines, piperidines, indolines, oxazolines, isooxazolines, lactones, dihydrofurans, and isoxazolidines. To enhance the reader’s interest, the review is structured into different sections covering the selenylation of aliphatic sp2/sp carbon and cyclic sp2 carbon, and then is further subdivided into various heterocyclic molecules.
Graphical Abstract

























































































































Similar content being viewed by others
Data Availability
The information presented in this review article can be accessed on the internet through the provided references. The authors affirm that the data substantiating the conclusions drawn in this study are included in the article.
References
Akhoon SA, Naqvi T, Nisar S, Rizvi MA (2015) Synthetic organo-selenium compounds in medicinal domain. Asian J Chem. https://doi.org/10.14233/ajchem.2015.18834
Freudendahl DM, Shahzad SA, Wirth T (2009) Recent advances in organoselenluim chemistry. Eur J Org Chem. https://doi.org/10.1002/ejoc.200990025
Kumar S, Sharma N, Maurya IK et al (2016) Facile synthesis, structural evaluation, antimicrobial activity and synergistic effects of novel imidazo[1,2- a ]pyridine based organoselenium compounds. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2016.07.076
May SW, Pollock SH (1998) Selenium-based antihypertensives. Drugs. https://doi.org/10.2165/00003495-199856060-00001
Mal’tseva VN, Goltyaev MV, Turovsky EA, Varlamova EG (2022) Immunomodulatory and anti-inflammatory properties of selenium-containing agents: their role in the regulation of defense mechanisms against COVID-19. Int J Mol Sci. https://doi.org/10.3390/ijms23042360
Ibrahim M, Muhammad N, Naeem M, Deobald AM, Kamdem JP, Rocha JBT (2015) In vitro evaluation of glutathione peroxidase (GPx)-like activity and antioxidant properties of an organoselenium compound. Toxicol In Vitro. https://doi.org/10.1016/j.tiv.2015.03.017
Radomska D, Czarnomysy R, Radomski D, Bielawski K (2021) Selenium compounds as novel potential anticancer agents. Int J Mol Sci. https://doi.org/10.3390/ijms22031009
Plano D, Karelia DN, Pandey MK, Spallholz JE, Amin S, Sharma AK (2016) Design, synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J Med Chem. https://doi.org/10.1021/acs.jmedchem.5b01503
Barbosa NV, Nogueira CW, Nogara PA, de Bem AF, Aschner M, Rocha JBT (2017) Organoselenium compounds as mimics of selenoproteins and thiol modifier agents. Metallomics. https://doi.org/10.1039/c7mt00083a
Domínguez-Álvarez E, Gajdács M, Spengler G et al (2016) Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg Med Chem Lett. https://doi.org/10.1016/j.bmcl.2016.04.064
Weglarz-Tomczak E, Tomczak JM, Talma M, Burda-Grabowska M, Giurg M, Brul S (2021) Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci Rep. https://doi.org/10.1038/s41598-021-83229-6
Sancineto L, Mariotti A, Bagnoli L et al (2015) Design and synthesis of diselenobisbenzamides (DISeBAs) as nucleocapsid protein 7 (NCp7) inhibitors with anti-HIV activity. J Med Chem. https://doi.org/10.1021/acs.jmedchem.5b01183
Vilela F, Vobecka Z, Skabara PJ (2009) Organic conductors containing selenium and tellurium. PATAI’S Chem Funct Groups. https://doi.org/10.1002/9780470682531.pat0714
Chhabria S, Desai K (2016) Selenium nanoparticles and their applications. Encyclopedia of nanoscience and nanotechnology. American Scientific Publishers, Valencia, pp 1–32
Xia J, Li T, Lu C, Xu H (2018) Selenium-containing polymers: perspectives toward diverse applications in both adaptive and biomedical materials. Macromolecules. https://doi.org/10.1021/acs.macromol.8b01597
Cao W, Wang L, Xu H (2015) Selenium/tellurium containing polymer materials in nanobiotechnology. Nano Today. https://doi.org/10.1016/j.nantod.2015.11.004
Calvez L, Ma HL, Lucas J, Zhang XH (2007) Selenium-based glasses and glass ceramics transmitting light from the visible to the far-IR. Adv Mater. https://doi.org/10.1002/adma.200601962
Naumov AV (2010) Selenium and tellurium: state of the markets, the crisis, and its consequences. Metallurgist. https://doi.org/10.1007/s11015-010-9280-7
Duan Y, Luo G, Jung B, Kaushik V, Batchelor B, Abdel-Wahab A (2017) Photochemical degradation of arsenic and selenium with advanced reduction processes—effects of reagents. Environ Eng Sci. https://doi.org/10.1089/ees.2016.0513
Zhong W, Sun F, He G (2020) Photochemical preparation of selenium-rGO composite for application in photocatalytic degradation. IOP Conf Ser: Mater Sci Eng. https://doi.org/10.1088/1757-899X/730/1/012040
Gao X, Gao T, Zhang L (2003) Solution-solid growth of α-monoclinic selenium nanowires at room temperature. J Mater Chem. https://doi.org/10.1039/B209399E
Kurokawa Y, Nakagawa T, Maeda S et al (2015) High-sensitivity image sensor with stacked structure comprising crystalline selenium photoconductor, crystalline OS FET, and CMOS FET. IISW 348–351
Shaw RF, Ghosh AK (1980) Selenium heterostructure solar cells. Solar Cells 1:431
Xu Z, Fan Q, Meng X et al (2017) Selenium-containing medium bandgap copolymer for bulk heterojunction polymer solar cells with high efficiency of 9.8%. Chem Mater. https://doi.org/10.1021/acs.chemmater.7b00729
Ruck M, Locherer F (2015) Coordination chemistry of homoatomic ligands of bismuth, selenium and tellurium. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2014.10.010
Cargnelutti R, Schumacher RF, Belladona AL, Kazmierczak JC (2021) Coordination chemistry and synthetic approaches of pyridyl-selenium ligands: a decade update. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2020.213537
Conrad O, Jansen C, Krebs B (1998) Boron-sulfur and boron-selenium compounds-from unique molecular structural principles to novel polymeric materials. Angew Chem Int Ed. https://doi.org/10.1002/(SICI)1521-3773(19981217)37:23%3C3208::AID-ANIE3208%3E3.0.CO;2-5
Xu H, Cao W, Zhang X (2013) Selenium-containing polymers: promising biomaterials for controlled release and enzyme mimics. Acc Chem Res. https://doi.org/10.1021/ar4000339
Roeinfard M, Zahedifar M, Darroudi M, Khorsand Zak A, Sadeghi E (2020) Preparation and characterization of selenium-decorated graphene quantum dots with high afterglow for application in photodynamic therapy. Luminescence. https://doi.org/10.1002/bio.3798
Kim YW, Bae SM, Liu HB, Kim IW, Chun HJ, Ahn WS (2012) Selenium enhances the efficacy of Radachlorin mediated-photodynamic therapy in TC-1 tumor development. Oncol Rep. https://doi.org/10.3892/or.2012.1820
Sun C, Gao S, Tan Y, Zhang Z, Xu H (2021) Side-chain selenium-grafted polymers combining antiangiogenesis treatment with photodynamic therapy and chemotherapy. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.1c00254
Li Q, Zhang Y, Chen Z et al (2020) Organoselenium chemistry-based polymer synthesis. Org Chem Front. https://doi.org/10.1039/D0QO00640H
Ullah H, Liu G, Yousaf B et al (2019) A comprehensive review on environmental transformation of selenium: recent advances and research perspectives. Environ Geochem Health. https://doi.org/10.1007/s10653-018-0195-8
Nogueira CW, Barbosa NV, Rocha JB (2021) Toxicology and pharmacology of synthetic organoselenium compounds: an update. Arch Toxicol. https://doi.org/10.1007/s00204-021-03003-5
Luo D, Wu G, Yang H et al (2016) Copper-catalyzed three-component reaction for regioselective aryl-and heteroarylselenation of indoles using selenium powder. J Org Chem. https://doi.org/10.1021/acs.joc.6b00229
Becht JM, Le Drian C (2011) Formation of carbon-sulfur and carbon-selenium bonds by palladium-catalyzed decarboxylative cross-couplings of hindered 2, 6-dialkoxybenzoic acids. J Org Chem. https://doi.org/10.1021/jo200344w
Beletskaya IP, Ananikov VP (2011) Transition-metal-catalyzed C−S, C−Se, and C−Te bond formation via cross-coupling and atom-economic addition reactions. Chem Rev. https://doi.org/10.1021/cr100347k
Laitinen RS, Oilunkaniemi R, Chivers T (2019) Introduction of selenium and tellurium into reaction systems. Phys Sci Rev. https://doi.org/10.1515/9783110529340-001
Pop A, Silvestru C, Silvestru A (2019) Organoselenium and organotellurium compounds containing chalcogen-oxygen bonds in organic synthesis or related processes. Phys Sci Rev. https://doi.org/10.1515/psr-2018-0061
Nogueira CW, Zeni G, Rocha JB (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev. https://doi.org/10.1021/cr0406559
Santi C, Tidei C (2014) In comprehensive organic synthesis II, 2nd edn. Elsevier, Amsterdam
Freudendahl DM, Santoro S, Shahzad SA, Santi C, Wirth T (2009) Green chemistry with selenium reagents: development of efficient catalytic reactions. Angew Chem Int Ed. https://doi.org/10.1002/anie.200903893
Ma W, Kaplaneris N, Fang X, Gu L, Mei R, Ackermann L (2020) Chelation-assisted transition metal-catalysed C-H chalcogenylations. Org Chem Front. https://doi.org/10.1039/C9QO01497G
Ren YM, Cai C, Yang RC (2013) Molecular iodine-catalyzed multicomponent reactions: an efficient catalyst for organic synthesis. RSC Adv. https://doi.org/10.1039/C3RA23461D
Liu D, Lei A (2015) Iodine-catalyzed oxidative coupling reactions utilizing C-H and X-H as nucleophiles. Chem Asian J. https://doi.org/10.1002/asia.201403248
Singh FV, Wirth T (2014) Hypervalent iodine-catalyzed oxidative functionalizations including stereoselective reactions. Chem Asian J. https://doi.org/10.1002/asia.201301582
Breugst M, von der Heiden D (2018) Mechanisms in iodine catalysis. Chem Eur J. https://doi.org/10.1002/chem.201706136
Senadi GC, Guo BC, Hu WP, Wang JJ (2016) Iodine-promoted cyclization of N-propynyl amides and N-allyl amides via sulfonylation and sulfenylation. Chem Commun. https://doi.org/10.1039/C6CC05138C
Siddaraju Y, Prabhu KR (2017) Iodine-catalyzed cross dehydrogenative coupling reaction: sulfenylation of enaminones using dimethyl sulfoxide as an oxidant. J Org Chem. https://doi.org/10.1021/acs.joc.7b00073
Wu W, An Y, Li J, Yang S, Zhu Z, Jiang H (2017) Iodine-catalyzed cascade annulation of alkynes with sodium arylsulfinates: assembly of 3-sulfenylcoumarin and 3-sulfenylquinolinone derivatives. Org Chem Front. https://doi.org/10.1039/C7QO00326A
He Y, Liu S, Wen P et al(2016) Iodine-catalyzed regioselective sulfenylation of indoles with thiols in water. ChemistrySelect. https://doi.org/10.1002/slct.201600257
Nandeshwarappa B, Sadashiv S (eds) (2020) Heterocycles—synthesis and biological activities. IntechOpen, London
Vieira AA, Azeredo JB, Godoi M, Santi C, da Silva Junior EN, Braga AL (2015) Catalytic chalcogenylation under greener conditions: A solvent-free sulfur-and seleno-functionalization of olefins via I2/DMSO oxidant system. J Org Chem. https://doi.org/10.1021/jo502621a
Shang Z, Chen Q, Xing L, Zhang Y, Wait L, Du Y (2019) in situ Formation of RSCl/ArSeCl and their oxidative coupling with enaminone derivatives under transition-metal free conditions. Adv Synth Catal. https://doi.org/10.1002/adsc.201900940
Meng XJ, Zhong PF, Wang YM, Wang HS, Tang HT, Pan YM (2020) Electrochemical difunctionalization of olefines: access to selenomethyl-substituted cyclic ethers or lactones. Adv Synth Catal. https://doi.org/10.1002/adsc.201901115
Zeng X, Chen L (2019) Organo-selenium mediated regio-and stereoselective iodoselenylation of alkynes in an aqueous medium: simple access to (E)-β-iodoalkenyl selenides. Org Biomol Chem. https://doi.org/10.1039/C9OB00524B
Ni P, Tan J, Zhao W, Huang H, Deng GJ (2019) Metal free three-component selenopheno [2, 3-b] indole formation through double C–H selenylation with selenium powder. Adv Synth Catal. https://doi.org/10.1002/adsc.201901023
Ji XM, Zhou SJ, Chen F, Zhang XG, Tang RY (2015) Direct sulfenylation of imidazoheterocycles with disulfides in an iodine-hydrogen peroxide system. Synthesis. https://doi.org/10.1055/s-0034-1379941
Bhalla A, Bari SS, Bhalla J, Khullar S, Mandal S (2016) Facile synthesis of novel halogenated 4-pyrazolylspirocyclic-β-lactams: versatile heterocyclic synthons. Tetrahedron Lett. https://doi.org/10.1016/j.tetlet.2016.05.047
Bhalla A, Modi G, Yadav P, Kumar P, Bari SS, Hundal G (2020) Stereoselective C-3 alkylation of trans-3-phenylsulfonyl-β-lactams with organic halides to access C-3 substituted β-lactams using sulfonyl moiety as an activating group. Tetrahedron Lett. https://doi.org/10.1016/j.tetlet.2020.152098
Ghanghas P, Sharma M, Desai D et al (2022) Selenium-based novel epigenetic regulators offer effective chemotherapeutic alternative with wider safety margins in experimental colorectal cancer. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02659-5
Kaur R, Desai D, Amin S et al (2023) Selenocoxib-3, a novel anti-inflammatory therapeutic effectively resolves colitis. Mol Cell Biochem. https://doi.org/10.1007/s11010-022-04532-y
Garg A, Desai D, Bhalla A, Thakur S, Rastogi P, Kaushal N (2023) SelSA-1, a novel HDAC inhibitor demonstrates enhanced chemotherapeutic potential by redox modulation. Sci Rep. https://doi.org/10.1038/s41598-023-36555-w
Narula D, Bari SS, Singh G, Sharma R, Garg A, Bhalla A (2023) Serendipitous discovery of a regioselective synthesis of novel benzoyloxy substituted phenyl/benzyl-sulfanyl/selenylbisesters, 3-benzoyloxy-3-(phenylsulfanyl)-β-lactams and their antimicrobial evaluation. J Sulfur Chem. https://doi.org/10.1080/17415993.2023.2233652
Saini P, Bari SS, Yadav P, Khullar S, Mandal SK, Bhalla A (2022) Synthesis of C2-Formamide(thiophene)pyrazolyl- C4’-carbaldehyde and their transformation to Schiff’s bases and stereoselective trans -β-lactams: mechanistic and theoretical insights. ChemistrySelect. https://doi.org/10.1002/slct.202202172
Saini P, Thakur S, Garg A, Bari SS, Bhalla A (2023) Stereoselective synthesis of trans -benzo[d ]oxazolyl)phenyl substituted β-lactams decorated with C-3 thio/seleno rich motifs: synthetic intermediates for diverse heterocycles. J Sulfur Chem. https://doi.org/10.1080/17415993.2023.2206968
Azeredo JB, Braga AL, Santi C (2020) Solvent-free asymmetric alkoxy-selenylation of styrenes using I2/DMSO catalytic system. In: 1st International Electronic Conference on Catalysis Sciences. https://doi.org/10.3390/ECCS2020-07563
Yao J, Hu D, Zhang J-Q et al (2022) Ring-opening selenation of cyclopropanol for the selective synthesis of β-hydroxy-substituted selenylated ketones. J Org Chem. https://doi.org/10.1021/acs.joc.2c02004
Maity P, Ranu BC (2017) Iodine-catalyzed synthesis of chalcogenophenes by the reaction of 1, 3-dienyl bromides and potassium selenocyanate/potassium sulfide (KSeCN/K2S). Adv Synth Catal. https://doi.org/10.1002/adsc.201701232
Chen W, Zhu X, Wang F, Yang Y, Deng G, Liang Y (2020) Iodine-catalyzed three-component cascade reaction for the synthesis of substituted 2-Phenylnaphtho [1, 3] selenazoles under transition-metal-free conditions. J Org Chem. https://doi.org/10.1021/acs.joc.9b03154
Kim Y, Kim DY (2019) Synthesis of β-selenylated ketones via iodine-mediated selenylation/1, 2-carbon migration sequences of alkenyl alcohols. Tetrahedron Lett. https://doi.org/10.1016/j.tetlet.2019.05.014
Zhong S, Liu Y, Cao X, Wan JP (2017) KIO3-catalyzed domino C (sp2)-H bond sulfenylation and C−N bond oxygenation of enaminones toward the synthesis of 3-sulfenylated chromones. ChemCatChem. https://doi.org/10.1002/cctc.201601273
Rafique J, Saba S, Schneider AR, Franco MS, Silva SM, Braga AL (2017) Metal-and solvent-free approach to access 3-Se/S-chromones from the cyclization of enaminones in the presence of dichalcogenides catalyzed by KIO3. ACS Omega. https://doi.org/10.1021/acsomega.7b00445
Yu JM, Cai C (2018) Iodine (III)-mediated intramolecular sulfeno-and selenofunctionalization of β, γ-unsaturated tosyl hydrazones and oximes. Org Biomol Chem. https://doi.org/10.1039/C7OB02892J
Wang PF, Yi W, Ling Y, Ming L, Liu GQ, Zhao Y (2021) Preparation of selenofunctionalized heterocycles via iodosobenzene-mediated intramolecular selenocyclizations of olefins with diselenides. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2021.02.050
Zhou CF, Zhang YQ, Ling Y, et al (2022) Time-economical synthesis of selenofunctionalized heterocycles via I2O5-mediated selenylative heterocyclization. Org Biomol Chem. https://doi.org/10.1039/D1OB02196F
Tao S, Huo A, Gao Y, Zhang X, Yang J, Du Y (2022) PhICl2-Mediated regioselective and electrophilic oxythio/selenocyanation of o-(1-alkynyl)benzoates: access to biologically active s/secn-containing isocoumarins. Front Chem. https://doi.org/10.3389/fchem.2022.859995
Zhang HY, Zeng TT, Xie ZB et al (2022) Aerial oxygen-driven selenocyclization of o-vinylanilides mediated by coupled Fe3+/Fe2+ and I2/I− redox cycles. Molecules. https://doi.org/10.3390/molecules27217386
Fang JD, Yan XB, Zhou L, Wang YZ, Liu XY (2019) Synthesis of 3-organoselenyl-2H-coumarins from propargylic aryl ethers via oxidative radical cyclization. Adv Synth Catal. https://doi.org/10.1002/adsc.201801565
Ai Z, Xiao J, Li Y, Guo B, Du Y, Zhao K (2020) Metal-free synthesis of 3-chalcogenyl chromones from alkynyl aryl ketones and diorganyl diselenides/disulfides mediated by PIFA. Org Chem Front. https://doi.org/10.1039/D0QO01175D
Win KM, Sonawane AD, Koketsu M (2021) Synthesis of selenated tetracyclic indoloazulenes via iodine and diorganyl diselenides. Org Biomol Chem. https://doi.org/10.1039/D1OB00268F
Saba S, Rafique J, Braga AL (2016) DMSO/iodine-catalyzed oxidative C-Se/C-S bond formation: a regioselective synthesis of unsymmetrical chalcogenides with nitrogen-or oxygen-containing arenes. Catal Sci Technol. https://doi.org/10.1039/C5CY01503K
Zhou Z, He X (2018) A simple method for the preparation of arylselanyl anilines. Synth Commun. https://doi.org/10.1080/00397911.2018.1513531
Saba S, Rafique J, Braga AL (2015) Synthesis of unsymmetrical diorganyl chalcogenides under greener conditions: use of an iodine/DMSO system, solvent-and metal-free approach. Adv Synth Catal. https://doi.org/10.1002/adsc.201500024
Kaushik N, Kaushik N, Attri P, Kumar N, Kim C, Verma A, Choi E (2013) Biomedical importance of indoles. Molecules. https://doi.org/10.3390/molecules18066620
Chuai H, Zhang SQ, Bai H et al (2021) Small molecule selenium-containing compounds: recent development and therapeutic applications. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2021.113621
Zhang S, An B, Li J et al (2017) Synthesis and evaluation of selenium-containing indole chalcone and diarylketone derivatives as tubulin polymerization inhibition agents. Org Biomol Chem. https://doi.org/10.1039/C7OB01655G
Azeredo JB, Godoi M, Martins GM, Silveira CC, Braga AL (2014) A solvent-and metal-free synthesis of 3-chacogenyl-indoles employing DMSO/I2 as an eco-friendly catalytic oxidation system. J Org Chem. https://doi.org/10.1021/jo5000779
Wen Z, Xu J, Wang Z et al (2015) 3-(3, 4, 5-Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: microwave-assisted synthesis and biological evaluation. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2014.11.024
Chen SQ, Wang QM, Xu PC, Ge SP, Zhong P, Zhang XH (2016) Iodine-promoted selective 3-selanylation and 3-sulfenylation of indoles with dichalcogenides under mild conditions. Phosphorus Sulfur Silicon Relat Elem. https://doi.org/10.1080/10426507.2014.999068
Luz EQ, Seckler D, Araujo JS et al (2019) Fe (III)-catalyzed direct C3 chalcogenylation of indole: the effect of iodide ions. Tetrahedron. https://doi.org/10.1016/j.tet.2019.01.037
Song WH, Shi J, Chen X, Song G (2020) Silver-catalyzed remote C5-H selenylation of indoles. J Org Chem. https://doi.org/10.1021/acs.joc.0c00921
Vieira BM, Thurow S, Brito J et al (2015) Sonochemistry: an efficient alternative to the synthesis of 3-selanylindoles using CuI as catalyst. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2015.05.012
Yang Y, Li W, Ying B, Liao H, Shen C, Zhang P (2016) Catalyst-triggered highly selective C−S and C−Se bond formation by C−H activation. ChemCatChem. https://doi.org/10.1002/cctc.201600589
Gao YT, Liu SD, Cheng L, Liu L (2021) A fast and direct iodide-catalyzed oxidative 2-selenylation of tryptophan. Chem Commun. https://doi.org/10.1039/D1CC00700A
Zhang X, Wang C, Jiang H, Sun L (2018) Convenient synthesis of selenyl-indoles via iodide ion-catalyzed electrochemical C-H selenation. Chem Commun. https://doi.org/10.1039/C8CC04543G
Ansari AJ, Sharma S, Pathare RS, Gopal K, Sawant DM, Pardasani RT (2016) Solvent-free multicomponent synthesis of biologically-active fused-imidazo heterocycles catalyzed by reusable Yb(OTf)3 under microwave irradiation. ChemistrySelect. https://doi.org/10.1002/slct.201600241
Chitrakar R, Rawat D, Sistla R, Vadithe LN, Subbarayappa A (2021) Design, synthesis and anticancer activity of sulfenylated imidazo-fused heterocycles. Bioorg Med Chem Lett. https://doi.org/10.1016/j.bmcl.2021.128307
Bagdi AK, Hajra A (2016) Design, synthesis, and functionalization of imidazoheterocycles. Chem Rec. https://doi.org/10.1002/tcr.201600057
Akritopoulou-Zanze I, Wakefield BD, Gasiecki A et al (2011) Scaffold oriented synthesis, Part 4: design, synthesis and biological evaluation of novel 5-substituted indazoles as potent and selective kinase inhibitors employing heterocycle forming and multicomponent reactions. Bioorg Med Chem Lett. https://doi.org/10.1016/j.bmcl.2011.01.001
Thakur A, Pereira G, Patel C, Chauhan V, Dhaked RK, Sharma A (2020) Design, one-pot green synthesis and antimicrobial evaluation of novel imidazopyridine bearing pyran bis-heterocycles. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.127686
Bavetsias V, Crumpler S, Sun C et al (2012) Optimization of imidazo[4,5- b ]pyridine-based kinase inhibitors: identification of a dual flt3/aurora kinase inhibitor as an orally bioavailable preclinical development candidate for the treatment of acute myeloid leukemia. J Med Chem. https://doi.org/10.1021/jm300952s
Teli P, Sahiba N, Sethiya A, Soni J, Agarwal S (2022) Imidazole derivatives: impact and prospects in antiviral drug discovery. Imidazole-Based Drug Discovery. https://doi.org/10.1016/B978-0-323-85479-5.00001-0
Rafique J, Saba S, Rosário AR, Braga AL (2016) Regioselective, solvent-and metal-free chalcogenation of imidazo [1, 2-a] pyridines by employing I2/DMSO as the catalytic oxidation system. Chem Eur J. https://doi.org/10.1002/chem.201600800
Yi R, Liu S, Gao H, Liang Z, Xu X, Li N (2020) Iodine-promoted direct thiolation (selenylation) of imidazole with disulfides (diselenide): a convenient and metal-free protocol for the synthesis of 2-arylthio (seleno) imidazole. Tetrahedron. https://doi.org/10.1016/j.tet.2020.130951
Dey A, Hajra A (2019) Iodine-catalyzed selenylation of 2 H-indazole. J Org Chem. https://doi.org/10.1021/acs.joc.9b02199
Obah Kosso AR, Kabri Y, Broggi J, Redon S, Vanelle P (2020) Sequential regioselective diorganochalcogenations of imidazo [1, 2-a] pyrimidines using I2/H3PO4 in dimethylsulfoxide. J Org Chem. https://doi.org/10.1021/acs.joc.9b02963
Bettanin L, Saba S, Doerner CV et al(2018) NH4I-catalyzed chalcogen (S/Se)-functionalization of 5-membered N-heteroaryls under metal-free conditions. Tetrahedron. https://doi.org/10.1016/j.tet.2018.05.084
Rafique J, Farias G, Saba S et al (2020) Selenylated-oxadiazoles as promising DNA intercalators: synthesis, electronic structure, DNA interaction and cleavage. Dyes Pigm. https://doi.org/10.1016/j.dyepig.2020.108519
Sarmah M, Chutia K, Dutta D, Gogoi P (2022) Overview of coumarin-fused-coumarins: synthesis, photophysical properties and their applications. Org Biomol Chem. https://doi.org/10.1039/D1OB01876K
Stefanachi A, Leonetti F, Pisani L, Catto M, Carotti A (2018) Coumarin: a natural, privileged and versatile scaffold for bioactive compounds. Molecules. https://doi.org/10.3390/molecules23020250
Phutdhawong W, Chuenchid A, Taechowisan T, Sirirak J, Phutdhawong WS (2021) Synthesis and biological activity evaluation of coumarin-3-carboxamide derivatives. Molecules. https://doi.org/10.3390/molecules26061653
Annunziata F, Pinna C, Dallavalle S, Tamborini L, Pinto A (2020) An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int J Mol Sci. https://doi.org/10.3390/ijms21134618
Sharifi-Rad J, Cruz-Martins N, López-Jornet P et al (2021) Natural coumarins: exploring the pharmacological complexity and underlying molecular mechanisms. Oxid Med Cell Longev. https://doi.org/10.1155/2021/6492346
Xu L, Zhao XY, Wu YL, Zhang W (2015) The study on biological and pharmacological activity of coumarins. https://doi.org/10.2991/ap3er-15.2015.33
Wu Y, Chen JY, Liao HR et al (2021) Electrochemical transient iodination and coupling for selenylated 4-anilinocoumarin synthesis. Green Synth Catal. https://doi.org/10.1016/j.gresc.2021.03.006
Song Z, Ding C, Wang S et al (2020) Metal-free regioselective C-H chalcogenylation of coumarins/(hetero) arenes at ambient temperature. Chem Commun. https://doi.org/10.1039/C9CC09001K
Mani R, Natesan V (2018) Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochem. https://doi.org/10.1016/j.phytochem.2017.09.016
Bahadori M, Baharara J, Amini E (2016) Anticancer properties of chrysin on colon cancer cells, in vitro and in vivo with modulation of caspase-3, -9, bax and sall4. Iran J Biotechnol. https://doi.org/10.15171/ijb.1374
Song JH, Kwon BE, Jang H et al (2015) Antiviral activity of chrysin derivatives against coxsackievirus b3 in vitro and in vivo. Biomol Ther. https://doi.org/10.4062/biomolther.2015.095
Suresh Babu K, Hari Babu T, Srinivas PV, Hara Kishore K, Murthy USN, Rao JM (2006) Synthesis and biological evaluation of novel C (7) modified chrysin analogues as antibacterial agents. Bio Med Chem Lett. https://doi.org/10.1016/j.bmcl.2005.09.009
Omonga N, Zia Z, Ghanbour H et al (2021) Facile synthesis and biological evaluation of chrysin derivatives. J Chem Res. https://doi.org/10.1177/17475198211057467
Wang QQ, Cheng N, Yi WB, Peng SM, Zou XQ (2014) Synthesis, nitric oxide release, and α-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorg Med Chem 22(5):1515–1521. https://doi.org/10.1016/j.bmc.2014.01.038
Byun EB, Song HY, Kim WS et al (2021) Chrysin derivative cm1 and exhibited anti-inflammatory action by upregulating toll-interacting protein expression in lipopolysaccharide-stimulated RAW264.7 macrophage cells. Molecules. https://doi.org/10.3390/molecules26061532
Fonseca SF, Padilha NB, Thurow S et al (2017) Ultrasound-promoted copper-catalyzed synthesis of bis-arylselanyl chrysin derivatives with boosted antioxidant and anticancer activities. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2017.06.007
Kaur T, Bhandari DD (2022) Annotated review on various biological activities of quinoline molecule. Biointerface Res Appl Chem. https://doi.org/10.33263/BRIAC134.355
de la Guardia C, Stephens D, Dang H, Quijada M, Larionov O, Lleonart R (2018) Antiviral activity of novel quinoline derivatives against dengue virus serotype 2. Molecules. https://doi.org/10.3390/molecules23030672
Guan YF, Liu XJ, Yuan XY et al (2021) Design, synthesis, and anticancer activity studies of novel quinoline-chalcone derivatives. Molecules. https://doi.org/10.3390/molecules26164899
Zajdel P, Marciniec K, Maślankiewicz A et al (2013) Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2/D3 receptors. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2012.11.042
Kumar Gupta S, Mishra A (2016) Synthesis, characterization & screening for anti-inflammatory & analgesic activity of quinoline derivatives bearing azetidinones scaffolds. Antiinflamm Antiallergy Agents Med Chem 15(1):31–43. https://doi.org/10.2174/1871523015666160210124545
Herraiz T, Guillén H, González-Peña D, Arán VJ (2019) Antimalarial quinoline drugs inhibit β-hematin and increase free hemin catalyzing peroxidative reactions and inhibition of cysteine proteases. Sci Rep. https://doi.org/10.1038/s41598-019-51604-z
Kumar V, Banert K, Ray D, Saha B (2019) An atom-economical and regioselective metal-free C-5 chalcogenation of 8-aminoquinolines under mild conditions. Org Biomol Chem. https://doi.org/10.1039/C9OB02235J
Beukeaw D, Noikham M, Yotphan S (2019) Iodine/persulfate-promoted site-selective direct thiolation of quinolones and uracils. Tetrahedron. https://doi.org/10.1016/j.tet.2019.130537
Liu X, Wang Y, Song D, Wang Y, Cao H (2020) Electrochemical regioselective selenylation/oxidation of N-alkylisoquinolinium salts via double C (sp 2)-H bond functionalization. Chem Commun. https://doi.org/10.1039/D0CC06778D
Ghosh P, Nandi AK, Chhetri G, Das S (2018) Generation of ArS-and ArSe-substituted 4-quinolone derivatives using sodium iodide as an inducer. J Org Chem. https://doi.org/10.1021/acs.joc.8b01426
Sharma V, Chitranshi N, Agarwal AK (2014) Significance and biological importance of pyrimidine in the microbial world. Int J Med Chem. https://doi.org/10.1155/2014/202784
Zarenezhad E, Farjam M, Iraji A (2021) Synthesis and biological activity of pyrimidines-containing hybrids: focusing on pharmacological application. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.129833
Pałasz A, Cież D (2015) In search of uracil derivatives as bioactive agents. Uracils and fused uracils. Synthesis, biological activity and applications. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2014.10.008
Kezin VA, Matyugina ES, Novikov MS et al (2022) New derivatives of 5-substituted uracils: potential agents with a wide spectrum of biological activity. Molecules. https://doi.org/10.3390/molecules27092866
Ghosh P, Chhetri G, Das S (2021) Metal free C-3 chalcogenation (sulfenylation and selenylation) of 4 H-pyrido [1, 2-a] pyrimidin-4-ones. RSC Adv. https://doi.org/10.1039/D1RA00834J
Chen J, Xiao Y, You X, Li S, Fu Y, Ouyang Y (2023) Electrochemical oxidative selenation of 4 h -pyrido-[1,2- a ]-pyrimidin-4-ones with diorganyldiselenides. ChemistrySelect. https://doi.org/10.1002/slct.202203879
Chen JY, Zhong CT, Gui QW et al (2021) Practical and sustainable approach for clean preparation of 5-organylselanyl uracils. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2020.09.034
Li XD, Gao YT, Sun YJ et al (2019) A NaI/H2O2-mediated sulfenylation and selenylation of unprotected uracil and its derivatives. Org lett. https://doi.org/10.1021/acs.orglett.9b02183
Dai H, Li YQ, Du D et al (2008) Synthesis and biological activities of novel pyrazole oxime derivatives containing a 2-chloro-5-thiazolyl moiety. J Agric Food Chem. https://doi.org/10.1021/jf802429x
Assali M, Abualhasan M, Sawaftah H, Hawash M, Mousa A (2020) Synthesis, biological activity, and molecular modeling studies of pyrazole and triazole derivatives as selective COX-2 inhibitors. J Chem. https://doi.org/10.1155/2020/6393428
Sayed AEDH, Zaki RM, El-Dean AMK, Abdulrazzaq AY (2015) The biological activity of new thieno[2,3-c]pyrazole compounds as anti-oxidants against toxicity of 4-nonylphenol in Clarias gariepinus. Toxicol Rep. https://doi.org/10.1016/j.toxrep.2015.10.008
Faria JV, Vegi PF, Miguita AGC, dos Santos MS, Boechat N, Bernardino AMR (2017) Recently reported biological activities of pyrazole compounds. Bioorg Med Chem. https://doi.org/10.1016/j.bmc.2017.09.035
Wang J, Liu Y, Yan J (2018) Iodine-mediated synthesis of 4-selanylpyrazoles. New J Chem. https://doi.org/10.1039/C8NJ02629G
Marinescu M, Popa C-V (2022) Pyridine compounds with antimicrobial and antiviral activities. Int J Mol Sci 23(10):5659. https://doi.org/10.3390/ijms23105659
De S, Kumar SKA, Shah SK et al (2022) Pyridine: the scaffolds with significant clinical diversity. RSC Adv. https://doi.org/10.1039/D2RA01571D
Islam MdB, Islam MdI, Nath N et al (2023) Recent advances in pyridine scaffold: focus on chemistry, synthesis, and antibacterial activities. Biomed Res Int. https://doi.org/10.1155/2023/9967591
Ge W, Zhu X, Wei Y (2013) Aerobic multicomponent tandem synthesis of 3-sulfenylimidazo [1, 2-a] pyridines from ketones, 2-aminopyridines, and disulfides. Eur J Org Chem. https://doi.org/10.1002/ejoc.201300905
Jiang N, Fang Y, Fang Y, Wang SY, Ji SJ (2019) Iodine-promoted one pot reaction of pyridin-2-amine with arylmethyl ketone and selenosulfonate: synthesis of 3-(alkylselanyl)-2-arylimidazo [1, 2-a] pyridine under transition-metal free conditions. Org Chem Front. https://doi.org/10.1039/C8QO01245H
Li J, Liu X, Deng J et al (2020) Electrochemical diselenylation of indolizines via intermolecular C–Se formation with 2-methylpyridines, α-bromoketones and diselenides. Chem Commun. https://doi.org/10.1039/C9CC08784B
Bellina F, Rossi R (2006) Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 62(31):7213–7256. https://doi.org/10.1016/j.tet.2006.05.024
Bhardwaj V, Gumber D, Abbot V, Dhiman S, Sharma P (2015) Pyrrole: a resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. https://doi.org/10.1039/C4RA15710A
Ivan BC, Barbuceanu SF, Hotnog CM et al (2022) New pyrrole derivatives as promising biological agents: design, synthesis, characterization, in silico, and cytotoxicity evaluation. Int J Mol Sci. https://doi.org/10.3390/ijms23168854
Li Petri G, Spanò V, Spatola R et al (2020) Bioactive pyrrole-based compounds with target selectivity. Eur J of Med Chem. https://doi.org/10.1016/j.ejmech.2020.112783
Peglow TJ, Costa GP, Duarte LF et al (2019) Ultrasound-promoted one-pot synthesis of mono-or bis-substituted organylselanyl pyrroles. J Org Chem. https://doi.org/10.1021/acs.joc.9b00405
Silva LT, Azeredo JB, Saba S, Rafique J, Bortoluzzi AJ, Braga AL (2017) Solvent-and metal-free chalcogenation of bicyclic arenes using I2/DMSO as non-metallic catalytic system. Eur J Org Chem. https://doi.org/10.1002/ejoc.201700744
Jana A, Panday AK, Mishra R, Parvin T, Choudhury LH (2017) Synthesis of thio and selenoethers of cyclic β-Hydroxy carbonyls and amino uracils: a metal-free regioselective I2/DMSO mediated reaction. ChemistrySelect. https://doi.org/10.1002/slct.201702066
Rodrigues J, Saba S, Joussef AC, Rafique J, Braga AL (2018) KIO3-Catalyzed C (sp2)-H bond selenylation/sulfenylation of (Hetero) arenes: synthesis of chalcogenated (Hetero) arenes and their evaluation for anti-Alzheimer activity. Asian J Org Chem. https://doi.org/10.1002/ajoc.201800346
Ding C, Yu Y, Yu Q et al (2018) NIS/TBHP induced regioselective selenation of (hetero) arenes via direct C−H functionalization. ChemCatChem. https://doi.org/10.1002/cctc.201801548
Meirinho AG, Pereira VF, Martins GM et al (2019) Electrochemical oxidative C (sp2)-H bond selenylation of activated arenes. Eur J Org Chem. https://doi.org/10.1002/ejoc.201900992
Gu L, Fang X, Weng Z, Lin J, He M, Ma W (2019) PIDA-promoted selective C5 C-H selenylations of indolines via weak interactions. Adv Synth Catal. https://doi.org/10.1002/adsc.201900766
Funding
A.B. gratefully acknowledges the financial support for this work from the Department of Science and Technology (DST), New Delhi, Government of India, Project No. SR/FT/CS-037/2010 dated 28-10-2010, FIST-II/PURSE-II (DST), and UGC-CAS, Panjab University, Chandigarh. P.K. acknowledges the financial support from the Council of Scientific and Industrial Research (CSIR) New Delhi vides Award No- F.No.09/135(0823)2018-EMR-I.
Author information
Authors and Affiliations
Contributions
Pankaj Kumar: conceptualization, data collection and interpretation, and original draft preparation. Aman Bhalla: supervision, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, P., Bhalla, A. Reaction Pattern and Mechanistic Aspects of Iodine and Iodine-Based Reagents in Selenylation of Aliphatic, Aromatic, and (Hetero)Cyclic Systems. Top Curr Chem (Z) 382, 12 (2024). https://doi.org/10.1007/s41061-024-00459-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-024-00459-8