Abstract
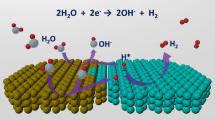
Searching for catalyst materials with high intrinsic activity for water oxidation holds the key to numerous clean energy technologies. Hydroxide semiconductors are electrochemically active to drive oxygen evolution reaction (OER), but suffer from poor electronic conductivity, restricting their intrinsic electrocatalytic activity. Here, a semimetallic hydroxide material was designed as efficient OER catalyst with both improved electronic conductivity and intrinsic electrocatalytic activity. By cationic doping and anionic vacancy manipulation, the NiFe layered double hydroxide (LDH) semiconductor was turned into semi-metallic with two orders of magnitude lower resistivity. Consequently, the semi-metallic LDH (SM LDH) array electrode exhibited an intrinsically improved OER activity with a low overpotential of 195 mV at 10 mA cm−2 and a low Tafel slope of 40.9 mV dec−1 in alkaline medium, outperforming commercial RuO2 catalysts (316 mV, 99.6 mV dec−1) under the same test condition. In-depth Raman and first-principles calculations demonstrated that the enhanced OER intrinsic activity of SM LDH was associated with the high electronic conductivity, which promoted the formation and stabilization of high-valence metal sites in oxyhydroxide intermediates. These finding suggest semi-metallic hydroxides as an advanced electrode material with both fascinating electric and catalytic properties.

摘要
寻找具有高本征活性的水氧化催化剂材料对许多清洁能源技术 的发展至关重要. 氢氧化物半导体对析氧反应具有一定的电催化活性. 然而, 该材料导电性较差, 限制着其电催化本征活性的提升. 本文提出 一种兼具高导电性和高催化活性的半金属氢氧化物析氧电催化材料. 通过阳离子掺杂和阴离子空位协同作用, 镍铁水滑石半导体可转化为 半金属材料, 其电阻率降低了两个数量级. 相应半金属氢氧化物阵列电 极的电催化活性显著提升, 在10 mA cm−2电流密度下其析氧过电势仅 为195 mV, Tafel斜率仅为40.9 mV dec−1, 显著优于商用RuO2催化剂 (316 mV, 99.6 mV dec−1). 原位拉曼光谱和理论计算结果表明, 半金属 氢氧化物可在较低过电位下转化为羟基氧化物中间体, 有助于高价态 金属活性位点的形成与稳定, 从而提升材料的析氧本征活性. 本研究表 明, 兼具优异导电性和催化活性的半金属氢氧化物可作为先进的电极 材料.
Similar content being viewed by others
References
Mefford JT, Akbashev AR, Kang M, et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature, 2021, 593: 67–73
Kang J, Qiu X, Hu Q, et al. Valence oscillation and dynamic active sites in monolayer NiCo hydroxides for water oxidation. Nat Catal, 2021, 4: 1050–1058
Shi H, Wang T, Liu J, et al. A sodium-ion-conducted asymmetric electrolyzer to lower the operation voltage for direct seawater electrolysis. Nat Commun, 2023, 14: 3934
Zhang Y, Cai Z, Zhao Y, et al. Superaerophilic copper nanowires for efficient and switchable CO2 electroreduction. Nanoscale Horiz, 2019, 4: 490–494
Xiong P, Tan J, Lee H, et al. Two-dimensional carbon-based heterostructures as bifunctional electrocatalysts for water splitting and metal-air batteries. Nano Mater Sci, 2022, doi: https://doi.org/10.1016/j.nanoms.2022.10.001
Chen M, Kitiphatpiboon N, Feng C, et al. Recent progress in transition-metal-oxide-based electrocatalysts for the oxygen evolution reaction in natural seawater splitting: A critical review. eScience, 2023, 3: 100111
Zhou Y, Li C, Zhang Y, et al. Controllable thermochemical generation of active defects in the horizontal/vertical MoS2 for enhanced hydrogen evolution. Adv Funct Mater, 2023, 33: 2304302
Li G, Duan X, Liu X, et al. Locking active Li metal through localized redistribution of fluoride enabling stable Li-metal batteries. Adv Mater, 2023, 35: 2207310
Zhang N, Hu Y, An L, et al. Surface activation and Ni-S stabilization in NiO/NiS2 for efficient oxygen evolution reaction. Angew Chem Int Ed, 2022, 61: e202207217
Chen YF, Li JH, Liu TT, et al. Constructing robust NiFe LDHs–NiFe alloy gradient hybrid bifunctional catalyst for overall water splitting: one-step electrodeposition and surface reconstruction. Rare Met, 2023, 42: 2272–2283
Xie R, Luo W, Zou L, et al. Low-temperature synthesis of colloidal few-layer WTe2 nanostructures for electrochemical hydrogen evolution. Discover Nano, 2023, 18: 44
Jia B, Zhang B, Cai Z, et al. Construction of amorphous/crystalline heterointerfaces for enhanced electrochemical processes. eScience, 2023, 3: 100112
Cai Z, Bi Y, Hu E, et al. Single-crystalline ultrathin Co3O4 nanosheets with massive vacancy defects for enhanced electrocatalysis. Adv Energy Mater, 2017, 8: 1701694
Lee YJ, Park SK. Synergistically coupling of Ni–Fe LDH arrays with hollow Co–Mo sulfide nanotriangles for highly efficient overall water splitting. Rare Met, 2024, 43: 522–532
Zhu H, Sun S, Hao J, et al. A high-entropy atomic environment converts inactive to active sites for electrocatalysis. Energy Environ Sci, 2023, 16: 619–628
Xiao Z, Zhou W, Yang B, et al. Tuned d-band states over lanthanum doped nickel oxide for efficient oxygen evolution reaction. Nano Mater Sci, 2023, 5: 228–236
Xu X, Wang S, Guo S, et al. Cobalt phosphosulfide nanoparticles encapsulated into heteroatom-doped carbon as bifunctional electrocatalyst for Zn-air battery. Adv Powder Mater, 2022, 1: 100027
Lin R, Kang L, Zhao T, et al. Identification and manipulation of dynamic active site deficiency-induced competing reactions in electrocatalytic oxidation processes. Energy Environ Sci, 2022, 15: 2386–2396
Duan X, Getaye Sendeku M, Zhang D, et al. Tungsten-doped NiFe-layered double hydroxides as efficient oxygen evolution catalysts. Acta Physico Chim Sin, 2023, 0: 2303055
Han X, Li N, Baik JS, et al. Sulfur mismatch substitution in layered double hydroxides as efficient oxygen electrocatalysts for flexible zinc–air batteries. Adv Funct Mater, 2023, 33: 2212233
Deng R, Guo M, Wang C, et al. Recent advances in cobalt phosphide-based materials for electrocatalytic water splitting: From catalytic mechanism and synthesis method to optimization design. Nano Mater Sci, 2022, doi: https://doi.org/10.1016/j.nanoms.2022.04.003
Chen L, Wang H, Tan L, et al. PEO-PPO-PEO induced holey NiFe-LDH nanosheets on Ni foam for efficient overall water-splitting and urea electrolysis. J Colloid Interface Sci, 2022, 618: 141–148
Wang L, Zhang L, Ma W, et al. In situ anchoring massive isolated Pt atoms at cationic vacancies of α-NixFe1−x(OH)2 to regulate the electronic structure for overall water splitting. Adv Funct Mater, 2022, 32: 2203342
Hu R, Wei L, Xian J, et al. Microwave shock process for rapid synthesis of 2D porous La0.2Sr0.8CoO3 perovskite as an efficient oxygen evolution reaction catalyst. Acta Physico Chim Sin, 2023, 0: 2212025
Duan X, Li T, Jiang X, et al. Catalytic applications of single-atom metal-anchored hydroxides: Recent advances and perspective. Mater Rep-Energy, 2022, 2: 100146
Xie Q, Cai Z, Li P, et al. Layered double hydroxides with atomic-scale defects for superior electrocatalysis. Nano Res, 2018, 11: 4524–4534
Wang H, Chen L, Tan L, et al. Electrodeposition of NiFe-layered double hydroxide layer on sulfur-modified nickel molybdate nanorods for highly efficient seawater splitting. J Colloid Interface Sci, 2022, 613: 349–358
Wang Z, Wang C, Ye L, et al. MnOx film-coated NiFe-LDH nanosheets on Ni foam as selective oxygen evolution electrocatalysts for alkaline seawater oxidation. Inorg Chem, 2022, 61: 15256–15265
Bi Y, Cai Z, Zhou D, et al. Understanding the incorporating effect of Co2+/Co3+ in NiFe-layered double hydroxide for electrocatalytic oxygen evolution reaction. J Catal, 2018, 358: 100–107
Luo M, Cai Z, Wang C, et al. Phosphorus oxoanion-intercalated layered double hydroxides for high-performance oxygen evolution. Nano Res, 2017, 10: 1732–1739
Cai Z, Zhou D, Wang M, et al. Introducing Fe2+ into nickel-iron layered double hydroxide: local structure modulated water oxidation activity. Angew Chem Int Ed, 2018, 57: 9392–9396
Tan L, Yu J, Wang C, et al. Partial sulfidation strategy to NiFe-LDH@FeNi2S4 heterostructure enable high-performance water/sea-water oxidation. Adv Funct Mater, 2022, 32: 2200951
Huang C, Zhou Q, Duan D, et al. The rapid self-reconstruction of Fe-modified Ni hydroxysulfide for efficient and stable large-current-density water/seawater oxidation. Energy Environ Sci, 2022, 15: 4647–4658
Xiong X, Cai Z, Zhou D, et al. A highly-efficient oxygen evolution electrode based on defective nickel-iron layered double hydroxide. Sci China Mater, 2018, 61: 939–947
Bao Y, Lian C, Huang K, et al. Generating high-valent iron-oxo ≡FeIV=O complexes in neutral microenvironments through peroxymonosulfate activation by Zn–Fe layered double hydroxides. Angew Chem Int Ed, 2022, 61: e202209542
Yuan Z, Bak SM, Li P, et al. Activating layered double hydroxide with multivacancies by memory effect for energy-efficient hydrogen production at neutral pH. ACS Energy Lett, 2019, 4: 1412–1418
Xin K, Yang W, Xia J, et al. Research progress of ultra-wide bandgap two-dimensional semiconductor materials and devices. Sci Sin-Phys Mech Astron, 2022, 52: 297302
Kumar N, Shekhar C, Wu SC, et al. Observation of pseudo-two-dimensional electron transport in the rock salt-type topological semi-metal LaBi. Phys Rev B, 2016, 93: 241106
Zhao Y, Zhang X, Jia X, et al. Sub-3 nm ultrafine monolayer layered double hydroxide nanosheets for electrochemical water oxidation. Adv Energy Mater, 2018, 8: 1703585
Faid AY, Barnett AO, Seland F, et al. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim Acta, 2020, 361: 137040
Qiu Z, Tai CW, Niklasson GA, et al. Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting. Energy Environ Sci, 2019, 12: 572–581
Jing C, Yuan T, Li L, et al. Electrocatalyst with dynamic formation of the dual-active site from the dual pathway observed by in situ Raman spectroscopy. ACS Catal, 2022, 12: 10276–10284
Lan L, Li Q, Gu G, et al. Hydrothermal synthesis of γ-MnOOH nanorods and their conversion to MnO2, Mn2O3, and Mn3O4 nanorods. J Alloys Compd, 2015, 644: 430–437
Enkhtuvshin E, Yeo S, Choi H, et al. Surface reconstruction of Ni–Fe layered double hydroxide inducing chloride ion blocking materials for outstanding overall seawater splitting. Adv Funct Mater, 2023, 33: 2214069
Trześniewski BJ, Diaz-Morales O, Vermaas DA, et al. In situ observation of active oxygen species in Fe-containing Ni-based oxygen evolution catalysts: the effect of pH on electrochemical activity. J Am Chem Soc, 2015, 137: 15112–15121
Friebel D, Louie MW, Bajdich M, et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J Am Chem Soc, 2015, 137: 1305–1313
Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem, 2012, 33: 580–592
Zhou L, Zhang C, Zhang Y, et al. Host modification of layered double hydroxide electrocatalyst to boost the thermodynamic and kinetic activity of oxygen evolution reaction. Adv Funct Mater, 2021, 31: 2009743
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22205068 and 22109144), the “CUG Scholar” Scientific Research Funds at China University of Geosciences (Wuhan) (2022118), and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (162301202673). We appreciate eceshi (www.eceshi.com) and Shiyanjia Lab (www.shiyanjia.com) for the PPMS analyses. Thanks to the in-situ Raman electrochemical cell from Hefei In-situ Technology. Co., Ltd.
Author information
Authors and Affiliations
Contributions
Author contributions Wang J, Zhou C and Cai Z conceived the project. Wang J, Gao Q, Han B, Sun R and Zhao C performed the experiments. Wang J, Jamesh MI, Hsu HY and Cai Z wrote the manuscript. Cai Z and Zhou C supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Jing Wang received her Bachelor and Master’s degrees from China University of Geoscience (Wuhan) in 2019 and 2022, respectively. She is pursuing her doctoral degree under the supervision of Prof. Zhao Cai at China University of Geoscience (Wuhan). Her research interests mainly focus on transition metal-based nanomaterials for electrocatalysis.
Zhao Cai gained his BS degree and PhD in Chemistry from Beijing University of Chemical Technology in 2012 and 2018, respectively. After working as a visiting scholar at Yale University and postdoctoral researcher at Wuhan National Laboratory for Optoelectronics, he joined China University of Geosciences (Wuhan) as a professor of chemistry in 2022. His research focuses on developing novel transition metal nanostructures for key energy conversion and storage processes, such as electrocatalysis and aqueous batteries.
Supplementary Information
Rights and permissions
About this article
Cite this article
Wang, J., Jamesh, MI., Gao, Q. et al. Semimetallic hydroxide materials for electrochemical water oxidation. Sci. China Mater. 67, 1551–1558 (2024). https://doi.org/10.1007/s40843-023-2802-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2802-8