Abstract
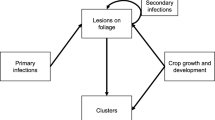
A model describing an early blight epidemic and the growth dynamics of tomato interacting together is developed. The model is formulated as a set of differential equations for the rate of change in the amount of healthy H, diseased Y and defoliated D area in the disease situation, and healthy H DF and defoliated D DF in the disease-free situation. Model parameters were estimated through fitting the model to experimental data obtained from glasshouse experiments which comprised tomato plants inoculated with A. solani at three inoculation times; early (t INOC = 23 days after transplanting (DAT)), intermediate (33 DAT) and late (43 DAT) in experiment 1 and t INOC = 22, 30 and 38 DAT in experiment 2. Defoliation rates were 2.5 times higher for late inoculated (older) plants compared with the early inoculated (younger) plants. Values of the logistic rate parameter for disease increase were about three-fold higher in the late inoculations (0.380, 0.305 day−1) when compared with the early inoculations (0.151, 0.095 day−1). Based on the good fit of the model outputs to observed data with R 2 > 0.995, the model can be considered as offering a good description of the dynamic interaction between the early blight epidemic and growth of tomato.


Similar content being viewed by others
References
Allorent D, Savary S (2005) Epidemiological characteristics of angular leaf spot of bean: a systems analysis. Eur J Plant Pathol 113:329–341
Allorent D, Willocquet L, Sartorato A, Savary S (2005) Quantifying and modelling the mobilisation of inoculum from diseased leaves and infected defoliated tissues in epidemics of angular leaf spot of bean. Eur J Plant Pathol 113:377–394
Aust HJ, Hoyningen-Huene JV (1986) Microclimate in relation to epidemics of powdery mildew. Annu Rev Phytopathol 24:491–510
Barnwal MK, Kotasthane A, Magculia N, Mukherjee PK, Savary S, Sharma AK, Singh HB, Singh US, Sparks AH, Variar M, Zaidi N (2013) A review on crop losses, epidemiology and disease management of rice brown spot to identify research priorities and knowledge gaps. Eur J Plant Pathol 136:443–457
Basu PK (1974) Measuring early blight, its progress and influence on fruit losses in nine tomato cultivars. Can Plant Dis Surv 54:45–51
Bennett FG (1981) The expression of resistance to powdery mildew infection in winter wheat cultivars. II. Adult plant resistance. Ann Appl Biol 98:305–317
Blanco FF, Folegatti MV (2005) Estimation of leaf area for greenhouse cucumber by linear measurements under salinity and grafting. Sci Agric 62:305–309
Calonnec A, Cartolaro P, Naulin JM, Bailey D, Langlais M (2008) A host–pathogen simulation model: powdery mildew of grapevine. Plant Pathol 57:493–508
Chaerani R, Voorrips RE (2006) Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J Gen Plant Pathol 72:335–347
Chaerani R, Groenwold R, Stam P, Voorrips RE (2007a) Assessment of early blight (Alternaria solani) resistance in tomato using a drop inoculation method. J Gen Plant Pathol 73:96–103
Chaerani R, Smulders MJM, van der Linden CG, Vosman B, Stam P, Voorrips RE (2007b) QTL identification for early blight resistance (Alternaria solani) in a Solanum lycopersicum × S. Arcanum cross. Theor Appl Genet 114:439–450
Cook RJ, Yarham DJ (1998) Epidemiology in sustainable systems. In: Jones DG (ed) The epidemiology of plant diseases. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 260–277
Datar VV, Mayee CD (1981) Assessment of losses in tomato yield due to early blight. Indian Phytopathol 34:191–195
De Wolf ED, Isard SA (2007) Disease cycle approach to plant disease prediction. Annu Rev Phytopathol 45:203–220
Denissen CJM (1993) Components of adult plant resistance to leaf rust in wheat. Euphytica 70:131–140
Gleason ML, Ricker MD, MacNab AA, East DA, Pitblado RE, Latin RX (1995) Disease-warning systems for processing tomatoes in eastern North America: Are we there yet? Plant Dis 79:113–121
Godoy CV, Amorim L, Bergamin Filho A, Silva HP, Silva WJ, Berger RD (2003) Temporal progress of southern rust in maize under different environmental conditions. Fitopatol Bras 28:273–278
Jasinski J (1999) TOMCAST for Ohio, Indiana and Michigan. Available at: www.2.ag.ohiostate.edu/~vegnet/tomcats/tomfrm.htm. Accessed on April 10 2013
Jeger MJ (1986) The potential of analytic compared with simulation approaches to modeling in plant disease epidemiology. In: Leonard KJ, Fry WE (eds) Plant disease epidemiology. Population dynamics and management, vol 1. MacMillan Publishing Co, New York, USA, pp 255–281
Johnson KB, Teng PS (1990) Coupling a disease progress model for early blight to a model of potato growth. Phytopathology 80:416–425
Jones JP (1991) Early blight. In: Jones JB, Jones JP, Stall RE, Zitter TA (eds) Compendium of tomato diseases. APS Press, St. Paul MN, USA, pp 13–14
Koo J (2002) Modeling the impacts of climate variability on tomato disease management and production. Available at: www.etd.fcla.edu/UF/UFE1000136/koo_j.pdf. Accessed on July 15 2013
Kranz J, Jörg E (1989) The synecological approach in plant disease epidemiology. Rev Trop Plant Pathol 6:27–38
Lawrence CB, Singh NP, Qiu J, Gardener RG, Tuzun S (2000) Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as elicitor release mechanism. Physiol Mol Plant Pathol 57:211–220
Ma H, Singh RP (1996) Expression of adult resistance to stripe rust at different growth stages of wheat. Plant Dis 80:375–379
Madden L, Pennypacker SP, MacNab AA (1978) FAST, a forecast system for Alternaria solani on tomato. Phytopathology 68:1354–1358
Majid RF, Heather LM, Hamid A (2008) Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit Rev Plant Sci 27:75–107
Melching JS, Dowler WM, Koogle DL, Royer MH (1988) Effect of plant and leaf age on susceptibility of soybean to soybean rust. Can J Plant Pathol 10:30–35
Mersha Z, Hau B (2008) Effects of bean rust (Uromyces appendiculatus) epidemics on host dynamics of common bean (Phaseolus vulgaris). Plant Pathol 57:674–686
Mersha Z, Hau B (2011) Reciprocal effects of host and disease dynamics in the bean rust pathosystems. J Plant Dis Protect 118:54–62
Nash AF, Gardner RG (1988) Tomato early blight resistance in a breeding line derived from Lycopersicon hirsutum PI 126445. Plant Dis 72:206–209
Nelson SC, Campbell CL (1993) Disease progress, defoliation and spatial pattern in a multiple-pathogen disease complex on white clover. Phytopathology 83:419–429
Ojiambo PS, Scherm H (2005) Temporal progress of Septoria leaf spot on rabbiteye blueberry (Vaccinium ashei). Plant Dis 89:1090–1096
Pandey KK, Pandey PK, Kallo G, Banerjee MK (2003) Resistance to early blight of tomato with respect to various parameters of disease epidemics. J Gen Plant Pathol 69:364–371
Pretorius ZA, Rijkenberg FHJ, Wilcoxson RD (1988) Effects of growth stage, leaf position and temperature on adult plant resistance of wheat infected by Puccinia recondita f. sp. tritici. Plant Pathol 37:36–44
Rossi V, Racca P, Giosuè S, Pancaldi D, Alberti I (1997) A simulation model for the development of brown rust epidemics in winter wheat. Eur J Plant Pathol 103:453–465
Rotem J (1994) The genus Alternaria: biology, epidemiology, and pathogenicity. APS Press, St. Paul MN, USA
Rotem J, Reichert I (1964) Dew–a principal moisture factor enabling early blight epidemics in a semi arid region of Israel. Plant Dis Rep 48:211–215
Savary S, Willocquet L, Teng PS (1997) Modelling sheath blight epidemics on rice tillers. Agric Syst 55:359–384
Schwarz D, Klaring HP (2001) Allometry to estimate leaf area tomato. J Plant Nutr 24:1291–1309
Sherf AF, MacNab AA (1986) Vegetable diseases and their control. Wiley Interscience, New York NY, USA
Sinden SL, Goth RW, O’brien MJ (1972) Effect of potato alkaloids on the growth of Alternaria solani and their possible role as resistance factors in potatoes. Phytopathology 63:303–307
Thal WM, Campbell CL (1988) Analysis of progress of alfalfa leaf spot epidemics. Phytopathology 78:389–395
Van Maanen A, Xu M (2003) Modelling plant disease epidemics. Eur J Plant Pathol 109:669–682
Vloutoglou I, Kalogerakis SN (2000) Effects of inoculum concentration, wetness duration and plant age on development of early blight (Alternaria solani) and on shedding of leaves in tomato plants. Plant Pathol 49:339–345
Waggoner PE (1986) Progress curves of foliar diseases: their interpretation and use. In: Leonard KJ, Fry WE (eds) Plant disease epidemiology. Population dynamics and management, vol 1. MacMillan Publishing Co, New York NY, USA, pp 3–37
Waggoner PE, Berger RD (1987) Defoliation, disease, and growth. Phytopathology 77:393–398
Waggoner PE, Horsfall JG (1969) EPIDEM: a simulator of plant disease written for a computer. Conn Agric Exp Station Bull 698:1–80
Walker JC (1957) Plant pathology. McGraw-Hill Book Co, New York NY, USA
Willocquet L, Allorent D, Savary S (2004) Quantitative analysis of two important epidemiological features in the common bean – Phaeoisariopsis griseola pathosystem. Fitopatol Bras 29:676–679
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Harald Scherm
Rights and permissions
About this article
Cite this article
Chelal, J., Al Masri, A. & Hau, B. Modelling the interaction between early blight epidemics and host dynamics of tomato. Trop. plant pathol. 40, 77–87 (2015). https://doi.org/10.1007/s40858-015-0021-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-015-0021-0