Abstract
Since December 2019, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused a global pandemic named coronavirus disease-19 (COVID-19) and resulted in a worldwide economic crisis. Utilizing the spike-like protein on its surface, the SARS-CoV-2 binds to the receptor angiotensin-converting enzyme 2 (ACE2), which highly expresses on the surface of many cell types. Given the crucial role of ACE2 in the renin–angiotensin system, its engagement by SARS-CoV-2 could potentially result in endothelial cell perturbation. This is supported by the observation that one of the most common consequences of COVID-19 infection is endothelial dysfunction and subsequent vascular damage. Furthermore, endothelial dysfunction is the shared denominator among previous comorbidities, including hypertension, kidney disease, cardiovascular diseases, etc., which are associated with an increased risk of severe disease and mortality in COVID-19 patients. Several vaccines and therapeutics have been developed and suggested for COVID-19 therapy. The present review summarizes the relationship between ACE2 and endothelial dysfunction and COVID-19, also reviews the most common comorbidities associated with COVID-19, and finally reviews several categories of potential therapies against COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In December 2019, unknown pandemic pneumonia emerged around the world and became a global and epochal challenge resulting in a near-complete halt in economic and social activities (Guan et al. 2020). The first cases of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) were reported from Wuhan and rapidly spread around the world (Zhu et al. 2020a, b). SARS-CoV-2 has a meaningful lethality compared to other coronaviruses such as SARS-CoV and Middle East Respiratory Syndrome (MERS)-CoV (Ksiazek et al. 2003; Peiris et al. 2003), due to high infectivity. The disease caused by SARS-CoV-2 was named as coronavirus disease 2019 (COVID-19). The most common symptoms reported in patients with COVID-19 include cough, fever, dyspnea, fatigue, myalgia, and shortness of breath (Huang et al. 2020a, b; Rodriguez-Morales et al. 2020). The infection of the lungs, the most important target of SARS-CoV-2, may result in acute respiratory distress syndrome (ARDS) (Mason 2020).
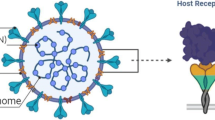
There is a high affinity between the virus and angiotensin-converting enzyme 2 (ACE2) as the primary receptor to enter the host cells through endocytosis (Hulswit et al. 2016). Many viruses have developed different mechanisms to interact and attach with host cells and have employed more than one type of receptor molecule (Bhella 2015). This is also the case for SARS-CoV-2 that employ more than one type of receptor. Although ACE2 is currently known as the principal target for entry of SARS-CoV-2 to the host cell, there has been growing evidence that different types of receptors are involved in cell entry (Amraei et al. 2020; Cantuti-Castelvetri et al. 2020; Daly et al. 2020; Wang et al. 2020a, b, c, d, e). CD147 (Basigin or EMMPRIN) is a transmembrane glycoprotein (Cui et al. 2018) and belongs to the immunoglobulin superfamily. Previous studies showed that CD147-antagonistic peptides had an inhibitory effect on SARS-CoV, indicating it is a functional factor in facilitating the SARS-CoV entry to host cells (Chen et al. 2005). A recent study identified CD147-spike protein as a novel route for SARS-CoV-2 invasion to host cells (Wang et al. 2020a, b, c, d, e). A monoclonal blocking antibody against the extracellular b1b2 domain of neuropilin 1 (NRP1) was shown to effectively inhibit SARS-CoV-2 infection, suggesting NRP1 as another gate for the virus (Cantuti-Castelvetri et al. 2020). Data from this study also suggested that NRP1 is involved in the enhanced tropism and spreading of SARS-CoV-2. In addition, Amraie et al. (2020) showed that CD209L (L-SIGN) and CD209 (DC-SIGN) are alternative cellular receptors for SARS-CoV-2.
The endothelial cells (ECs) can be infected by SARS-CoV-2 (Ackermann et al. 2020; Colmenero et al. 2020; Menter et al. 2020; Varga et al. 2020), which results in endothelial dysfunction. Commonly, EC dysfunction (ECD) is a denominator of comorbidities associated with COVID-19. Non-communicable diseases (NCDs), such as hypertension, have been considered crucial health issues in recent years. There is a concern that COVID-19 as a communicable disease (CD) might cause a secondary pandemic of NCDs. In this regard, there is a deep worry about emerging a new disease entity, the CDs and NCDs assembly without any boundary (Shibata et al. 2020). Numerous investigations are progressing to distinguish and develop efficient drugs and therapeutic strategies to treat COVID-19 (Ragia and Manolopoulos 2020).
Among several identified cellular receptors for SARS-CoV-2, ACE2 is the main focus of this review. We also summarize the properties of functional and dysfunctional endothelium and ECD-associated events that occur in COVID-19. Then, we review the most common comorbidities correlated with COVID-19 infection. Finally, we summarize vaccines, therapeutics, and strategies to treat COVID-19.
2 Novel Severe Acute Respiratory Syndrome Coronavirus-19
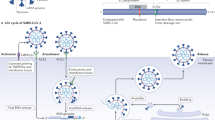
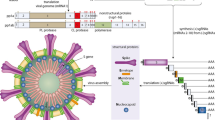
The term “corona” is the Latin word meaning crown, and this virus is named coronavirus because it has a crown-like surface created by the surface binding glycoproteins spikes. Taxonomically, SARS CoV-2 belongs to the realm Riboviria, order Nidovirales, family Coronaviridae, genus Betacoronavirus, and the species severe acute respiratory syndrome-related coronavirus (CSG 2020). SARS-CoV-2 is a single-stranded RNA coronavirus with four fundamental structural proteins, including envelope (E), matrix/membrane (M), nucleocapsid (N), and spike (S) (Fig. 1) (Perlman and Netland 2009; Helmy et al. 2020; Zhu et al. 2020a, b). In SARS-CoV-2, the 3′ end of the viral genome encodes the structural proteins. The S protein binds to human ACE2 on the host cell membrane, mediating the fusion of the virus and the host cell membrane and known as the primary determinant of CoVs tropism (Hulswit et al. 2016). After receptor recognition, the S protein is cleaved into two subunits, S1 and S2, to facilitate virus entry into the cell (Shang et al. 2020). This proteolysis relies on human transmembrane protease, serine 2 (TMPRSS2) (Sanders et al. 2020). The receptor-binding domain (RBD) has located on the S1 subunit, allowing direct viral binding to the peptidase domain of ACE2. The S2 subunit probably engages in viral and cellular membrane fusion. The 5' end of the viral RNA encodes two necessary precursor polyproteins for generating non-structural proteins participating in the replication complex. The polyproteins are cleaved to the non-structural proteins by two viral proteases, 3C-like or main protease (3CLpro or Mpro) and papain-like protease (PLpro) (Helmy et al. 2020).
3 Angiotensin-Converting Enzyme 2
3.1 Structure and Function
Angiotensin-converting enzyme 2 (EC 3.4.17.23) belongs to the ACE family, which all are zinc metallopeptidase. The ACE2 receptor consists of an N-terminal signal peptide, a peptidase domain (PD) with a HEXXH zinc-binding motif, a C-terminal collectrin-like domain (CLD), and a hydrophobic transmembrane region followed by a cytoplasmic segment (Fig. 2a, b) (Donoghue et al. 2000; Zhang et al. 2001). Endogenous disintegrin metalloproteinase 17 (ADAM-17) can perform ectodomain shedding of ACE2 to produce a soluble form of ACE2 (Lambert et al. 2005). In contrast, calmodulin (CALM) interacts with ACE2 and inhibits its ectodomain shedding (Lambert et al. 2008a, b).
ACE is a ubiquitous protein with 42% sequence identity and 61% sequence similarity with ACE2 (Tipnis et al. 2000). However, subtle changes in the active site residues of two enzymes (Fig. 2c) give rise to significant differences in their substrate specificity and reactivity (Rice et al. 2004; Towler et al. 2004). The renin–angiotensin system (RAS) comprises ACE2 and various regulatory enzymes and effector peptides that serve as the key regulators of vascular function under physiological and pathophysiological conditions (Lambert et al. 2008a, b; Crowley et al. 2005). Angiotensinogen is an α-glycoprotein substrate of the RAS released from the liver (Menard et al. 1983; Deschepper 1994; Hall 2003), whose renin-mediated hydrolysis produces inactive decapeptide angiotensin I, Ang I (Ang (1-10)), which itself is cleaved by ACE to produce an active vasoconstrictor octapeptide Ang II (Ang (1-8)) (Fig. 3) (Amraei and Rahimi 2020). In vitro, ACE2 cleaves Ang I and octapeptide Ang II to produce Ang (1-9) and heptapeptide Ang (1-7), respectively (Tipnis et al. 2000; Vickers et al. 2002). The efficiency of ACE2 to produce Ang (1-7) from Ang II is remarkably higher than to generate Ang (1-9) from Ang I. Notably, Ang (1-9) is not cleaved by ACE2, but it is cleaved by ACE or neprilysin (NEP). Despite the higher affinity of Ang (1-9) for ACE, this peptide is hydrolyzed preferentially by NEP rather than ACE (Rice et al. 2004). The Ang II binds to and activates Angiotensin-2 type 1 receptor 1 (AT1) and Angiotensin II type 1 receptor 2 (AT2). The AT1 activation leads to the activation of an excess of kinases, e.g., c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase (MAPK), that regulate vasoconstriction, inflammation, and fibrotic remodeling, while activation of AT2 stimulates various phosphatases (e.g., protein tyrosine phosphatases (PTP) and protein phosphatase 2 (PP2A)), resulting in vasodilation and growth inhibition (Lin and Pan 2008; Karnik et al. 2015).
Generally, Ang (1-7) and Ang II have contrasting effects; Ang II acts as a vasoconstrictor while Ang (1-7) acts as a vasodilator (Ferrario et al. 1997). It has been reported that Ang (1-7) reduces lung inflammation, fibrosis, and pulmonary arterial hypertension (Wagenaari et al. 2013; Meng et al. 2014; Magalhaes et al. 2015). Additionally, in vivo evidence supports the role of ACE2 in the human heart and reducing levels of Ang II and increasing levels of Ang (1-7) by ACE2 (Zisman et al. 2003; Rice et al. 2004).
MAS is a G protein-coupled receptor (GPCR) that was initially described as an oncogene (Young et al. 1986). The Ang (1-7) specifically binds to the MAS receptor on the MAS-transfected cells (Santos et al. 2003). MAS activation stimulates phosphoinositide 3 kinase (PI3K)/AKT axis and subsequent activation of eNOS (Fig. 3). Furthermore, the activation of GPCR induces the activation of phospholipases A (PLA) and C (PLC) that result in generating arachidonic acid and stimulating intracellular calcium, respectively (Bader et al. 2014; Solinski et al. 2014). Altogether, the activation of these pathways regulates some events in endothelial cells, including anti-fibrosis and anti-inflammatory responses and vasodilation. MAS acts similar to a physiological antagonist of the AT1 receptor through the heterodimerization with it, thereby inhibiting AT1-induced Ang II functions (Kostenis et al. 2005).
3.2 Expression and Viral Tropism
Viral tissue tropism refers to the cells and tissues of a host that support the virus’s growth. One of the influencing factors on tissue tropism is the presence of cellular receptors, permitting viral entry into host cells. The SARS-CoV-2 displays strong binding to cell-associated and soluble ACE2 receptors expressed in many organs such as the lung, kidneys, heart, intestine, brain, and liver (Kuba et al. 2010; South et al. 2020). Furthermore, it can infect human blood vessels and kidney organoids via ACE2, indicating viral tropism for vascularized tissues (Monteil et al. 2020). The presence of the ACE2 receptor on a wide range of cell types, including pneumocytes and macrophages, as well as smooth muscle and arterial endothelial cells of almost all organs (Hamming et al. 2004) may explain the multi-organ failure in some patients with severe COVID-19 infection (Kuba et al. 2010; Clerkin et al. 2020).
3.3 Gene Polymorphism, Gender Susceptibility, and Genetic Susceptibility
Susceptibility to SARS-CoV-2 and COVID-19 disease outcomes may be affected by gene polymorphism, mRNA expression, and protein polymorphism of human ACE2. ACE2 gene polymorphism is reported in the Chinese, Canadian, and Indian populations, which was correlated with hypertension and pathological variations in blood pressure (Niu et al. 2007; Fan et al. 2009; Chen et al. 2010, 2016; Malard et al. 2013; Patnaik et al. 2014; Luo et al. 2019). Similarly, ACE polymorphism was reported in African–Americans with hypertension (Duru et al. 1994), suggesting that it regulates the RAS pathway. Sequence alignment and comparison of the 10 human ACE2 proteins and 4 various ACE2 isoforms available in GeneBank were shown 100% identity among the complete ACE2 sequences and a deletion in the CLD domain or truncation in the transmembrane domain in different isoforms of ACE2. However, the role of these isoforms in SARS-CoV-2 infection and COVID-19 outcomes remains uncertain (Devaux et al. 2020). A recent investigation of the functional coding variants and the allele frequency in the ACE2 gene from genome databases revealed 32 coding variants of ACE2 among different populations and one variant with a truncation Gln300X in China populations. Also, the distributions of seven hotspot variants in various populations were observed (Cao et al. 2020). In this study, no mutation was found in the binding residues of S-protein of coronavirus in different populations. However, another investigation showed that several ACE2 variants may decrease the association between ACE2 and the S-protein in SARS-CoV or NL63 (Li et al. 2005). ACE2 polymorphism might create the chance that some people could have less susceptibility to SARS-CoV-2 infection than others. ACE2 polymorphism might be correlated with the less susceptibility of some people to SARS-CoV-2 infection.
Given that ACE2 acts as a cellular doorway for SARS-CoV-2, a higher expression level of ACE2 causes more SARS-CoV-2 infection. Various studies indicated that the ACE2 expression in the lung is higher in men than women, as well as in the Asian population than in Caucasian and African American populations (Sun et al. 2020). Based on a similar observation, higher levels of Ang (1-7) were reported in males compared with females (Gwathmey et al. 2008). Also, in another investigation, the males had higher expression levels of ACE2 in the lungs than the females (Zhao et al. 2020), although the ACE2 activity shows no difference between the males and females (Fernández-Atucha et al. 2017). Five cell types in the male lung were reported to express ACE2, while two to four cell types in the female lung do so (Zhao et al. 2020). In agreement with these findings, in a statistical study including 1099 patients, the SARS-CoV-2 infected males (58.1%) were somewhat, but not statistically significant, higher than the SARS-CoV-2 infected females (41.9%) (Guan et al. 2020). Some of the sex hormones might affect the homeostasis of RAS. In addition, the female sex hormones can influence ACE2 activity (Fernández-Atucha et al. 2017); estrogen has a positive effect on ACE2 activity, so that the ACE/ACE2 activity ratio in the female serum is less than that in the males (Hu et al. 2018). Furthermore, increasing progesterone during pregnancy may significantly induce upregulation of ACE2 expression in the reproductive system as well as the other organs (Levy et al. 2008; Neves et al. 2008). All clinical reports published to date indicate that men represent a significant percentage (66–75%) of the most severe cases of COVID-19.
4 COVID-19-Associated Endothelial Cell Dysfunction
ECs are infected by SARS-CoV-2, especially in the highly vascularized tissues (Ackermann et al. 2020; Colmenero et al. 2020; Khider et al. 2020; Menter et al. 2020; Pons et al. 2020; Puelles et al. 2020; Varga et al. 2020), suggesting the incidence of endothelial dysfunction in COVID-19. In this section, we compare the properties of a functional and dysfunctional endothelium and summarize the events following ECD in COVID-19 patients, including pro-inflammatory storm, thromboembolism, and coagulopathy.
4.1 Functional versus Dysfunctional Endothelium
The endothelium is a semi-permeable membrane and innermost layer of blood vessels, which forms an extensive interface between the blood and surrounding tissues for passaging substances and plays a critical role in preserving vascular homeostasis (Michiels 2003). The endothelium secretes several mediators required for regular vascular function, i.e., regulating vascular tone, coagulation, vascular cell growth, and immune responses (Levick 2013). The endothelium also preserves an excellent balance between anti- and pro-thrombotic phases under physiological conditions.
EC dysfunction is known as an imbalance between relaxing and contracting factors or pro- and anti-coagulant mediators (De Meyer and Herman 1997) and involves disruption of the vasoactive role of endothelial cells in regulating tissue perfusion (Szmitko et al. 2003). ECD encompasses various modifications of functional phenotype, which are critical for regulating hemostasis, thrombosis, and inflammatory reactions within blood vessels (Chesterman 1988; Wu et al. 1988; Gimbrone Jr 2016). Furthermore, it may cause the development and maintenance of high blood pressure (Hedner et al. 2000). The succession of cellular events leading to ECD can depict two types: (1) reversible EC activation, including (a) type I EC activation or EC stimulation, which involves the release of stored proteins independent of de novo protein synthesis, and (b) type II EC activation, which involves de novo synthesis and secretion of proteins; (2) irreversible EC injury including (a) endothelial apoptosis, and (b) endothelial necrosis (Fig. 4) (Zhang et al. 2020a, b, c, d, e). To date, many molecules are suggested as biomarkers of EC dysfunction. Some of these biomarkers solely originated from the activated ECs. In contrast, other biomarkers are not endothelial-specific and derived from other activated cell types, e.g., platelets, neutrophils, macrophages, and T lymphocytes. Therefore, a variety of reactions and events are expected from these biomarkers. The biomarkers of ECD are summarized in Table 1.
4.2 COVID-19-Associated Pro-inflammatory Storm
The high activation of the innate immune response against the virus induces the overexpression of pro-inflammatory cytokines, resulting in a “cytokine storm” (Liu et al. 2016). Toll-like receptors (TLRs) are the principal players in innate immunity and involved in recognizing molecular patterns from SARS-CoV-2 (such as viral proteins and single-stranded RNA) to produce pro-inflammatory responses (Fitzgerald et al. 2001; Takeuchi and Akira 2009; Chakraborty et al. 2020; Choudhury and Mukherjee 2020; Moreno-Eutimio et al. 2020). A highly pathogenic SARS-CoV-2 infection ascribed to IL-6, resulting from enhancing virus replication mainly in the lower respiratory tract (Ulhaq and Soraya 2020). Furthermore, an increased level of ferritin and IL-6 was found in non-survivors compared to survivors in the recent outbreak of SARS-CoV-2 in China (Huang et al. 2020a, b).
Based on a recent meta-analysis data, the mean concentrations of IL-6 in patients with complicated COVID-19 were 2.9-fold higher than those with an uncomplicated disease course (Coomes and Haghbayan 2020). Studies have exhibited that IL-6 is correlated with activation of the coagulation cascade, vascular leakage, and cardiomyopathy (Levi et al. 2003; Kanda and Takahashi 2004). The association of serum SARS-CoV-2 RNA level with elevated IL-6 concentration and poor prognosis implicates that multiple organ dysfunction in severe COVID-19 patients might be at least partly due to a direct viral invasion (Chen et al. 2020a, b). In another study, the COVID-19 severity attributed to the overproduction of pro-inflammatory cytokines IL-6, IL-1β, IL-2, IL-7, IL-10, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) (Mehta et al. 2020). The cytokine storm leading to the vascular endothelial cell apoptosis (loss of integrity) resulted in increased vascular permeability and vascular leakage, lung microvascular dysfunction, alveolar edema, and eventually hypoxia (De Lorenzo et al. 2020). Furthermore, pro-inflammatory cytokines upregulate the adhesion molecules to capture inflammatory cells from the circulation, leading to endothelial activation, procoagulant, and pro-adhesive alterations, deteriorating microvascular flow, and therefore tissue perfusion (De Lorenzo et al. 2020). The sequence of cellular events leading to the hypercoagulation state observed in patients with severe COVID-19 can be summarized as (1) SARS-CoV-2 infection, (2) cytokine storm, (3) endothelial activation followed by platelet activation, (4) the expression of tissue factor (TF) and the subsequent exposure of TF to the blood, (5) the activation of the extrinsic coagulation pathway together with the decreasing endogenous anticoagulant levels and increasing plasminogen activator inhibitor-1 (PAI-1) levels, and finally, (6) an extra production of thrombin and fibrinolysis shutdown (Levi and van der Poll 2017; Beristain-Covarrubias et al. 2019; Schmitt et al. 2019).
4.3 COVID-19-Associated Thromboembolism and Coagulopathy
During endothelial dysfunction, the endothelium properties, including thrombotic and coagulant, would be changed, which may shift the homeostasis of endothelium toward a pro-inflammatory and pro-thrombotic phenotype (Chousterman et al. 2017). The vascular ECs and skin ECs were found to be infected by SARS-CoV-2 (Ackermann et al. 2020; Colmenero et al. 2020; Menter et al. 2020; Varga et al. 2020), and circulating ECs are elevated in patients with COVID-19 admitted to the hospital (Khider et al. 2020). In addition to the respiratory tract, SARS-CoV-2 viral load has been discerned in highly vascularized tissues, including the heart, kidneys, liver, and brain (Pons et al. 2020; Puelles et al. 2020). Infection caused by SARS-CoV-2 has adverse effects on endothelium, correlating with EC apoptosis, suggesting the endothelium may become dysfunctional in COVID-19 (Varga et al. 2020; Wichmann et al. 2020). The hyper-inflammatory and procoagulatory states in COVID-19 indicate that the endothelium serves as a target and an effector participating in thrombosis and inflammation (Klok et al. 2020).
The thrombus formation results in alveolar damage and microcirculatory disturbance, thereby respiratory dysfunction in COVID-19. Both alveolar damage and microcirculatory disturbance correlated with thrombus formation participate in respiratory dysfunction in COVID-19. However, the commonly embolic event is due to the deep vein thrombus. In some cases, in situ formation in the pulmonary arteries can be responsible for pulmonary dysfunction. In another study, despite the absence of clinical presentations of thromboembolism, the autopsy examination of all COVID-19 patients showed thrombus establishment in small- and mid-sized pulmonary arteries (Lax et al. 2020).
The analysis of conventional coagulation tests in COVID-19 patients provided important data, including antithrombin activity (AT), fibrinogen, fibrin degradation product (FDP), D-dimer, prothrombin time (PT), and activated partial thromboplastin time (APTT) (Tang et al. 2020). Although coagulation laboratory tests, including PT, APTT, and platelet count, are often normal and aren’t practical indicators of the thrombotic risk, increased D-dimer implicating that D-dimer monitoring is critical in COVID-19 coagulopathy. The typical COVID-19-associated coagulopathy can be diagnosed by elevated D-dimer, fibrinogen, and VWF levels. The increasing levels of VWF, to 3–4 times normal amounts, have been reported in patients with COVID-19 (Keith et al. 2020; Zachariah et al. 2020). Furthermore, factor VIII and angiopoietin 2, stored in Weibel–Palade bodies, are released in response to SARS-CoV-2 infection (Streetley et al. 2019; Escher et al. 2020; Helms et al. 2020; Smadja et al. 2020). Angiopoietin 2 represses anticoagulatory, anti-inflammatory, and anti-apoptotic signaling induced by angiopoietin 1; therefore, it acts as an antagonist for angiopoietin 1 (Uchimido et al. 2019). Altogether, these studies emphasize the prothrombotic and procoagulatory characteristics of COVID-19.
5 COVID-19-Associated Comorbidities
Hypertension, heart failure, global cardiovascular diseases, and diabetes mellitus are associated with the severity of COVID-19 and the mortality of COVID-19 patients (Hu et al. 2020; Hessami et al. 2020; Su et al. 2020). Therefore, a correlation exists between comorbidities and the increased risk of COVID-19 severity. The common denominator among all these comorbidities is pre-existing endothelial dysfunction (Fig. 4). In this section, the most common comorbidities among COVID-19 patients, including aging, obesity, hypertension, diabetes, renal dysfunction, and cardiovascular diseases, are summarized.
5.1 Aging
Clinical data from COVID-19 patients indicate that children have lower infection rates and better clinical outcomes than adults (Guan et al. 2020; Li et al. 2020a, b, c). The lower susceptibility of children to COVID-19 than adults ascribed to the lower expression of ACE2 (Chen et al. 2016). The ACE2 expression and activity during the development of human children are obscure (Dong et al. 2020). Aging might be associated with the poor prognosis and pathological progression of COVID-19. The results from a study comprising a cohort of 1099 patients with laboratory-confirmed COVID-19 indicated that the severe patients and the non-survivors were significantly higher than the non-severs and the survivors (Yang et al. 2020a, b). The effect of aging on ACE2 activity might depend on gender (Fernández-Atucha et al. 2017; Hu et al. 2018). An investigation of the ACE2 activity in humans indicated that although the ACE2 activity had a considerable difference between the young and aged females, it was not different in the young and aged males. Another study reported the upper ACE2 activity in the aged females compared to the younger ones (Fernández-Atucha et al. 2017). Thus, the activity of ACE2 in women is controversial during aging, maybe due to sample number or genetic diversity.
5.2 Hypertension and Cardiovascular Disease
ACE2 produces Ang (1-7), thereby regulating blood pressure by altering vascular tone and function. The RAS dysregulation mainly implicates hypertension pathogenesis and progression (Riet et al. 2015). ACE2 was shown to decrease blood pressure in hypertension animal models (Kuba et al. 2010). Furthermore, decreased ACE2 levels were found in the lung tissues of patients with idiopathic pulmonary-associated hypertension (Zhang et al. 2018). Similarly, ACE2 declined in kidneys, blood vessels, and the brain of the models of hypertension (Xia et al. 2013; Mendoza-Torres et al. 2015). Given that ACE2 is known as the main SARS-CoV-2 entry receptor and the protective role of ACE2 in lungs (Imai et al. 2005) and heart (Crackower et al. 2002) tissue injuries, ACE2 plays a role in the development and progression of COVID-19 complications. In 2020, a high severity rate was observed in COVID-19 patients with hypertension (Guan et al. 2020). Also, it has to note that older COVID-19 patients with hypertension might have dysregulated ACE2 expression/function that inclines them to severe disease and mortality (Li et al. 2020a, b, c; Verity et al. 2020; Wang et al. 2020a, b, c, d, e; Wu and McGoogan 2020).
A multivariable-adjusted analysis consisting of 487 Chinese COVID-19 patients revealed that age over 50, male gender, and hypertension are independent factors for COVID-19 severity on admission (Shi et al. 2020a, b, c). In another study involving 548 Chinese inpatients, the prevalence of hypertension was significantly higher in patients with severe COVID-19 compared to non-severe cases (Li et al. 2020a, b, c). In contrast, a study in France showed that hypertension is not associated with COVID-19 severity (Simonnet et al. 2020). Shibata et al. (2020) believe that evidence is yet insufficient and speculate that the high prevalence of hypertension among patients with severe and fatal COVID-19 may be due to the vulnerability of older individuals to SARS-CoV-2 infection. To date, there is no clear evidence approved that hypertension is a critical factor for developing the severe disease in COVID-19 patients as hypertension is not located in the list of risk factors of the Centers for Disease Control and Prevention (CDC) for COVID-19 severity (Shibata et al. 2020).
Cardiovascular complications seem the riskiest and most lethal among different consequences of severe COVID-19. There is a reciprocal relationship between viral respiratory infections and cardiovascular diseases; on the one side, viral respiratory infectious diseases can increase the risk of cardiovascular events; on the other side, the underlying cardiovascular comorbidities raise the risk of mortality among patients with infection (Annamaria et al. 2020). Acute myocardial injury is the most commonly reported cardiovascular complication of COVID-19 infection proved by elevated cardiac biomarkers, including cardiac troponins and ECG changes (Bansal 2020). The incidence of myocardial injury was reported 7–28% and fluctuated among hospitalized patients (Bhatraju et al. 2020; Clerkin et al. 2020; Guo et al. 2020; Lippi et al. 2020; Shi et al. 2020a, b, c). Therefore, myocardial cell injury due to the direct viral attack to the myocardium and vascular endothelium is one of the suggested mechanisms for cardiovascular injury in COVID-19. Another assumption is the influence of tissue hypoxia, coronary plaque destabilization, and microthrombogenesis induced by the systematic inflammation and correlated with cytokine storm (Clerkin et al. 2020).
Acute cardiac injury and heart failure have been suggested as predictors of COVID-19 severity and outcome (Huang et al. 2020a, b). Some studies found a correlation between elevated troponin levels and a more severe clinical course and worse outcomes in Chinese hospitalized patients (Guo et al. 2020; Lippi et al. 2020; Shi et al. 2020a, b, c; Wang et al. 2020a, b, c, d, e). In contrast, in a study accomplished in the USA, elevated troponin levels on ICU admission were in only 15% of fatally ill COVID-19 patients, with a 50% of mortality rate in the cohort as a whole (Bhatraju et al. 2020). A cohort study involving 191 hospitalized Chinese COVID-19 patients found heart failure in half of the fatal cases and only 12% of survivors (Zhou et al. 2020). Data from a recent study have emphasized that genetic susceptibility to COVID-19-related cardiac events is a potential contributor to the high mortality among African American patients with COVID-19 (Giudicessi et al. 2020). Hachim et al. (2020) performed in silico analysis of publicly available transcriptomic datasets to elucidate the potential molecular pathways and the endothelium role in the pathogenesis of cardiac and vascular injuries in COVID-19. Their analysis using the SARS-CoV-2-infected cardiomyocyte-derived dataset revealed downregulating four cardioprotective genes, including MRPS11, HIKESHI, NDUFB7, and NDUFA4L2. Furthermore, they showed that three genes, including SON DNA and RNA binding protein (SON), O-linked N-acetylglucosamine [GlcNAc] transferase (OGT), and RAR-related orphan receptor A (RORA), were shared by all the venous thromboembolism, heart failure, and acute coronary syndrome, although they differentially expressed in the peripheral blood of patients with every three conditions. Besides, evaluating the expression of these genes in healthy blood endothelial cells using the dataset GSE17078 showed a significantly lower SON, OGT, and RORA expression in African Americans than Caucasians. As the results are indicated, the downregulation of cardio-protective genes may likely play a role in cardiovascular events in patients with COVID-19, and probably the expression pattern of the SON, OGT, and RORA genes participates in genetic susceptibility to cardiovascular injury observed in COVID-19 patients. Recent evidence suggests that the endothelial dysfunction because of a direct viral invasion is unifying the occurrence of cardiovascular events and other pre-existing comorbidities in severe COVID-19. However, the complex interaction between cytokines and coagulation storms within the vessels is beyond everything else that can irreparably compromise the endothelium integrity and its anti-inflammatory and antithrombotic properties (Zheng et al. 2020).
5.3 Renal Dysfunction
The ACE2 expression and activity are enhanced in the kidneys (Kuba et al. 2010), and the kidneys may be a major target organ of SARS-CoV-2 infection. The urine samples from some COVID-19 patients were found positive for SARS-CoV-2 (Guan et al. 2020). Acute kidney injury was reported in more than 20% of COVID-19 patients with a fatal disease and correlated with a higher risk of death in COVID-19 patients (Cheng et al. 2020; Fanelli et al. 2020; Richardson et al. 2020; Yang et al. 2020a, b). The expression level of ACE2 decreased in both acute kidney injury (Fang et al. 2013) and several models of chronic kidney disease, which disturbed the RAS homeostasis in the kidneys (Kuba et al. 2010; Soler et al. 2013). Therefore, it is plausible that the decreased ACE2 expression in persons with acute renal disease, on the one hand, and the decreased ACE2 activity due to viral binding, on the other hand, could potentially worsen kidney injury in patients with simultaneously COVID-19 and chronic kidney disease. In agreement, an independent risk factor for mortality in hospitalized patients was renal dysfunction (Cheng et al. 2020). These studies nominate acute kidney injury as one of the most critical complications that happen in COVID-19 patients. Nevertheless, further investigation into the implication of ACE2 in renal injury and clinical outcomes of the COVID-19 patients with chronic kidney diseases as comorbidity is needed.
5.4 Obesity and Diabetes
The adipose tissue directly secretes various inflammatory products, and obesity represents a low-grade inflammation state. Hyperplastic or hypertrophied adipose tissue releases various factors such as inflammatory cytokines, transforming growth factor-β (TGF-β), hemostatic proteins (plasminogen activator inhibitor-1; PAI-1), proteins affecting blood pressure (angiotensinogen), angiogenic molecules (vascular endothelial growth factor; VEGF), etc. (Divella et al. 2016). The main adipose tissue-derived inflammatory cytokines consist of TNFα, IL-1, and IL-6. According to the CDC report, people with a body mass index (BMI) of ≥ 40 (severe obesity) display a higher risk of severe COVID-19 (Hageman 2020).
ACE2 has expressed in pancreatic beta cells (Bindom and Lazartigues 2009; Blodgett et al. 2015; Shoemaker et al. 2015; Roca-Ho et al. 2017; Wang et al. 2017; Xuan et al. 2018). Earlier studies showed that ACE2 is a potential therapeutic target to improve microcirculation in the islets of Langerhans (Lu et al. 2014). A diverse range of harmful stimuli participates in vascular complications in diabetes including pro-inflammatory cytokines, chemokines, adhesion molecules, and transcription factors (Forbes and Cooper 2013). Type 2 diabetes mellitus (T2DM) is correlated with a chronic systemic inflammation state, and it has been clear that the levels of circulating inflammatory markers elevate in patients with diabetes (Festa et al. 2000; Vozarova et al. 2001). Previously, it is found that SARS-CoV damaged the endocrine part of the pancreas and higher fasting plasma glucose (FPG), hyperglycemia, was an independent predictor for mortality and morbidity in SARS-CoV patients and associated with the higher levels of ACE2 expression in the pancreas, suggesting that SARS-CoV can cause lesions in the pancreatic islets (Yang et al. 2010). It is likely that SARS-CoV-2 also could cause new-onset diabetes either by a direct action on the islets or by increasing insulin resistance. It was reported that diabetes mellitus is a common comorbidity in COVID-19 patients (Arentz et al. 2020; Bornstein et al. 2020; Gentile et al. 2020; Muniyappa and Gubbi 2020; Myers et al. 2020; Rayman et al. 2020). Most of the available evidence is related to type 2 diabetes mellitus and does not discern between the major types of diabetes mellitus (Lim et al. 2020). Changes in insulin requirements are apparently correlated with inflammatory cytokines levels in diabetic patients with COVID-19 (Lim et al. 2020).
An analysis of 1099 Chinese patients with laboratory-confirmed SARS-CoV-2 infection revealed that 7.4% of all patients, 16.2% of patients with severe disease, and 26.9% of patients experiencing a primary composite endpoint of ICU admission and mechanical ventilation had coexisting diabetes (Guan et al. 2020). In agreement, some meta-analyses confirmed the negative effects of diabetes on disease severity or progression in patients with COVID-19 (Fadini et al. 2020; Hu et al. 2020; Li et al. 2020a, b, c). One of the meta-analyses reported that the incidence of diabetes was twofold higher in ICU/severe cases than in non-ICU/severe cases of patients with COVID-19 (Li et al. 2020a, b, c). Another study showed that more than 66% of COVID-19 patients that did not survive had diabetes (Remuzzi and Remuzzi 2020). Altogether, these studies intimate that coexisting pancreatic dysfunction is a risk factor in COVID-19 patients and also suggest the incidence of new-onset diabetes in these patients.
6 Prevention of SARS-CoV-2 Infection
Vaccination is critical for the prevention of SARS-CoV-2 and for overcoming the pandemic. The development of covid-19 vaccines is based on seven platforms: inactivated vaccine, viral vector, mRNA, protein subunit, virus-like particle (VLP), DNA, and live attenuated vaccines (Li et al. 2022).
6.1 Inactivated Vaccines
Inactivated vaccines use in vitro-cultured viruses (SARS-CoV-2), which are inactivated by chemical substances, heat, or radiation (Forchette et al. 2021). The vaccine can maintain the whole virus as an immunogen (Li et al. 2022). Through injection into the body, it activates immune responses of the human body and produces a wide range of antibodies. The WHO has approved two types of inactivated vaccines: BBIBP-CorV(Sinopharm), CoronaVac (Sinovac), and COVAXIN (Bharat) (Li et al. 2022).
6.2 Viral Vector Vaccines
Viral vector vaccines utilize attenuated viruses such as adenovirus as a vector to deliver genetic material of viral proteins (e.g., DNA of s protein) into the body (Forchette et al. 2021). When adenovirus-based vaccines are injected, COVID-19 spike protein is produced and provokes the immune system and induces Th1 cell responses. Besides adenovirus, vesicular stomatitis virus (VSV) can also be genetically engineered for COVID-19 vaccine production (Li et al. 2022). The WHO has approved two types of viral vector vaccines: Ad26.COV2.S (Johnson & Johnson) and AZD1222 (AstraZeneca-University of Oxford).
6.3 mRNA Vaccines
mRNA vaccines are acquired from mRNA, encapsulated by lipid nanoparticles (LNP) or other delivery systems (Forchette et al. 2021). The mRNA vaccine contains genetic code to make SARS-CoV-2 S protein; when it is injected into the body, COVID-19 virus spike protein is created and recognized by the immune system and induces Th1 cell responses, germinal center cell responses and produces specific antibodies against the COVID-19 virus (Forchette et al. 2021). The capsulation of mRNA with LNP can transfer mRNA into cells efficiently and provoke a strong immune response; therefore, it is used in most mRNA vaccines. The WHO approved mRNA vaccines: mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) (Li et al. 2022).
6.4 Protein Subunit Vaccine
Protein subunit vaccines include viral proteins or peptides (spike proteins) as the antigen, which are expressed systemically by several protein expression systems such as yeast, bacteria, insect, or mammalian cells. Also, this type of vaccine needs an adjuvant to signal antigen-presenting cells to provoke strong immune responses. (Li et al. 2022; Forchette et al. 2021; Heidary et al. 2022; Alshrari et al. 2021). The WHO has approved only one COVID-19 protein subunit vaccine for emergency use: NVX-CoV2373 (Novavax).
6.5 VLP Vaccines
VLP vaccines are made up of non-infectious and non-replicating virus-like particles of SARS-COV-2 (such as S proteins) expressed in vitro. VLP vaccines do not contain genetic material but assume the function and structure of the virus (antigen covering a shell structure), stimulate immune responses, and produce antibodies (Li et al. 2022; Forchette et al. 2021). Plant-based VLPs have the potential to use as a COVID-19 vaccine; they are not approved yet but are in clinical trials and preclinical stages. They show immunogenicity and safety in human clinical trials (Chen et al. 2013).
6.6 DNA Vaccines
DNA vaccines consist of a recombinant plasmid containing a gene encoding the viral proteins or polypeptides of SARS-CoV-2. Through injection of DNA vaccines, spike protein is produced and activates the immune responses. The WHO has not approved any COVID-19 DNA vaccine for emergency use (Li et al. 2022).
6.7 Live Attenuated Vaccines
Live attenuated vaccines are viruses acquired from reverse genetics to reduce virulence, so they function as non- or weak pathogenic antigens. The main processes for vaccine production include virulence gene knockout and codon pair deoptimization (CPD). The CPD method induces extensive cellular, humoral, and innate immunity responses in recipients against viral proteins. The WHO has not approved any COVID-19 live attenuated vaccine for emergency use (Li et al. 2022).
The features of different COVID-19 vaccines are shown in Table 2.
7 Potential Strategies and Therapeutic Agents for COVID-19 Treatment
Although vaccine development is a major focus for preventing SARS-CoV-2, other therapeutic approaches, including antiviral drugs and monoclonal antibodies, have been utilized and researched to treat patients with COVID-19. This section highlights some pre-existing US Food and Drug Administration (FDA)-approved drugs and potential therapeutic future strategies (Fig. 5).
7.1 RAS Inhibitors and ACE2 Activators
ACE inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are common antihypertensive drugs developed to inhibit RAS and to decrease the adverse effects of Ang II by reducing its production (ACEIs) or downstream effects (ARBs). It has been exhibited that both ACEI and ARBs improve endothelial dysfunction (Shahin et al. 2011; Li et al. 2014). Furthermore, they may reduce the tissue factor expression and hence procoagulatory states in endothelial cells and other cell types (Müller et al. 2000). Several experimental animal models have exhibited inconsistent findings correlated with the effects of ACEIs and ARBs on ACE2 levels or activity in tissue. While some have shown that RAS inhibitors upregulate ACE2 expression (Ferrario et al. 2005), others showed no effect. Against attainable animal studies, only a few studies investigated the effect of ACEIs and ARBs on ACE2 levels or ACE2 activity in humans. An earlier study assessed the impact of ACEI and ARB treatment on the intestinal gene expression of ACE2 (Vuille-dit-Bille et al. 2015). The results indicated that the mRNA expression level of ACE2 was increased 1.9-fold in patients treated with ACEIs compared to non-treated controls, although ACE2 expression levels showed no significant differences in patients treated with ARBs compared to the non-treated group. Furuhashi et al. (2015) performed a cohort study consist Japanese hypertensive patients to investigate the effects of RAS inhibitors on urinary ACE2 levels. They found that although Ang II receptor blocker olmesartan increased urinary ACE2 levels, other ARBs and the ACE inhibitor enalapril did not significantly affect the treatment groups. These conflicting results suggested that ACEIs or ARBs effects on ACE2 expression depend on tissue kind and clinical state.
On the one hand, if RAS inhibitors upregulate ACE2 expression in viral infection, theoretically it can increase the opportunity of viral entry into organs and susceptibility to infection. On the other hand, it is noteworthy that ACE2, as a natural RAS inhibitor, via the production of vasodilator angiotensin (1-7), participates in the natural host response against pulmonary infection (Sodhi et al. 2019). Thus, it is plausible that RAS inhibition participates in lung protection against SARA-CoV-2 infection (Imai et al. 2005; Kuba et al. 2005). The effect of RAS inhibitors on worse outcomes and mortality was investigated in Iranian COVID-19 diabetic patients (Aghaaliakbari et al. 2020). Data showed that ACEI use was correlated with a higher mortality rate in diabetic patients with COVID-19, although the ARB's use did not affect the survival rate. Interestingly, the use of neither ACEI nor ARBs did not correlate with mortality in non-diabetic COVID-19 patients. However, the results showed the adverse effect of using ACEIs in diabetic COVID-19 patients but could not support such an effect in the COVID-19 patients' outcomes treated with ARBs. Nevertheless, more studies are needed to confirm the effect of ACEIs on the death risk in diabetic COVID-19 patients. Given that no valid/enough clinical data affirm the hypothesis that RAS inhibitors enhance the risk of COVID-19 or disease severity, the International Society of Hypertension and other knowledgeable societies have already recommended that the use of ACEIs and ARBs must be continued in high-risk patients during the pandemic (Shibata et al. 2020). More examination is needed to determine if RAS inhibitors are advantageous or disadvantageous in COVID-19 treatment.
Increasing the ACE2 activity or decreasing the effect of the classical RAS through opposing Ang II effects is an approach to reconstructing vascular dysfunction and other pathological diseases (Qaradakhi et al. 2020). The increase in ACE2 activity protects against lung damage and reduces the inflammatory response in lung injury (Fang et al. 2019; Peiró and Moncada 2020). It has been found that xanthenone (XNT) and diminazene aceturate (DIZE) activate ACE2. DIZE is an antitrypanosomal agent that is commercially available. Because of its vasorelaxation, hypotensive, and anti-inflammatory properties and its ability to augment ACE2, DIZE has been shown beneficial cardioprotective effects in various experimental models of diseases (Velkoska et al. 2016). The protective effect of ACE2 activation on pulmonary injury, including ARDS, indicated that developing and utilizing ACE2 activators can be a potential therapeutic strategy against COVID-19.
7.2 Recombinant ACE2, ADAM-17 Enhancers/Activators, and Calmodulin Antagonists
SARS-CoV-2 utilizes ACE2 as a doorway to cellular entry, resulting in the systematic shortage of ACE2. It has shown that recombinant ACE2 (rACE2) can protect against diabetic nephrology, hypertension (Kuba et al. 2010), and pulmonary injury (Imai et al. 2005). Given that ACE2 is the crucial receptor for SARS-CoV-2 infection, it has been suggested that inhibiting ACE2/SARS-CoV-2 interaction could be a promising pharmacologic target for treating patients with COVID-19. Despite anchored ACE2 may enable SARS-CoV-2 to enter cells, circulating ACE2 (detached form) may restrict SARS-CoV-2 entry into pulmonary endothelial cells by attaching itself to the virus. Treatment of COVID-19 patients with a human recombinant soluble ACE2 (hrsACE2) is already under clinical trials (Zhang et al. 2020a, b, c, d, e). Furthermore, ADAM-17 contributes to the cleavage of ACE2 ectodomain, and the stimulation of ADAM-17 expression could result in increasing ACE2 shedding and elevating soluble ACE2 levels (Lambert et al. 2005). This introduces ADAM-17 as an attractive target for reducing the SARS-CoV-2 infectivity, suggesting each agent that could upregulate or activate (such as 5-fluorouracil) ADAM-17 will probably have a potential role in COVID-19 treatment (Kyula et al. 2010). It was exhibited that estradiol upregulates ADAM-17 expression in human non-small cell lung cancer (Ren et al. 2015). If estradiol shows such a positive effect on ADAM-17 levels in COVID-19 patients, it will mean higher ACE2 shedding in women and probably provide another plausible explanation for lower susceptibility to SARS-CoV-2 and severity of COVID-19 in women compared to men (Ragia and Manolopoulos 2020). Overall, using soluble ACE2 as a decoy receptor could potentially increase anchored ACE2 receptors available for converting Ang II to vasodilator Ang (1-7), resulting in reduced hypertension and inflammation.
The function of various cell surface proteins regulates by their ectodomain release, including cytokines, growth factors, and enzymes such as ACE2, etc. (Lambert et al. 2008a, b). It has been exhibited that CALM, a ubiquitous calcium-binding protein, interacts with ACE2 and inhibits its ADAM17-dependent ectodomain shedding. It has also shown that CALM inhibitors such as calmidazolium stimulate the shedding of the ACE2 ectodomain in a dose- and time-dependent manner (Lambert et al. 2008a, b). Phenothiazine antipsychotic agents, such as melatonin, trifluoperazine, chlorpromazine, etc., interact with CALM and inhibit its function (Roufogalis 1985; Soto‐Vega et al. 2004). Melatonin is protective against virus-related diseases such as acute lung injury or ARDS and has a high safety profile (Zhang et al. 2020a, b, c, d, e). Melatonin has been suggested as a potential adjuvant therapy in COVID-19 due to its advantageous effects, including anti-inflammatory, antioxidant, and immunomodulatory (Zhang et al. 2020a, b, c, d, e). Tamoxifen is a triphenylethylene antiestrogen that binds to estrogen receptors and modifies their processing. There is evidence that tamoxifen is also a CALM antagonist and inhibits the activation of cAMP phosphodiesterase by CALM (Lam 1984). Thus, it is feasible that the expression of ACE2 has been affected by tamoxifen. Toremifene is another CALM antagonist that, besides tamoxifen, has been exhibited for SARS-CoV and MERS-CoV inhibition (Dyall et al. 2014). Thus, it is likely that both tamoxifen and toremifene could also inhibit SARS-CoV-2.
7.3 TMPRSS2 Inhibitors
Intravascular thrombosis and coagulation more damage to the endothelium and can participate in endothelial inflammation and dysfunction in COVID-19 patients (Chousterman et al. 2017; Evans et al. 2020). Several clinical reports implicated developing thrombotic complications despite prophylactic anticoagulation in COVID-19 patients (Klok et al. 2020; Thachil et al. 2020), suggesting the necessity of supplemental therapy to impede thrombosis. The use of synthetic serine protease inhibitors such as nafamostat mesylate and camostat mesylate, and physiologic anticoagulants such as protein C (Richardson et al. 2008) and antithrombin (Iba et al. 2018) can be a potential therapy against COVID-19. As previously mentioned, TMPRSS2 cleaves and activates the spike protein of SARS-CoV-2 and is essential for viral uptake (Sanders et al. 2020). Therefore, TMPRSS2 inhibition could be a potential therapy against SARS-CoV-2 infection. Camostat mesylate is a potent TMPRSS2 inhibitor and was recently confirmed to obstruct SARS-CoV-2 entry into lung cells (Hoffmann et al. 2020a, b). Nafamostat mesylate is another TMPRSS2 inhibitor with therapeutic potential for COVID-19 treatment. It has reported that the nafamostat efficacy to inhibit SARS-CoV-2 entry into host cells is significantly higher (approximately 15-fold) than camostat (Hoffmann et al. 2020a, b). Both camostat and nafamostat mesylate have been used to treat diseases unrelated to coronavirus and thus are readily available. However, TMPRSS2 expression in microvascular ECs normally is below the detection level, but it actively has increased during angiogenic or tubulogenic responses (Aimes et al. 2003). Furthermore, the TMPRSS2 activity, similar to other serine proteases, is regulated by nitrosylation (Stamler et al. 2001). Thus, the activation of endothelial nitric oxide synthase (eNOS) and following production of nitric oxide (NO) may likely affect viral infection. Nevertheless, additional studies are required to better understand the physiological expression and function of TMPRSS2 in adult ECs.
7.4 Statins
Statins are conventional cholesterol-lowering drugs used for improving vascular endothelial function and the prevention of atherosclerotic cardiovascular diseases (Adhyaru and Jacobson 2018; Dastghaib et al. 2020). Different mechanisms have been introduced to improve endothelial function mediated by statin, including reducing oxidized low-density lipoprotein cholesterol, inducing eNOS expression and nitric oxide release, decreasing C-reactive protein (CRP) levels, inhibition of NF-κB, the high mobility group box 1(HMGB1)/TLR4 pathway and other signaling pathways (Hölschermann et al. 2006; Peymani et al. 2021). CRP is an acute-phase protein and belongs to the pentraxin family of proteins, and its circulating concentrations increase in response to inflammation (Pepys and Hirschfield 2003). Thus, reducing CRP levels by statins implicated the anti-inflammatory properties of these drugs independent of their cholesterol-lowering effects. Statins exhibited advantageous effects in hypertensive patients, with normal cholesterol levels, due to their anti-inflammatory properties (Peymani et al. 2021). Statins reduce the tissue factor expression in endothelial cells, and these drugs may impede EC activation and exhibit antithrombotic and anticoagulant effects (Lee et al. 2020). Other suggested mechanisms to elucidate the antithrombotic property of statin include decreasing platelet aggregation and increasing thrombomodulin expression in endothelial cells (Lee et al. 2020). The ability of statins to prevent endothelial dysfunction introduces these as promising drugs for obstructing vascular damage in COVID-19. Furthermore, some properties of statins, including availability, low cost, and safety, suggest their application as part of COVID-19 treatment.
Recently, a small observational study has been performed on patients with a pre-existing chronic cardiovascular disease and with an incidence of COVID-19 (Rossi et al. 2020). However, the results showed a trend toward reducing mortality risk in patients taking statin compared to patients without statin, but the effect of statins was not substantially significant. Besides, the risk of mortality was not significantly different between the subgroup of high-intensity statins and the subgroup of low- or moderate-intensity statins. Further analysis was performed to investigate whether the pharmacokinetic characteristics of statins were able to affect the mortality rate. The comparison of the survival curves demonstrated a significant reduction in mortality in the patients who assumed lipophilic statins compared to patients who did not take statins and also patients who took hydrophilic statins. Against hydrophilic statins, which have some hardships in organ penetrating, lipophilic statins have a broad tissue distribution and reach throughout the body, providing a protective role against the virus. Therefore, hydrophilic statins have fewer anti-inflammatory effects compared to lipophilic statins.
A recent meta-analysis reported that statin use did not improve illness' severity or mortality in COVID-19 infections (Hariyanto and Kurniawan 2020). However, the statins decreased the risk of neutrophilia but did not influence the mortality in chronic renal disease patients with COVID-19 (Yang et al. 2020a, b). Data from a retrospective cohort study in Iran revealed an association, but not statistically significant, between statin use and a lower risk of morbidity and mortality (Peymani et al. 2021). Moreover, statin use reduced the necessity of being subjected to mechanical ventilation, and statin users displayed a more normal computed tomography (CT) scan result. In a similar retrospective cohort study in China, statin use correlated with a lower death rate compared to non-use in COVID-19 (Zhang et al. 2020a, b, c, d, e). Given that both statins and ARBs upregulate ACE2 activity and opposed endothelial dysfunction, a statin/ARB combination therapy might be considered for patients with severe COVID-19 infection (Fedson et al. 2020). Taken together, statin use and its capability in improving COVID-19 outcomes are debatable, and there is a necessity for prospective randomized controlled trials and comprehensive retrospective studies to more assess the potential pleiotropic effects of statin treatment in COVID-19.
7.5 SARS-CoV-2 Replication Inhibitors
Due to their critical role in processing and generating 16 non-structural proteins involved in SARS-CoV-2 replication, the SARS-CoV-2 proteases, including Mpro and PLpro, are other attractive targets for COVID-19 treatment. To date, several novels or repurposed drugs have been recognized that effectively interact with one or both the viral proteases, thereby inhibiting the processing of the replicase polyproteins and assembly of the viral transcription or replication complex (Neumaier et al. 2020). They can divide into two groups: (1) peptides that mimic part of the substrate of the proteases and (2) different small molecule drugs. In a recent study, Zhang et al. (2020a, b, c, d, e) developed an α-ketoamide inhibitor based on the crystal structure of unliganded SARS-CoV-2 Mpro and introduced it as a lead compound to treat COVID-19. In another recent study, Jin et al. (2020a, b) assayed more than 10,000 compounds by combining structure-based virtual and high-throughput screening. They identified six compounds that inhibit Mpro. Among these compounds, ebselen also showed a significant antiviral effect in cell-based assays. Furthermore, it has been exhibited that ebselen effectively inhibits SARS-CoV-2 PLpro (Weglarz-Tomczak et al. 2020). Recently, the antineoplastic agent carmofur has been shown to inhibit SARS-CoV-2 replication by inhibiting its Mpro (Jin et al. 2020a, b).
The 5-leader-UTR-RNA dependent RNA polymerase (RdRp) is one of the critical non-structural proteins involved in viral replication, which may be targeted by nucleoside analogues, such as remdesivir, molnupiravir, ribavirin, favipiravir, Galidesivir, paxlovid, and tocilizumab, to inhibit RNA synthesis and SARS-CoV-2 infection (Han et al. 2021). Remdesivir is an antiviral drug, acting as a nucleoside analog inhibiting the RNA-dependent RNA polymerase (RdRp) of coronaviruses (e.g., SARS-CoV-2), and is the primary antiviral approved by US Food and Drug Administration (FDA) for COVID-19 treatment in adults and pediatric patients aged 12 years and older and weighing at least 40 kg requiring hospitalization (Forchette et al. 2021; Alshrari et al. 2021). It is effective in shortening the time of recovery in patients who were hospitalized with COVID-19 and had evidence of lower respiratory tract infection. A combination therapy using remdesivir and GC376, an inhibitor of the Mpro, resulted in a synergistic antiviral effect against SARS-CoV-2 in Vero cells by inhibiting the replication of SARS-CoV-2 through different drug targets (Fu et al. 2020). A recent retrospective comparative study in the USA involving 2483 COVID-19 patients revealed that patients who received remdesivir recovered more rapidly than those who did not receive remdesivir (Garibaldi et al. 2021). In 2020, the FDA approved and recommended remdesivir for the treatment of adult and pediatric hospitalized patients with COVID-19 (aged > 12 years with a body weight of at least 40 kg) (Aleissa et al. 2020). Recent studies have also exhibited that ribavirin, a guanine derivative that was approved by FDA to treat the hepatitis C virus (HCV), respiratory syncytial virus, and Ebola virus (EBOV) infection, has antiviral activity against SARS-CoV-2 (Elfiky 2020; Wang et al. 2020a, b, c, d, e). Molnupiravir (also known as EIDD-2801/MK-4482) increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans (Kabinger et al. 2021). Molnupiravir has been primarily used to treat RNA viruses such as influenza and coronaviruses (Forchette et al. 2021). Recent studies have shown that molnupiravir reduces the risk of hospitalization and death in non-hospitalized adults with mild-to-moderate COVID-19 disease. Molnupiravir is only administered orally for a short period of about five days. Although it is well tolerated and safe in short-term use, it may produce mutations in human DNA in long-term use (Wen et al. 2022). Recent in vitro and clinical studies have exhibited the capability of favipiravir to inhibit SARS-CoV-2 replication (Choy et al. 2020), improve the symptoms of COVID-19 patients, and reduce the treatment period (Chen et al. 2020a, b). Galidesivir, which was developed to treat RNA viral infections, including HCV, significantly decreased the MERS-CoV and SARS-CoV loads, and improved survival (Taylor et al. 2016), also inhibited the replication of SARS-CoV-2 in animal models (Elfiky 2020). Paxlovid is an oral antiviral drug candidate for SARS-CoV-2 protease inhibitors developed by Pfizer. It is based on blocking the activity of SARS- COV-2-3Cl protease, which is needed for the SARS-COV-2 to replicate. Paxlovid is an oral antiviral drug candidate for SARS-CoV-2 protease inhibitors developed by Pfizer. It is based on blocking the activity of SARS-COV-2-3Cl protease, which is needed for the SARS-COV-2 to replicate. Paxlovid is a combination of PF-07321332 and ritonavir. Ritonavir helps slow down the metabolism or breakdown of PF-07321332 so that it remains active longer at higher concentrations in the body. Paxlovid reduces the risk of hospitalization or death by 89% (Wen et al. 2022). Further investigations to find compounds inhibiting different stages of SARS-CoV-2 replication can help to COVID-19 treatment.
7.6 Other Potential Drugs
Many Chinese herbal medicines (CHMs) have demonstrated antiviral effects through different mechanisms, including inhibiting S protein-ACE2 interaction, binding to RdRp and inhibiting viral replication, anti-inflammatory effects, etc. (Han et al. 2021). Recently, the Lianhuaqingwen capsule, a traditional Chinese medicine commonly used to treat viral diseases such as influenza, was demonstrated to inhibit the replication of SARS-CoV-2 and its inflammatory activity in vitro (Runfeng et al. 2020). Liquiritin is one of the major flavonoids in Glycyrrhiza uralensis that showed therapeutic effects against COVID-19 by impeding the SARS-CoV-2 replication in Vero E6 cells (Zhu et al. 2020a, b). Scutellariae, a widely used antiviral herb, inhibited the replication of SARS-CoV-2 by interfering with SARS-CoV-2 protease Mpro in Vero cells (Liu et al. 2021). Although recent computational studies and some databases have identified some CHM as potential therapeutic drugs against COVID-19, experimental and clinical studies still require to validate the antiviral effects of these predicted herbs against COVID-19 (Han et al. 2021).
Some drugs, including chloroquine (CQ) and hydroxychloroquine (HCQ), exert antiviral effects by inhibiting viral entry, inhibiting viral replication, and diminishing the inflammatory response induced by SARS-CoV-2. Although several clinical studies exhibited that CQ was effective against SARS-CoV-2 infections (Gao et al. 2020; Huang et al. 2020a, b), other clinical studies reported that HCQ had a more therapeutic effect against COVID-19 than CQ (Yao et al. 2020). A clinical study exhibited a synergistic effect of HCQ and azithromycin against COVID-19 in a cohort of French patients (Andreani et al. 2020; Gautret et al. 2020). Although some experimental and clinical studies indicated potential therapeutic effects of CQ and HCQ to treat SARS-CoV-2 infection, others exhibited that these drugs failed to improve the clinical outcomes of COVID-19 (Chowdhury et al. 2020; Elavarasi et al. 2020; Kumar et al. 2021). These may be due to different disease stages in the clinical trials, different treatment times, and dosages (Han et al. 2021).
Antibody-based therapy is another strategy to treat viral diseases, including COVID-19. The production of specific antibodies is an adaptive immune response in COVID-19 patients. Tocilizumab (TCZ) is a monoclonal antibody (mAb) that inhibits the interleukin-6 (IL-6) receptor; FDA approves it for treating cytokine release syndrome and for emergency use to treat patients hospitalized with COVID19. One of the critical cytokines described in the cytokine storm induced by COVID-19 is IL-6, which has a vital role in systemic inflammation (Petrelli et al. 2021). In addition, the WHO has recommended the use of tocilizumab and sarilumab plus corticosteroids to treat severe COVID-19 (Hwang et al. 2022). The antibodies from patients with COVID-19 can bind to the SARS-CoV-2-S protein or -RBD and inhibit their interactions with ACE2 receptors (Han et al. 2021). Some of these antibodies, such as CB6LALA, ADG20, CT-P59, AZD8895, etc., are in phase 2 or 3 clinical trials in order to treat COVID-19 (Shi et al. 2020a, b, c; Valdez-Cruz et al. 2021). Recently, a clinical trial examined the efficacy of neutralizing antibody LY-CoV555 in COVID-19 patients. The results indicated that patients who received LY-CoV555 had slightly lower symptom severity and viral load than those who had received the placebo (Chen et al. 2021). Canakinumab, a human monoclonal antibody targeting IL-1β, caused rapid, long-lasting improvement in oxygenation levels in 60.3% of the patients with mild or severe non-intensive care COVID-19, which is better than that of the patients who were treated with standard HCQ plus lopinavir/ritonavir, a standard therapy (Katia et al. 2021).
8 Conclusion
SARS-CoV-2 has a high affinity to ACE2 as the primary for cellular entry. A correlation exists between SARS-CoV-2 tropism and the organs expressing ACE2, including the lung, kidneys, heart, etc. Besides, evidence suggests the correlation of the ACE2 expression with gender and genetic properties results in more susceptibility to COVID-19 in men than women, also in the Asian population than the Caucasian and African-American people. One of the most common causes of severe disease and mortality observed in COVID-19 patients is associated with SARS-CoV-2-induced ECD, also pre-existing endothelial dysfunction due to aging, hypertension, cardiovascular diseases, renal dysfunction, diabetes, and obesity. Alterations in ACE2 expression/function that happen in these comorbidities may be correlated with COVID-19 severity. Identifying and developing therapeutic strategies against COVID-19 are necessary to restrain this global pandemic. In this context, several potential therapeutic strategies have been suggested, including those that target the primary viral receptor or its ectodomain shedding, viral uptake, viral replication, etc. These include using RAS inhibitors, ACE2 activators, recombinant ACE2, ADAM-17 enhancers/activators, TMPRSS2 inhibitors, statins, calmodulin antagonists, SARS-CoV-2 replication inhibitors, CHMs, anti-inflammatory drugs, and neutralizing antibodies. Further investigations are needed to further develop these strategies and even identify new therapeutic strategies and drugs.
Change history
03 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40995-023-01424-8
References
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383(2):120–128. https://doi.org/10.1056/NEJMoa2015432
Adhyaru BB, Jacobson TA (2018) Safety and efficacy of statin therapy. Nat Rev Cardiol 15(12):757–769. https://doi.org/10.1038/s41569-018-0098-5
Aghaaliakbari F, Abbasi MA, Ranjbar M, Makiani MJ, Tameshkel FFS, Niya MHK et al (2020) Angiotensin converting enzyme inhibitors, a risk factor of poor outcome in diabetic patients with COVID-19 infection. Iran J Kidney Dis 14(6):482–487
Aimes R, Zijlstra A, Hooper J, Ogbourne S, Sit M-L, Fuchs S et al (2003) Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost 89(3):561–572. https://doi.org/10.1055/s-0037-1613388
Aleissa MM, Silverman EA, Paredes Acosta LM, Nutt CA, Richterman A, Marty FM et al (2020) New perspectives on antimicrobial agents: remdesivir treatment for COVID-19. Antimicrob Agents Chemother 65(1):e01814-01820. https://doi.org/10.1128/AAC.01814-20
Alshrari AS, Hudu SA, Imran M, Asdaq SM, Ali AM, Rabbani SI (2021) Innovations and development of COVID-19 vaccines: a patent review. J Infect Public Health 15(1):123–131
Amraei R, Rahimi N (2020) COVID-19, renin–angiotensin system and endothelial dysfunction. Cells 9(7):1652. https://doi.org/10.3390/cells9071652
Amraei R, Napoleon M, Yin W, Berrigan J, Suder E, Zhao G et al (2020) CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. ACS Cent Sci 7:1156–1165. https://doi.org/10.1021/acscentsci.0c01537
Andreani J, Bideau ML, Duflot I, Jardot P, Rolland C, Boxberger M et al (2020) In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog 145:104228. https://doi.org/10.1016/j.micpath.2020.104228
Annamaria V, Rosetta R, Chiara C, Giuseppina B (2020) COVID-19 and cardiovascular consequences: is the endothelial dysfunction the hardest challenge? Thromb Res 196:143–151. https://doi.org/10.1016/j.thromres.2020.08.039
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M et al (2020) Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323(16):1612–1614. https://doi.org/10.1001/jama.2020.4326
Bader M, Alenina N, Andrade-Navarro MA, Santos RA (2014) Mas and its related G protein-coupled receptors, Mrgprs. Pharmacol Rev 66(4):1080–1105. https://doi.org/10.1124/pr.113.008136
Bansal M (2020) Cardiovascular disease and COVID-19. Diabetes Metab Syndr 14(3):247–250. https://doi.org/10.1016/j.dsx.2020.03.013
Beristain-Covarrubias N, Perez-Toledo M, Thomas MR, Henderson IR, Watson SP, Cunningham AF (2019) Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol 10:2569. https://doi.org/10.3389/fimmu.2019.02569
Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK et al (2020) Covid-19 in critically ill patients in the Seattle region-case series. N Engl J Med 382(21):2012–2022. https://doi.org/10.1056/NEJMoa2004500
Bhella D (2015) The role of cellular adhesion molecules in virus attachment and entry. Philos Trans R Soc B Biol Sci 370(1661):20140035. https://doi.org/10.1098/rstb.2014.0035
Bindom SM, Lazartigues E (2009) The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol 302(2):193–202. https://doi.org/10.1016/j.mce.2008.09.020
Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ et al (2015) Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64(9):3172–3181. https://doi.org/10.2337/db15-0039
Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO (2020) Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol 16(6):297–298. https://doi.org/10.1038/s41574-020-0353-9
Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S et al (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370(6518):856–860. https://doi.org/10.1126/science.abd2985
Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X et al (2020) Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 6(1):1–4. https://doi.org/10.1038/s41421-020-0147-1
Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Agoramoorthy G (2020) Consider TLR5 for new therapeutic development against COVID-19. J Med Virol 92(11):2314–2315. https://doi.org/10.1002/jmv.25997
Chen Q, Lai H (2013) Plant-derived virus-like particles as vaccines. Hum Vaccin Immunother 9(1):26–49
Chen Z, Mi L, Xu J, Yu J, Wang X, Jiang J et al (2005) Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis 191(5):755–760. https://doi.org/10.1086/427811
Chen Q, Tang X, Yu C, Chen D, Tian J, Cao Y et al (2010) Correlation of angiotensin-converting enzyme 2 gene polymorphism with antihypertensive effects of benazepril. Beijing Da Xue Xue Bao Yi Xue Ban 42(3):293–298
Chen Y, Liu D, Zhang P, Zhong J, Zhang C, Wu S et al (2016) Impact of ACE2 gene polymorphism on antihypertensive efficacy of ACE inhibitors. J Hum Hypertens 30(12):766–771. https://doi.org/10.1038/jhh.2016.24
Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y et al (2020b) Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 71(8):1937–1942. https://doi.org/10.1093/cid/ciaa449
Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J et al (2021) SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 384(3):229–237. https://doi.org/10.1056/NEJMoa2029849
Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y et al (2020a). Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv https://doi.org/10.1101/2020.03.17.20037432
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5):829–838. https://doi.org/10.1016/j.kint.2020.03.005
Chesterman CN (1988) Vascular endothelium, haemostasis and thrombosis. Blood Rev 2(2):88–94
Choudhury A, Mukherjee S (2020) In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol 92(10):2105–2113. https://doi.org/10.1002/jmv.25987
Chousterman BG, Swirski FK, Weber GF (2017) Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 39:517–528. https://doi.org/10.1007/s00281-017-0639-8
Chowdhury MS, Rathod J, Gernsheimer J (2020) A rapid systematic review of clinical trials utilizing chloroquine and hydroxychloroquine as a treatment for COVID-19. Acad Emerg Med 27(6):493–504. https://doi.org/10.1111/acem.14005
Choy K-T, Wong AY-L, Kaewpreedee P, Sia SF, Chen D, Hui KPY et al (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res 178:104786. https://doi.org/10.1016/j.antiviral.2020.104786
Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A et al (2020) COVID-19 and cardiovascular disease. Circulation 141(20):1648–1655. https://doi.org/10.1161/CIRCULATIONAHA.120.046941
Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D et al (2020) SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 183(4):729–737. https://doi.org/10.1111/bjd.19327
Coomes EA, Haghbayan H (2020) Interleukin-6 in COVID-19: a systematic review and meta-analysis. J Med Virol 30(6):1–9. https://doi.org/10.1002/rmv.2141
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE et al (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417(6891):822–828. https://doi.org/10.1038/nature00786
Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ et al (2005) Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin–angiotensin system. J Clin Investig 115(4):1092–1099. https://doi.org/10.1172/JCI23378
CSG of the International Committee on Taxonomy of Viruses (2020) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544. https://doi.org/10.1038/s41564-020-0695-z
Cui J, Huang W, Wu B, Jin J, Jing L, Shi WP et al (2018) N-glycosylation by N-acetylglucosaminyltransferase V enhances the interaction of CD147/basigin with integrin β1 and promotes HCC metastasis. J Pathol 245(1):41–52. https://doi.org/10.1002/path.5054
Daly JL, Simonetti B, Klein K, Chen K-E, Williamson MK, Antón-Plágaro C et al (2020) Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370(6518):861–865. https://doi.org/10.1126/science.abd3072
Dastghaib S, Shojaei S, Mostafavi-Pour Z, Sharma P, Patterson JB, Samali A et al (2020) Simvastatin induces unfolded protein response and enhances temozolomide-induced cell death in glioblastoma cells. Cells 9(11):2339. https://doi.org/10.3390/cells9112339
De Meyer GR, Herman AG (1997) Vascular endothelial dysfunction. Prog Cardiovasc Dis 39(4):325–342. https://doi.org/10.1016/S0033-0620(97)80031-X
De Lorenzo A, Escobar S, Tibiriçá E (2020) Systemic endothelial dysfunction: a common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr Metab Cardiovasc Dis 30(8):1401–1402. https://doi.org/10.1016/j.numecd.2020.05.007
Deschepper CF (1994) Angiotensinogen: hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int 46(6):1561–1563. https://doi.org/10.1038/ki.1994.446
Devaux CA, Rolain J-M, Raoult D (2020) ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect 53(3):425–435. https://doi.org/10.1016/j.jmii.2020.04.015
Divella R, De Luca R, Abbate I, Naglieri E, Daniele A (2016) Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer 7(15):2346. https://doi.org/10.7150/jca.16884
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z et al (2020) Epidemiology of COVID-19 among children in China. Pediatrics. https://doi.org/10.1542/peds.2020-0702
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N et al (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87(5):e1–e9. https://doi.org/10.1161/01.RES.87.5.e1
Duru K, Farrow S, Wang J-M, Lockette W, Kurtz T (1994) Frequency of a deletion polymorphism in the gene for angiotensin converting enzyme is increased in African–Americans with hypertension. Am J Hypertens 7(8):759–762. https://doi.org/10.1093/ajh/7.8.759
Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J et al (2014) Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 58(8):4885–4893. https://doi.org/10.1128/AAC.03036-14
Elavarasi A, Prasad M, Seth T, Sahoo RK, Madan K, Nischal N et al (2020) Chloroquine and hydroxychloroquine for the treatment of COVID-19: a systematic review and meta-analysis. J Gen Intern Med 35(11):3308–3314. https://doi.org/10.1007/s11606-020-06146-w
Elfiky AA (2020) Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci 253:117592. https://doi.org/10.1016/j.lfs.2020.117592
Escher R, Breakey N, Lämmle B (2020) ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res 192:174–175. https://doi.org/10.1016/j.thromres.2020.05.032
Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z et al (2020) Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc Res 116(14):2177–2184. https://doi.org/10.1093/cvr/cvaa230
Fadini G, Morieri M, Longato E, Avogaro A (2020) Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest 43(6):867–869. https://doi.org/10.1007/s40618-020-01236-2
Fan X-h, Wang Y-b, Wang H, Sun K, Zhang W-l, Song X-d et al (2009) Polymorphisms of angiotensin-converting enzyme (ACE) and ACE2 are not associated with orthostatic blood pressure dysregulation in hypertensive patients. Acta Pharmacol Sin 30(9):1237–1244. https://doi.org/10.1038/aps.2009.110
Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C et al (2020) Acute kidney injury in SARS-CoV-2 infected patients. Crit Care 24:1–3. https://doi.org/10.1186/s13054-020-02872-z
Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V et al (2013) Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS ONE 8(8):e71433. https://doi.org/10.1371/journal.pone.0071433
Fang Y, Gao F, Liu Z (2019) Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM-INT J MED 112(12):914–924. https://doi.org/10.1093/qjmed/hcz206
Fedson DS, Opal SM, Rordam OM (2020) Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. MBio. https://doi.org/10.1128/mBio.00398-20
Fernández-Atucha A, Izagirre A, Fraile-Bermúdez AB, Kortajarena M, Larrinaga G, Martinez-Lage P et al (2017) Sex differences in the aging pattern of renin–angiotensin system serum peptidases. Biol Sex Differ 8(1):1–8. https://doi.org/10.1186/s13293-017-0128-8
Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI (1997) Counterregulatory actions of angiotensin-(1–7). Hypertension 30(3):535–541. https://doi.org/10.1161/01.HYP.30.3.535
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA et al (2005) Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111(20):2605–2610. https://doi.org/10.1161/CIRCULATIONAHA.104.510461
Festa A, D’Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM (2000) Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102(1):42–47. https://doi.org/10.1161/01.CIR.102.1.42
Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G et al (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413(6851):78–83. https://doi.org/10.1038/35092578
Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1):137–188. https://doi.org/10.1152/physrev.00045.2011
Forchette L, Sebastian W, Liu T (2021) A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr Med Sci 41(6):1037–1051
Fu L, Ye F, Feng Y, Wang Q, Wu Y, Zhao C et al (2020) Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun 11(1):1–8. https://doi.org/10.1038/s41467-020-18233-x
Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K et al (2015) Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 28(1):15–21. https://doi.org/10.1093/ajh/hpu086
Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. https://doi.org/10.5582/bst.2020.01047
Garibaldi BT, Wang K, Robinson ML, Zeger SL, Bandeen-Roche K, Wang M-C et al (2021) Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA NETW OPEN 4(3):e213071–e213071. https://doi.org/10.1001/jamanetworkopen.2021.3071
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob 56(1):105949. https://doi.org/10.1016/j.ijantimicag.2020.105949
Gentile S, Strollo F, Ceriello A (2020) COVID-19 infection in Italian people with diabetes: lessons learned for our future (an experience to be used). Diabetes Res Clin Pract. https://doi.org/10.1016/j.diabres.2020.108137
Gimbrone MA Jr, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4):620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Giudicessi JR, Roden DM, Wilde AA, Ackerman MJ (2020) Genetic susceptibility for COVID-19–associated sudden cardiac death in African Americans. Heart Rhythm 17(9):1487–1492. https://doi.org/10.1016/j.hrthm.2020.04.045
Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720. https://doi.org/10.1056/NEJMoa2002032
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T et al (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 5(7):811–818. https://doi.org/10.1001/jamacardio.2020.1017
Gwathmey TM, Shaltout HA, Nixon PA, O’shea TM, Rose JC, Washburn LK et al (2008) Gender differences in urinary ACE and ACE2 activities in adolescents. FASEB J 22(1):940.6-940.6. https://doi.org/10.1096/fasebj.22.1_supplement.940.6
Hachim MY, Al Heialy S, Senok A, Hamid Q, Alsheikh-Ali A (2020) Molecular basis of cardiac and vascular injuries associated with COVID-19. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2020.582399
Hageman JR (2020) The coronavirus disease 2019 (COVID-19). Pediatr Ann 49(3):e99–e100. https://doi.org/10.3928/19382359-20200219-01
Hall JE (2003) Historical perspective of the renin–angiotensin system. Mol Biotechnol 24(1):27–39. https://doi.org/10.1385/MB:24:1:27
Hamming I, Timens W, Bulthuis M, Lely A, Navis GV, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/path.1570
Han F, Liu Y, Mo M, Chen J, Wang C, Yang Y, Wu J (2021) Current treatment strategies for COVID-19. Mol Med Rep 24(6):1–12. https://doi.org/10.3892/mmr.2021.12498
Hariyanto TI, Kurniawan A (2020) Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr 14(6):1613–1615. https://doi.org/10.1016/j.dsx.2020.08.023
Hedner T, Hansson L, Himmelmann A (2000) Endothelial dysfunction: a challenge for hypertension research. Blood Press 9:2
Heidary M, Kaviar VH, Shirani M, Ghanavati R, Motahar M, Sholeh M et al (2022) A comprehensive review of the protein subunit vaccines against COVID-19. Front Microbiol 14(13):927306
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X et al (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46(6):1089–1098. https://doi.org/10.1007/s00134-020-06062-x
Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA (2020) The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med 9(4):1225. https://doi.org/10.3390/jcm9041225
Hessami A, Shamshirian A, Heydari K, Pourali F, Alizadeh-Navaei R, Moosazadeh M et al (2020) Cardiovascular diseases burden in COVID-19: systematic reviewand meta-analysis. Am J Emerg Med 46:382–391. https://doi.org/10.1016/j.ajem.2020.10.022
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al (2020a) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e278. https://doi.org/10.1016/j.cell.2020.02.052
Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S (2020b) Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother 64(6):e00754-e820. https://doi.org/10.1128/AAC.00754-20
Hölschermann H, Schuster D, Parviz B, Haberbosch W, Tillmanns H, Muth H (2006) Statins prevent NF-κB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 185(2):240–245. https://doi.org/10.1016/j.atherosclerosis.2005.06.019
Hu Y, Li X, Wu N, Wang N, Qiu C, Li J (2018) Study on the correlation among sex, age and the activity of ACE, ACE2 and the ratio of ACE/ACE2. J Qiqihar Med Univ 39:884–887
Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q et al (2020) Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. https://doi.org/10.1016/j.jcv.2020.104371
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020a) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Huang M, Tang T, Pang P, Li M, Ma R, Lu J et al (2020b) Treating COVID-19 with chloroquine. J Mol Cell Biol 12(4):322–325. https://doi.org/10.1093/jmcb/mjaa014
Hulswit R, De Haan C, Bosch B-J (2016) Coronavirus spike protein and tropism changes. Adv Virus Res 96:29–57. https://doi.org/10.1016/bs.aivir.2016.08.004
Hwang YC, Lu RM, Su SC, Chiang PY, Ko SH, Ke FY et al (2022) Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J Biomed Sci 29(1):1–50
Iba T, Levy JH, Hirota T, Hiki M, Sato K, Murakami T et al (2018) Protection of the endothelial glycocalyx by antithrombin in an endotoxin-induced rat model of sepsis. Thromb Res 171:1–6. https://doi.org/10.1016/j.thromres.2018.09.042
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B et al (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436(7047):112–116. https://doi.org/10.1038/nature03712
Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y et al (2020a) Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 582(7811):289–293. https://doi.org/10.1038/s41586-020-2223-y
Jin Z, Zhao Y, Sun Y, Zhang B, Wang H, Wu Y et al (2020b) Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol 27(6):529–532. https://doi.org/10.1038/s41594-020-0440-6
Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS et al (2021) Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 28:740–746. https://doi.org/10.1038/s41594-021-00651-0
Kanda T, Takahashi T (2004) Interleukin-6 and cardiovascular diseases. Jpn Heart J 45(2):183–193. https://doi.org/10.1536/jhj.45.183
Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM et al (2015) Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimulis. Pharmacol Rev 67(4):754–819. https://doi.org/10.1124/pr.114.010454
Katia F, Myriam DP, Ucciferri C, Auricchio A, Nicola MD, Marchioni M et al (2021) Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun Inflamm Dis 9(2):399–405. https://doi.org/10.1002/iid3.400
Keith P, Day M, Perkins L, Moyer L, Hewitt K, Wells A (2020) A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care 24:128. https://doi.org/10.1186/s13054-020-2836-4
Khider L, Gendron N, Goudot G, Chocron R, Hauw-Berlemont C, Cheng C et al (2020) Curative anticoagulation prevents endothelial lesion in COVID-19 patients. J Thromb Haemost 18(9):2391–2399. https://doi.org/10.1111/jth.14968
Klok F, Kruip M, Van der Meer N, Arbous M, Gommers D, Kant K et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147. https://doi.org/10.1016/j.thromres.2020.04.013
Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM et al (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111(14):1806–1813. https://doi.org/10.1161/01.CIR.0000160867.23556.7D
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S et al (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348(20):1953–1966. https://doi.org/10.1056/NEJMoa030781
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B et al (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11(8):875–879. https://doi.org/10.1038/nm1267
Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM (2010) Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther 128(1):119–128. https://doi.org/10.1016/j.pharmthera.2010.06.003
Kumar J, Jain S, Meena J, Yadav A (2021) Efficacy and safety of hydroxychloroquine/chloroquine against SARS-CoV-2 infection: a systematic review and meta-analysis: Authors reply. J Infect Chemother 27(10):1539–1540. https://doi.org/10.1016/j.jiac.2021.02.021
Kyula JN, Van Schaeybroeck S, Doherty J, Fenning CS, Longley DB, Johnston PG (2010) Chemotherapy-induced activation of ADAM-17: a novel mechanism of drug resistance in colorectal cancer. Clin Cancer Res 16(13):3378–3389. https://doi.org/10.1158/1078-0432.CCR-10-0014
Lam H-YP (1984) Tamoxifen is a calmodulin antagonist in the activation of cAMP phosphodiesterase. Biochem Biophys Res Commun 118(1):27–32. https://doi.org/10.1016/0006-291X(84)91062-3
Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI et al (2005) Tumor necrosis factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280(34):30113–30119. https://doi.org/10.1074/jbc.M505111200
Lambert DW, Clarke NE, Hooper NM, Turner AJ (2008a) Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett 582(2):385–390. https://doi.org/10.1016/j.febslet.2007.11.085
Lambert DW, Hooper NM, Turner AJ (2008b) Angiotensin-converting enzyme 2 and new insights into the renin–angiotensin system. Biochem Pharmacol 75(4):781–786. https://doi.org/10.1016/j.bcp.2007.08.012
Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C et al (2020) Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 173(5):350–361. https://doi.org/10.7326/M20-2566
Lee KCH, Sewa DW, Phua GC (2020) Potential role of statins in COVID-19. Int J Infect Dis 96:615–617. https://doi.org/10.1016/j.ijid.2020.05.115
Levi M, van der Poll T (2017) Coagulation and sepsis. Thromb Res 149:38–44. https://doi.org/10.1016/j.thromres.2016.11.007
Levi M, Keller TT, van Gorp E, ten Cate H (2003) Infection and inflammation and the coagulation system. Cardiovasc Res 60(1):26–39. https://doi.org/10.1016/S0008-6363(02)00857-X
Levick JR (2013) An introduction to cardiovascular physiology. Butterworth-Heinemann, London. https://doi.org/10.1016/C2013-0-06523-1
Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C (2008) ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol 295(6):R1953–R1961. https://doi.org/10.1152/ajpregu.90592.2008
Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S et al (2005) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24(8):1634–1643. https://doi.org/10.1038/sj.emboj.7600640
Li S, Wu Y, Yu G, Xia Q, Xu Y (2014) Angiotensin II receptor blockers improve peripheral endothelial function: a meta-analysis of randomized controlled trials. PLoS ONE 9(3):e90217. https://doi.org/10.1371/journal.pone.0090217
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L et al (2020a) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109(5):531–538. https://doi.org/10.1007/s00392-020-01626-9
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y et al (2020b) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. https://doi.org/10.1056/NEJMoa2001316
Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y et al (2020c) Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 146(1):110–118. https://doi.org/10.1016/j.jaci.2020.04.006
Li M, Wang H, Tian L, Pang Z, Yang Q, Huang T et al (2022) COVID-19 vaccine development: milestones, lessons and prospects. Signal Transduct Target Ther 7(1):1–32
Lim S, Bae JH, Kwon H-S, Nauck MA (2020) COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. https://doi.org/10.1038/s41574-020-00435-4
Lin C-S, Pan C-H (2008) Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell Mol Life Sci 65(10):1489–1508. https://doi.org/10.1007/s00018-008-7408-8
Lippi G, Lavie CJ, Sanchis-Gomar F (2020) Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 63(3):390–391. https://doi.org/10.1016/j.pcad.2020.03.001
Liu Q, Y-h Z, Yang Z-q (2016) The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol 13(1):3–10. https://doi.org/10.1038/cmi.2015.74
Liu H, Ye F, Sun Q, Liang H, Li C, Li S et al (2021) Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J Enzym Inhib Med Chem 36(1):497–503. https://doi.org/10.1080/14756366.2021.1873977
Lu C-L, Wang Y, Yuan L, Li Y, Li X-Y (2014) The angiotensin-converting enzyme 2/angiotensin (1–7)/Mas axis protects the function of pancreatic β cells by improving the function of islet microvascular endothelial cells. Int J Mol Med 34(5):1293–1300. https://doi.org/10.3892/ijmm.2014.1917
Luo Y, Liu C, Guan T, Li Y, Lai Y, Li F et al (2019) Association of ACE2 genetic polymorphisms with hypertension-related target organ damages in south Xinjiang. Hypertens Res 42(5):681–689. https://doi.org/10.1038/s41440-018-0166-6
Magalhaes G, Rodrigues-Machado M, Motta-Santos D, Silva A, Caliari M, Prata L et al (2015) A ngiotensin-(1–7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br J Pharmacol 172(9):2330–2342. https://doi.org/10.1111/bph.13057
Malard L, Kakinami L, O’Loughlin J, Roy-Gagnon M-H, Labbe A, Pilote L et al (2013) The association between the angiotensin-converting enzyme-2 gene and blood pressure in a cohort study of adolescents. BMC Med Genet 14(1):1–7. https://doi.org/10.1186/1471-2350-14-117
Mason RJ (2020) Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 55:2000607. https://doi.org/10.1183/13993003.00607-2020
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Menard J, Bouhnik J, Clauser E, Richoux J, Corvol P (1983) Biochemistry and regulation of angiotensinogen. Clin Exp Hypertens 5(7–8):1005–1019. https://doi.org/10.3109/10641968309048838
Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, Azocar A, Castro PF, Jalil JE et al (2015) ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis 9(4):217–237. https://doi.org/10.1177/1753944715597623
Meng Y, Yu C-H, Li W, Li T, Luo W, Huang S et al (2014) Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am J Respir Cell Mol Biol 50(4):723–736. https://doi.org/10.1165/rcmb.2012-0451OC
Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N et al (2020) Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 77(2):198–209. https://doi.org/10.1111/his.14134
Michiels C (2003) Endothelial cell functions. J Cell Physiol 196(3):430–443. https://doi.org/10.1002/jcp.10333
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M et al (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181(4):905-913.e907. https://doi.org/10.1016/j.cell.2020.04.004
Moreno-Eutimio MA, López-Macías C, Pastelin-Palacios R (2020) Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect 22(4–5):226–229. https://doi.org/10.1016/j.micinf.2020.04.009
Müller DN, Mervaala EM, Dechend R, Fiebeler A, Park J-K, Schmidt F et al (2000) Angiotensin II (AT1) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy. Am J Pathol 157(1):111–122. https://doi.org/10.1016/S0002-9440(10)64523-3
Muniyappa R, Gubbi S (2020) COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 318(5):E736–E741. https://doi.org/10.1152/ajpendo.00124.2020
Myers LC, Parodi SM, Escobar GJ, Liu VX (2020) Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 323(21):2195–2198. https://doi.org/10.1001/jama.2020.7202
Neumaier F, Zlatopolskiy BD, Neumaier B (2020) Nuclear medicine in times of COVID-19: how radiopharmaceuticals could help to fight the current and future pandemics. Pharmaceutics 12(12):1247. https://doi.org/10.3390/pharmaceutics12121247
Neves LA, Stovall K, Joyner J, Valdés G, Gallagher PE, Ferrario CM et al (2008) ACE2 and ANG-(1–7) in the rat uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol 294(1):R151–R161. https://doi.org/10.1152/ajpregu.00514.2007
Niu W, Qi Y, Hou S, Zhou W, Qiu C (2007) Correlation of angiotensin-converting enzyme 2 gene polymorphisms with stage 2 hypertension in Han Chinese. Transl Res 150(6):374–380. https://doi.org/10.1016/j.trsl.2007.06.002
Patnaik M, Pati P, Swain SN, Mohapatra MK, Dwibedi B, Kar SK et al (2014) Association of angiotensin-converting enzyme and angiotensin-converting enzyme-2 gene polymorphisms with essential hypertension in the population of Odisha. India Ann Hum Biol 41(2):145–152. https://doi.org/10.3109/03014460.2013.837195
Peiris J, Lai S, Poon L, Guan Y, Yam L, Lim W et al (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361(9366):1319–1325. https://doi.org/10.1016/S0140-6736(03)13077-2
Peiró C, Moncada S (2020) Substituting angiotensin-(1–7) to prevent lung damage in SARS-CoV-2 infection? Circulation 141(21):1665–1666. https://doi.org/10.1161/CIRCULATIONAHA.120.047297
Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Investig 111(12):1805–1812. https://doi.org/10.1172/JCI18921
Perlman S, Netland J (2009) Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 7(6):439–450. https://doi.org/10.1038/nrmicro2147
Petrelli F, Cherri S, Ghidini M, Perego G, Ghidini A, Zaniboni A (2021) Tocilizumab as treatment for COVID-19: a systematic review and meta-analysis. World J Methodol 11(3):95
Peymani P, Dehesh T, Aligolighasemabadi F, Sadeghdoust M, Kotfis K, Ahmadi M et al (2021) Statins in patients with COVID-19: a retrospective cohort study in Iranian COVID-19 patients. Transl Med Commun 6(1):1–14. https://doi.org/10.1186/s41231-021-00082-5
Pons S, Fodil S, Azoulay E, Zafrani L (2020) The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 24(1):1–8. https://doi.org/10.1186/s13054-020-03062-7
Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L et al (2020) Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383(6):590–592. https://doi.org/10.1056/NEJMc2011400
Qaradakhi T, Gadanec LK, McSweeney KR, Tacey A, Apostolopoulos V, Levinger I et al (2020) The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin Exp Pharmacol Physiol 47(5):751–758. https://doi.org/10.1111/1440-1681.13251
Ragia G, Manolopoulos VG (2020) Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-020-02963-4
Rayman G, Lumb A, Kennon B, Cottrell C, Nagi D, Page E et al (2020) Guidelines for the management of diabetes services and patients during the COVID-19 pandemic. Diabet Med 37(7):1087–1089. https://doi.org/10.1111/dme.14316
Remuzzi A, Remuzzi G (2020) COVID-19 and Italy: what next? Lancet 395(10231):1225–1228. https://doi.org/10.1016/S0140-6736(20)30627-9
Ren J, Nie Y, Lv M, Shen S, Tang R, Xu Y et al (2015) Estrogen upregulates MICA/B expression in human non-small cell lung cancer through the regulation of ADAM17. Cell Mol Immunol 12(6):768–776. https://doi.org/10.1038/cmi.2014.101
Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM (2004) Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383(1):45–51. https://doi.org/10.1042/BJ20040634
Richardson MA, Gupta A, O’Brien LA, Berg DT, Gerlitz B, Syed S et al (2008) Treatment of sepsis-induced acquired protein C deficiency reverses angiotensin-converting enzyme-2 inhibition and decreases pulmonary inflammatory response. J Pharmacol Exp Ther 325(1):17–26. https://doi.org/10.1124/jpet.107.130609
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW et al (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323(20):2052–2059. https://doi.org/10.1001/jama.2020.6775
Roca-Ho H, Riera M, Palau V, Pascual J, Soler MJ (2017) Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci 18(3):563. https://doi.org/10.3390/ijms18030563
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP et al (2020) Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 34:101623. https://doi.org/10.1016/j.tmaid.2020.101623
Rossi R, Talarico M, Coppi F, Boriani G (2020) Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med 15(8):1573–1576. https://doi.org/10.1007/s11739-020-02504-y
Roufogalis BD (1985) Calmodulin antagonism. In: Marmé D (ed) Calcium and cell physiology. Springer, Berlin, Heidelberg, pp 148–169. https://doi.org/10.1007/978-3-642-70070-5_8
Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S et al (2020) Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 156:104761. https://doi.org/10.1016/j.phrs.2020.104761
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323(18):1824–1836. https://doi.org/10.1001/jama.2020.6019
Santos RA, e Silva ACS, Maric C, Silva DM, Machado RP, de Buhr I et al (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci 100(14):8258–8263. https://doi.org/10.1073/pnas.1432869100
Schmitt FCF, Manolov V, Morgenstern J, Fleming T, Heitmeier S, Uhle F et al (2019) Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care 9(1):1–15. https://doi.org/10.1186/s13613-019-0499-6
Shahin Y, Khan JA, Samuel N, Chetter I (2011) Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis 216(1):7–16. https://doi.org/10.1016/j.atherosclerosis.2011.02.044
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H et al (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581(7807):221–224. https://doi.org/10.1038/s41586-020-2179-y
Shi R, Shan C, Duan X, Chen Z, Liu P, Song J et al (2020a) A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 584(7819):120–124. https://doi.org/10.1038/s41586-020-2381-y
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F et al (2020b) Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China JAMA Cardiol 5(7):802–810. https://doi.org/10.1001/jamacardio.2020.0950
Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J (2020c) Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 24(1):1–4. https://doi.org/10.1186/s13054-020-2833-7
Shibata S, Arima H, Asayama K, Hoshide S, Ichihara A, Ishimitsu T et al (2020) Hypertension and related diseases in the era of COVID-19: a report from the Japanese society of hypertension task force on COVID-19. Hypertens Res 43(10):1028–1046. https://doi.org/10.1038/s41440-020-0515-0
Shoemaker R, Yiannikouris F, Thatcher S, Cassis L (2015) ACE2 deficiency reduces β-cell mass and impairs β-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab 309(7):E621–E631. https://doi.org/10.1152/ajpendo.00054.2015
Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A et al (2020) High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 28(7):1195–1199. https://doi.org/10.1002/oby.22831
Smadja DM, Guerin CL, Chocron R, Yatim N, Boussier J, Gendron N et al (2020) Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. https://doi.org/10.1007/s10456-020-09730-0
Sodhi CP, Nguyen J, Yamaguchi Y, Werts AD, Lu P, Ladd MR et al (2019) A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to Pseudomonas aeruginosa lung infection in mice. J Immunol 203(11):3000–3012. https://doi.org/10.4049/jimmunol.1900579
Soler MJ, Wysocki J, Batlle D (2013) ACE2 alterations in kidney disease. Nephrol Dial Transplant 28(11):2687–2697. https://doi.org/10.1093/ndt/gft320
Solinski HJ, Gudermann T, Breit A (2014) Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol Rev 66(3):570–597. https://doi.org/10.1124/pr.113.008425
Soto-Vega E, Meza I, Ramírez-Rodríguez G, Benitez-King G (2004) Melatonin stimulates calmodulin phosphorylation by protein kinase C. J Pineal Res 37(2):98–106. https://doi.org/10.1111/j.1600-079X.2004.00141.x
South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318:H1084–H1090. https://doi.org/10.1152/ajpheart.00217.2020
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106(6):675–683. https://doi.org/10.1016/S0092-8674(01)00495-0
Streetley J, Fonseca A-V, Turner J, Kiskin NI, Knipe L, Rosenthal PB et al (2019) Stimulated release of intraluminal vesicles from Weibel-Palade bodies. Blood Am Soc Hematol 133(25):2707–2717. https://doi.org/10.1182/blood-2018-09-874552
Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y et al (2020) Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98(1):219–227. https://doi.org/10.1016/j.kint.2020.04.003
Sun P, Lu X, Xu C, Sun W, Pan B (2020) Understanding of COVID-19 based on current evidence. J Med Virol 92(6):548–551. https://doi.org/10.1002/jmv.25722
Szmitko PE, Wang C-H, Weisel RD, Jeffries GA, Anderson TJ, Verma S (2003) Biomarkers of vascular disease linking inflammation to endothelial activation: part II. Circulation 108(17):2041–2048. https://doi.org/10.1161/01.CIR.0000089093.75585.98
Takeuchi O, Akira S (2009) Innate immunity to virus infection. Immunol Rev 227(1):75–86. https://doi.org/10.1111/j.1600-065X.2008.00737.x
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(4):844–847. https://doi.org/10.1111/jth.14768
Taylor R et al (2016) BCX4430–a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health 9(3):220–226
Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AJ (2015) Hypertension: renin–angiotensin–aldosterone system alterations. Circ Res 116(6):960–975. https://doi.org/10.1161/CIRCRESAHA.116.303587
Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M et al (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18(5):1023–1026. https://doi.org/10.1111/jth.14810
Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ (2000) A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275(43):33238–33243. https://doi.org/10.1074/jbc.M002615200
Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T et al (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279(17):17996–18007. https://doi.org/10.1074/jbc.M311191200
Uchimido R, Schmidt EP, Shapiro NI (2019) The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care 23(1):1–12. https://doi.org/10.1186/s13054-018-2292-6
Ulhaq ZS, Soraya GV (2020) Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect 50(4):382–383. https://doi.org/10.1016/j.medmal.2020.04.002
Valdez-Cruz NA, García-Hernández E, Espitia C, Cobos-Marín L, Altamirano C, Bando-Campos CG, Cofas-Vargas LF et al (2021) Integrative overview of antibodies against SARS-CoV-2 and their possible applications in COVID-19 prophylaxis and treatment. Microb Cell Factories 20(1):1–32. https://doi.org/10.1186/s12934-021-01576-5
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5
Velkoska E, Patel SK, Burrell LM (2016) Angiotensin converting enzyme 2 and diminazene: role in cardiovascular and blood pressure regulation. Curr Opin Nephrol Hypertens 25(5):384–395. https://doi.org/10.1097/MNH.0000000000000254
Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N et al (2020) Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 20(6):669–677. https://doi.org/10.1016/S1473-3099(20)30243-7
Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J et al (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277(17):14838–14843. https://doi.org/10.1074/jbc.M200581200
Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE (2001) Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 9(7):414–417. https://doi.org/10.1038/oby.2001.54
Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J et al (2015) Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 47(4):693–705. https://doi.org/10.1007/s00726-014-1889-6
Wagenaar GT, Laghmani EH, Fidder M, Sengers RM, De Visser YP, De Vries L et al (2013) Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol 305(5):L341–L351. https://doi.org/10.1152/ajplung.00360.2012
Wang H, Bender A, Wang P, Karakose E, Inabnet WB, Libutti SK et al (2017) Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas. Nat Commun 8(1):1–15. https://doi.org/10.1038/s41467-017-00992-9
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020a) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585
Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z et al (2020c) Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181(4):894-904.e899. https://doi.org/10.1016/j.cell.2020.03.045
Wang K, Chen W, Zhang Z, Deng Y, Lian J-Q, Du P et al (2020d) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5:283. https://doi.org/10.1038/s41392-020-00426-x
Wang W, Tang J, Wei F (2020e) Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol 92(4):441–447. https://doi.org/10.1002/jmv.25689
Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P et al (2020b) SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv https://doi.org/10.1101/2020.03.14.988345
Weglarz-Tomczak E, Tomczak JM, Talma M, Brul S (2020) Ebselen as a highly active inhibitor of PLProCoV2. BioRxiv. https://doi.org/10.1101/2020.05.17.100768
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y et al (2022) Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and paxlovid) for COVID-19: a meta-analysis. Ann Med 54(1):516–523
Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 173(4):268–277. https://doi.org/10.7326/M20-2003
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323(13):1239–1242. https://doi.org/10.1001/jama.2020.2648
Wu KK, Frasier-Scott K, Hatzakis H (1988) Endothelial cell function in hemostasis and thrombosis. Adv Exp Med Biol 242:127–133
Xia H, Sriramula S, Chhabra KH, Lazartigues E (2013) Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res 113(9):1087–1096. https://doi.org/10.1161/CIRCRESAHA.113.301811
Xuan X, Gao F, Ma X, Huang C, Wang Y, Deng H et al (2018) Activation of ACE2/angiotensin (1–7) attenuates pancreatic β cell dedifferentiation in a high-fat-diet mouse model. Metabolism 81:83–96. https://doi.org/10.1016/j.metabol.2017.12.003
Yang J-K, Lin S-S, Ji X-J, Guo L-M (2010) Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47(3):193–199. https://doi.org/10.1007/s00592-009-0109-4
Yang D, Xiao Y, Chen J, Chen Y, Luo P, Liu Q et al (2020a) COVID-19 and chronic renal disease: clinical characteristics and prognosis. QJM Int J Med 113(11):799–805. https://doi.org/10.1093/qjmed/hcaa258
Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y et al (2020b) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8(5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P et al (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71(15):732–739. https://doi.org/10.1093/cid/ciaa237
Young D, Waitches G, Birchmeier C, Fasano O, Wigler M (1986) Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45(5):711–719. https://doi.org/10.1016/0092-8674(86)90785-3
Zachariah U, Nair S, Goel A, Balasubramanian K, Mackie I, Elias E et al (2020) Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: consider low volume plasma exchange and low dose steroid. Thromb Res 192:2. https://doi.org/10.1016/j.thromres.2020.05.001
Zhang H, Wada J, Hida K, Tsuchiyama Y, Hiragushi K, Shikata K et al (2001) Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem 276(20):17132–17139. https://doi.org/10.1074/jbc.M006723200
Zhang J, DeFelice AF, Hanig JP, Colatsky T (2010) Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol Pathol 38(6):856–871. https://doi.org/10.1177/0192623310378866
Zhang J, Dong J, Martin M, He M, Gongol B, Marin TL et al (2018) AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit 198(4):509–520. https://doi.org/10.1164/rccm.201712-2570OC
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020a) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590. https://doi.org/10.1007/s00134-020-05985-9
Zhang J, Tecson KM, McCullough PA (2020b) Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med 21(3):315–319. https://doi.org/10.31083/j.rcm.2020.03.126
Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L et al (2020c) Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368(6489):409–412. https://doi.org/10.1126/science.abb3405
Zhang R, Wang X, Ni L, Di X, Ma B, Niu S et al (2020d) COVID-19: Melatonin as a potential adjuvant treatment. Life Sci 250:117583. https://doi.org/10.1016/j.lfs.2020.117583
Zhang X-J, Qin J-J, Cheng X, Shen L, Zhao Y-C, Yuan Y et al (2020e) In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab 32(2):176-187.e174. https://doi.org/10.1016/j.cmet.2020.06.015
Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W (2020) Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit 202(5):756–759. https://doi.org/10.1164/rccm.202001-0179LE
Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X (2020) COVID-19 and the cardiovascular system. Nat Rev Cardiol 17(5):259–260. https://doi.org/10.1038/s41569-020-0360-5
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Zhu J, Deng Y-Q, Wang X, Li X-F, Zhang N-N, Liu Z t al (2020a) An artificial intelligence system reveals liquiritin inhibits SARS-CoV-2 by mimicking type I interferon. https://doi.org/10.1101/2020.05.02.074021
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al (2020b) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. https://doi.org/10.1056/NEJMoa2001017
Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR et al (2003) Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation 108(14):1707–1712. https://doi.org/10.1161/01.CIR.0000094734.67990.99
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
The original Online version of this article was revised: The Journal title has been incorrectly published.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Assareh, E., Abbasi, M.A., Heidari, M. et al. A Review on COVID-19: Primary Receptor, Endothelial Dysfunction, Related Comorbidities, and Therapeutics. Iran J Sci 47, 1–25 (2023). https://doi.org/10.1007/s40995-022-01400-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-022-01400-8