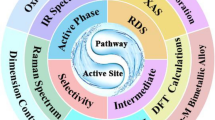
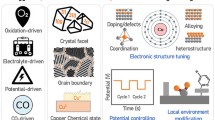
Abstract
Electrochemical CO2 reduction reaction (CO2RR) is a critical route to reduce the concentration of CO2 in the atmosphere and solve the energy crisis by converting CO2 into high-value chemicals and fuels. It is therefore crucial to rationally design efficient and cost-effective electrochemical catalysts. Copper (Cu) is found to be an excellent metal catalyst that can reduce CO2 to hydrocarbons and alcohols, especially C2+ products. However, there exist some problems (such as high overpotential and poor selectivity in CO2RR) that limit the application of Cu-based catalysts. In recent years, single-atom catalysts (SACs) have become an emerging research frontier in the field of heterogeneous catalysis due to their potential high activity, selectivity, and stability. Herein, the recent progress of various Cu-based SACs for CO2RR has been reviewed, especially on the regulatory strategies for the interaction of the active site with key reaction intermediates. This interaction is important for designing the active site to optimize the multi-electron reduction step and improve the catalytic performance. Meanwhile, different design strategies, including the regulation of metal centers, Cu-based single-atom alloy catalysts (SAAs), non-metal SACs, tandem catalysts, and composite catalysts, have also been discussed. Finally, the current research challenges and future developments of SACs in CO2RR have been summarized.

摘要
电化学二氧化碳还原反应(CO2RR)通过将CO2转化为高价值化学品和燃料来降低其在大气中的浓度, 是解决能源危机的重要途径. 因此, 合理设计高效、 经济的电化学催化剂至关重要. 研究发现, 铜(Cu)是一种性能优越的金属催化剂, 可将CO2还原成碳氢化合物和醇类, 尤其是C2+产物. 然而, Cu催化剂也存在一定问题(如过电位高和CO2RR选择性差等), 从而限制了其应用. 近年来, 单原子催化剂(SACs)因其具有高活性、高选择性和高稳定性, 已成为异相催化剂领域的新兴研究前沿. 本文总结了CO2RR的各种铜基SACs的最新研究进展, 尤其是活性位点与关键反应中间产物相互作用的调控策略. 这种相互作用对于设计活性位点以及优化多电子还原步骤和提高催化性能非常重要. 同时, 本文还讨论了金属中心调控、 铜基单原子合金催化剂、非金属SACs、 串联催化剂和复合催化剂等研究前沿. 最后, 总结了SACs应用于CO2RR的挑战与展望.
Similar content being viewed by others
References
Hepburn C, Adlen E, Beddington J, et al. The technological and economic prospects for CO2 utilization and removal. Nature, 2019, 575: 87–97
Zhu P, Wang H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat Catal, 2021, 4: 943–951
Garba MD, Usman M, Khan S, et al. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J Environ Chem Eng, 2021, 9: 104756
Ni Z, Liang H, Yi Z, et al. Research progress of electrochemical CO2 reduction for copper-based catalysts to multicarbon products. Coord Chem Rev, 2021, 441: 213983
Ye W, Guo X, Ma T. A review on electrochemical synthesized copper-based catalysts for electrochemical reduction of CO2 to C2+ products. Chem Eng J, 2021, 414: 128825–128841
De Luna P, Hahn C, Higgins D, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science, 2019, 364: eaav3506
Ross MB, De Luna P, Li Y, et al. Designing materials for electrochemical carbon dioxide recycling. Nat Catal, 2019, 2: 648–658
Wang X, Niu H, Liu Y, et al. Theoretical investigation on graphene-supported single-atom catalysts for electrochemical CO2 reduction. Catal Sci Technol, 2020, 10: 8465–8472
Zhang T, Verma S, Kim S, et al. Highly dispersed, single-site copper catalysts for the electroreduction of CO2 to methane. J Electroanal Chem, 2020, 875: 113862–113868
Zhou JH, Zhang YW. Metal-based heterogeneous electrocatalysts for reduction of carbon dioxide and nitrogen: Mechanisms, recent advances and perspective. React Chem Eng, 2018, 3: 591–625
Yue Z, Ou C, Ding N, et al. Advances in metal phthalocyanine based carbon composites for electrocatalytic CO2 reduction. ChemCatChem, 2020, 12: 6103–6130
Xie H, Wang T, Liang J, et al. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today, 2018, 21: 41–54
Zhang B, Zhang J. Rational design of Cu-based electrocatalysts for electrochemical reduction of carbon dioxide. J Energy Chem, 2017, 26: 1050–1066
Yang Q, Jiang Y, Zhuo H, et al. Recent progress of metal single-atom catalysts for energy applications. Nano Energy, 2023, 111: 108404
Rong P, Jiang YF, Wang Q, et al. Photocatalytic degradation of methylene blue (MB) with Cu1-ZnO single atom catalysts on graphenecoated flexible substrates. J Mater Chem A, 2022, 10: 6231–6241
Knezevic M, Hoang TH, Rashid N, et al. Recent development in metal halide perovskites synthesis to improve their charge-carrier mobility and photocatalytic efficiency. Sci China Mater, 2023, 66: 2545–2572
Yang D, Ni B, Wang X. Heterogeneous catalysts with well-defined active metal sites toward CO2 electrocatalytic reduction. Adv Energy Mater, 2020, 10: 2001142
Yuan JJ, Li HD, Wang QL, et al. Fabrication, characterization, and photocatalytic activity of double-layer TiO2 nanosheet films. Mater Lett, 2012, 81: 123–126
Yu Q, Lin R, Jiang L, et al. Fabrication and photocatalysis of ZnO nanotubes on transparent conductive graphene-based flexible substrates. Sci China Mater, 2018, 61: 1007–1011
Li LA, Cheng SH, Li HD, et al. Effect of nitrogen on deposition and field emission properties of boron-doped micro- and nano-crystalline diamond films. Nano-Micro Lett, 2010, 2: 154–159
Yu Q, Jiang L, Gao S, et al. The highly efficient photocatalysts of B-doped ZnO microspheres synthesized on PET-ITO flexible substrate. Ceramics Int, 2017, 43: 2864–2866
Yu Q, Ai T, Jiang L, et al. Efficient energy transfer in Eu-doped ZnO on diamond film. RSC Adv, 2014, 4: 53946–53949
Hülsey MJ, Baskaran S, Ding S, et al. Identifying key descriptors for the single-atom catalyzed CO oxidation. CCS Chem, 2022, 4: 3296–3308
Zhou Y, Shen X, Wang M, et al. The understanding, rational design, and application of high-entropy alloys as excellent electrocatalysts: A review. Sci China Mater, 2023, 66: 2527–2544
Meng Y, Kuang S, Liu H, et al. Recent advances in electrochemical CO2 reduction using copperbased catalysts. Acta Physico Chim Sin, 2020, 0: 2006034–0
Wang JJ, Li XP, Cui BF, et al. A review of non-noble metal-based electrocatalysts for CO2 electroreduction. Rare Met, 2021, 40: 3019–3037
Kim C, Bui JC, Luo X, et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat Energy, 2021, 6: 1026–1034
Liu X, Zhu L, Wang H, et al. Catalysis performance comparison for electrochemical reduction of CO2 on Pd-Cu/graphene catalyst. RSC Adv, 2016, 6: 38380–38387
Al-Saydeh SA, Zaidi SJ, El-Naas MH. Conversion of carbon dioxide: Opportunities and fundamental challenges. Am J Eng Appl Sci, 2018, 11: 138–153
Chen Y, Chen K, Fu J, et al. Recent advances in the utilization of copper sulfide compounds for electrochemical CO2 reduction. Nano Mater Sci, 2020, 2: 235–247
Yu Q, Jiang J, Jiang L, et al. Advances in green synthesis and applications of graphene. Nano Res, 2021, 14: 3724–3743
Yu Q, Li H, Wang Q, et al. Hydrothermal synthesis, characterization and properties of boron-doped ZnO sheets grown on p-diamond film. Mater Lett, 2014, 128: 284–286
Zhang W, Jiang M, Yang S, et al. In-situ grown CuOx nanowire forest on copper foam: A 3D hierarchical and freestanding electrocatalyst with enhanced carbonaceous product selectivity in CO2 reduction. Nano Res Energy, 2022, 1: e9120033
Li L, Hasan IM, Qiao J, et al. Copper as a single metal atom based photo-, electro- and photoelectrochemical catalyst decorated on carbon nitride surface for efficient CO2 reduction: A review. Nano Res Energy, 2022, 1: e9120015
Sun K, Ji Y, Liu Y, et al. Synergies between electronic and geometric effects of Mo-doped Au nanoparticles for effective CO2 electrochemical reduction. J Mater Chem A, 2020, 8: 12291–12295
Goyal A, Marcandalli G, Mints VA, et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. J Am Chem Soc, 2020, 142: 4154–4161
Liu G, Ariyarathna IR, Zhu Z, et al. Molecular-level electrocatalytic CO2 reduction reaction mediated by single platinum atoms. Phys Chem Chem Phys, 2022, 24: 4226–4231
He Q, Lee JH, Liu D, et al. Accelerating CO2 electroreduction to CO over Pd single-atom catalyst. Adv Funct Mater, 2020, 30: 2000407
Hori Y, Wakebe H, Tsukamoto T, et al. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim Acta, 1994, 39: 1833–1839
Yu Q. Theoretical studies of non-noble metal single-atom catalyst Ni1//MoS2: Electronic structure and electrocatalytic CO2 reduction. Sci China Mater, 2023, 66: 1079–1088
Kuhl KP, Hatsukade T, Cave ER, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J Am Chem Soc, 2014, 136: 14107–14113
Zoubir O, Atourki L, Ait Ahsaine H, et al. Current state of copper-based bimetallic materials for electrochemical CO2 reduction: A review. RSC Adv, 2022, 12: 30056–30075
Ma W, He X, Wang W, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem Soc Rev, 2021, 50: 12897–12914
Zhao Z, Lu G. Cu-based single-atom catalysts boost electroreduction of CO2 to CH3OH: First-principles predictions. J Phys Chem C, 2019, 123: 4380–4387
Nitopi S, Bertheussen E, Scott SB, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem Rev, 2019, 119: 7610–7672
Liu J, Duan S, Xu J, et al. Catalysis by supported single metal atoms. Microsc Microanal, 2016, 22: 860–861
Wang Y, Chen Z, Han P, et al. Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4. ACS Catal, 2018, 8: 7113–7119
Qiao B, Wang A, Yang X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem, 2011, 3: 634–641
Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat Rev Chem, 2018, 2: 65–81
Hannagan RT, Giannakakis G, Flytzani-Stephanopoulos M, et al. Single-atom alloy catalysis. Chem Rev, 2020, 120: 12044–12088
Back S, Lim J, Kim NY, et al. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem Sci, 2017, 8: 1090–1096
Pei G, Liu X, Chai M, et al. Isolation of Pd atoms by Cu for semihydrogenation of acetylene: Effects of Cu loading. Chin J Catal, 2017, 38: 1540–1548
Huang F, Deng Y, Chen Y, et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J Am Chem Soc, 2018, 140: 13142–13146
Liu W, Zhang L, Liu X, et al. Discriminating catalytically active FeNx species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C–H bond. J Am Chem Soc, 2017, 139: 10790–10798
Sun Q, Wang X, Wang H, et al. Crystal facet effects of platinum single-atom catalysts in hydrolytic dehydrogenation of ammonia borane. J Mater Chem A, 2022, 10: 10837–10843
Lin L, Zhou W, Gao R, et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature, 2017, 544: 80–83
Guo X, Fang G, Li G, et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science, 2014, 344: 616–619
Cao S, Yang M, Elnabawy AO, et al. Single-atom gold oxo-clusters prepared in alkaline solutions catalyse the heterogeneous methanol self-coupling reactions. Nat Chem, 2019, 11: 1098–1105
Lv M, Guo H, Shen H, et al. Fe3C cluster-promoted single-atom Fe, N doped carbon for oxygen-reduction reaction. Phys Chem Chem Phys, 2020, 22: 7218–7223
Chen S, Zhang N, Narváez Villarrubia CW, et al. Single Fe atoms anchored by short-range ordered nanographene boost oxygen reduction reaction in acidic media. Nano Energy, 2019, 66: 104164
Zhou HY, Li JC, Wen Z, et al. Tuning the catalytic activity of a single Mo atom supported on graphene for nitrogen reduction via Se atom doping. Phys Chem Chem Phys, 2019, 21: 14583–14588
Yang Y, Xue XX, Chen Q, et al. Doping single transition metal atom into PtTe sheet for catalyzing nitrogen reduction and hydrogen evolution reactions. J Chem Phys, 2019, 151: 144710
Ye S, Luo F, Zhang Q, et al. Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ Sci, 2019, 12: 1000–1007
Wu J, Han N, Ning S, et al. Single-atom tungsten-doped CoP nanoarrays as a high-efficiency pH-universal catalyst for hydrogen evolution reaction. ACS Sustain Chem Eng, 2020, 8: 14825–14832
Yang HB, Hung SF, Liu S, et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat Energy, 2018, 3: 140–147
Xiong Y, Dong J, Huang ZQ, et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat Nanotechnol, 2020, 15: 390–397
Gu H, Wu J, Zhang L. Recent advances in the rational design of single-atom catalysts for electrochemical CO2 reduction. Nano Res, 2022, 15: 9747–9763
Ren S, Yu Q, Yu X, et al. Graphene-supported metal single-atom catalysts: A concise review. Sci China Mater, 2020, 63: 903–920
Feng S, Yu Q, Ma X, et al. Hydroformylation over polyoxometalates supported single-atom Rh catalysts. NSO, 2023, 2: 20220064
Mitchell S, Vorobyeva E, Pérez-Ramírez J. The multifaceted reactivity of single-atom heterogeneous catalysts. Angew Chem Int Ed, 2018, 57: 15316–15329
Zhang B, Zhang J, Zhang F, et al. Selenium-doped hierarchically porous carbon nanosheets as an efficient metal-free electrocatalyst for CO2 reduction. Adv Funct Mater, 2020, 30: 1906194
Bai X, Zhao X, Zhang Y, et al. Dynamic stability of copper single-atom catalysts under working conditions. J Am Chem Soc, 2022, 144: 17140–17148
Yang XF, Wang A, Qiao B, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc Chem Res, 2013, 46: 1740–1748
Xue D, Xia H, Yan W, et al. Defect engineering on carbon-based catalysts for electrocatalytic CO2 reduction. Nano-Micro Lett, 2021, 13: 5
Liu H, Zhu Y, Ma J, et al. Recent advances in atomic-level engineering of nanostructured catalysts for electrochemical CO2 reduction. Adv Funct Mater, 2020, 30: 1910534
Kortlever R, Shen J, Schouten KJP, et al. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J Phys Chem Lett, 2015, 6: 4073–4082
Yang C, Chai J, Wang Z, et al. Recent progress on bismuth-based nanomaterials for electrocatalytic carbon dioxide reduction. Chem Res Chin Univ, 2020, 36: 410–419
Zhang YJ, Sethuraman V, Michalsky R, et al. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal, 2014, 4: 3742–3748
Zong X, Jin Y, Liu C, et al. Electrospun nanofibers for electrochemical reduction of CO2: A mini review. Electrochem Commun, 2021, 124: 106968
Xiao C, Zhang J. Architectural design for enhanced C2 product selectivity in electrochemical CO2 reduction using Cu-based catalysts: A review. ACS Nano, 2021, 15: 7975–8000
Zhang X, Huang H, Jin H, et al. Progress of the electrochemical reduction of CO2 in aqueous electrolyte. Chem Ind Eng Pro, 2015, 34: 4139–4144
Han N, Ding P, He L, et al. Promises of main group metal-based nanostructured materials for electrochemical CO2 reduction to formate. Adv Energy Mater, 2019, 10: 1902338
Zhang B, Zhang B, Jiang Y, et al. Single-atom electrocatalysts for multi-electron reduction of CO2. Small, 2021, 17: 2101443
Wang F, Zhang W, Wan H, et al. Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction. Chin Chem Lett, 2022, 33: 2259–2269
Wang Y, Liu J, Wang Y, et al. Tuning of CO2 reduction selectivity on metal electrocatalysts. Small, 2017, 13: 1701809
Tan X, Yu C, Ren Y, et al. Recent advances in innovative strategies for the CO2 electroreduction reaction. Energy Environ Sci, 2021, 14: 765–780
Wang G, Chen J, Ding Y, et al. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem Soc Rev, 2021, 50: 4993–5061
Umegaki T, Kojima Y, Omata K. Influence of pore structure of silica coated on copper-zinc oxide-based catalyst for carbon dioxide into methanol. Top Catal, 2021, 64: 576–581
Sun D, Xu X, Qin Y, et al. Rational design of Ag-based catalysts for the electrochemical CO2 reduction to CO: A review. ChemSusChem, 2020, 13: 39–58
Malik K, Singh S, Basu S, et al. Electrochemical reduction of CO2 for synthesis of green fuel. WIREs Energy Environ, 2017, 6: e244
Chen Q, Tsiakaras P, Shen P. Electrochemical reduction of carbon dioxide: Recent advances on Au-based nanocatalysts. Catalysts, 2022, 12: 1348
Li P, Bi J, Liu J, et al. In situ dual doping for constructing efficient CO2-to-methanol electrocatalysts. Nat Commun, 2022, 13: 1965
Wang Y, Han P, Lv X, et al. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule, 2018, 2: 2551–2582
Sun Z, Ma T, Tao H, et al. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials. Chem, 2017, 3: 560–587
Zhao Z, Zhang J, Lei M, et al. Reviewing the impact of halides on electrochemical CO2 reduction. Nano Res Energy, 2023, 2: e9120044
Jiang L, Yang Q, Xia Z, et al. Recent progress of theoretical studies on electro- and photo-chemical conversion of CO2 with single-atom catalysts. RSC Adv, 2023, 13: 5833–5850
Weng Z, Jiang J, Wu Y, et al. Electrochemical CO2 reduction to hydrocarbons on a heterogeneous molecular Cu catalyst in aqueous solution. J Am Chem Soc, 2016, 138: 8076–8079
Cai Y, Fu J, Zhou Y, et al. Insights on forming N,O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat Commun, 2021, 12: 586
Guan A, Chen Z, Quan Y, et al. Boosting CO2 electroreduction to CH4 via tuning neighboring single-copper sites. ACS Energy Lett, 2020, 5: 1044–1053
Karapinar D, Huan NT, Ranjbar Sahraie N, et al. Electroreduction of CO2 on single-site copper-nitrogen-doped carbon material: Selective formation of ethanol and reversible restructuration of the metal sites. Angew Chem Int Ed, 2019, 58: 15098–15103
Ye K, Cao A, Shao J, et al. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity. Sci Bull, 2020, 65: 711–719
Zheng T, Liu C, Guo C, et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat Nanotechnol, 2021, 16: 1386–1393
Li J, Zeng H, Dong X, et al. Selective CO2 electrolysis to CO using isolated antimony alloyed copper. Nat Commun, 2023, 14: 340
Li YC, Wang Z, Yuan T, et al. Binding site diversity promotes CO2 electroreduction to ethanol. J Am Chem Soc, 2019, 141: 8584–8591
Ren D, Gao J, Pan L, et al. Atomic layer deposition of ZnO on CuO enables selective and efficient electroreduction of carbon dioxide to liquid fuels. Angew Chem Int Ed, 2019, 58: 15036–15040
Vasileff A, Zhu Y, Zhi X, et al. Electrochemical reduction of CO2 to ethane through stabilization of an ethoxy intermediate. Angew Chem Int Ed, 2020, 59: 19362
Zhou Y, Che F, Liu M, et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat Chem, 2018, 10: 974–980
Ma W, Xie S, Liu T, et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C-C coupling over fluorine-modified copper. Nat Catal, 2020, 3: 478–487
Meng DL, Zhang MD, Si DH, et al. Highly selective tandem electroreduction of CO2 to ethylene over atomically isolated nickel-nitrogen site/copper nanoparticle catalysts. Angew Chem Int Ed, 2021, 60: 25485–25492
Xu C, Zhi X, Vasileff A, et al. Highly selective two-electron electrocatalytic CO2 reduction on single-atom Cu catalysts. Small Struct, 2020, 2: 2000058
Chen D, Zhang LH, Du J, et al. A tandem strategy for enhancing electrochemical CO2 reduction activity of single-atom Cu-S1N3 catalysts via integration with Cu nanoclusters. Angew Chem Int Ed, 2021, 60: 24022–24027
Yang H, Wu Y, Li G, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J Am Chem Soc, 2019, 141: 12717–12723
Shi G, Xie Y, Du L, et al. Constructing Cu-C bonds in a graphdiyne-regulated Cu single-atom electrocatalyst for CO2 reduction to CH4. Angew Chem Int Ed, 2022, 61: e202203569
Jung E, Shin H, Lee BH, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat Mater, 2020, 19: 436–442
Gu J, Peng Y, Zhou T, et al. Porphyrin-based framework materials for energy conversion. Nano Res Energy, 2022, 1: e9120009
Jeon HS, Kunze S, Scholten F, et al. Prism-shaped Cu nanocatalysts for electrochemical CO2 reduction to ethylene. ACS Catal, 2018, 8: 531–535
Gao D, Sinev I, Scholten F, et al. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. Angew Chem Int Ed, 2019, 58: 17047–17053
Zhao Y, Yang KR, Wang Z, et al. Stable iridium dinuclear heterogeneous catalysts supported on metal-oxide substrate for solar water oxidation. Proc Natl Acad Sci USA, 2018, 115: 2902–2907
Chen ZW, Chen LX, Yang CC, et al. Atomic (single, double, and triple atoms) catalysis: Frontiers, opportunities, and challenges. J Mater Chem A, 2019, 7: 3492–3515
Cao L, Luo Q, Liu W, et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat Catal, 2018, 2: 134–141
Bergmann A, Jones TE, Martinez Moreno E, et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat Catal, 2018, 1: 711–719
Jirkovský JS, Panas I, Ahlberg E, et al. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production. J Am Chem Soc, 2011, 133: 19432–19441
Qin R, Liu P, Fu G, et al. Strategies for stabilizing atomically dispersed metal catalysts. Small Methods, 2018, 2: 1700286
Zhang T, Walsh AG, Yu J, et al. Single-atom alloy catalysts: Structural analysis, electronic properties and catalytic activities. Chem Soc Rev, 2021, 50: 569–588
Zhou L, Martirez JMP, Finzel J, et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat Energy, 2020, 5: 61–70
Jiang JC, Chen JC, Zhao M, et al. Rational design of copper-based single-atom alloy catalysts for electrochemical CO2 reduction. Nano Res, 2022, 15: 7116–7123
Jiang Z, Sun W, Shang H, et al. Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions. Energy Environ Sci, 2019, 12: 3508–3514
Qin F, Chen W. Copper-based single-atom alloys for heterogeneous catalysis. Chem Commun, 2021, 57: 2710–2723
Zheng X, Ji Y, Tang J, et al. Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials. Nat Catal, 2018, 2: 55–61
Morimoto M, Takatsuji Y, Yamasaki R, et al. Electrodeposited Cu-Sn alloy for electrochemical CO2 reduction to CO/HCOO−. Electrocatalysis, 2017, 9: 323–332
Nie X, Esopi MR, Janik MJ, et al. Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew Chem Int Ed, 2013, 52: 2459–2462
Nie X, Luo W, Janik MJ, et al. Reaction mechanisms of CO2 electrochemical reduction on Cu(111) determined with density functional theory. J Catal, 2014, 312: 108–122
Ahmad T, Liu S, Sajid M, et al. Electrochemical CO2 reduction to C2+ products using Cu-based electrocatalysts: A review. Nano Res Energy, 2022, 1: e9120021
Aizawa H, Tsuneyuki S. First-principles study of CO bonding to Pt (111): Validity of the Blyholder model. Surf Sci, 1998, 399: L364–L370
Blyholder G. Molecular orbital view of chemisorbed carbon monoxide. J Phys Chem, 1964, 68: 2772–2777
Greiner MT, Jones TE, Beeg S, et al. Free-atom-like d states in single-atom alloy catalysts. Nat Chem, 2018, 10: 1008–1015
Hannagan RT, Giannakakis G, Réocreux R, et al. First-principles design of a single-atom-alloy propane dehydrogenation catalyst. Science, 2021, 372: 1444–1447
Gao Q, Yao B, Pillai HS, et al. Synthesis of core/shell nanocrystals with ordered intermetallic single-atom alloy layers for nitrate electroreduction to ammonia. Nat Synth, 2023, 2: 624–634
Li H, Liu T, Wei P, et al. High-rate CO2 electroreduction to C2+ products over a copper-copper iodide catalyst. Angew Chem Int Ed, 2021, 60: 14329–14333
Yu J, Wang J, Ma Y, et al. Recent progresses in electrochemical carbon dioxide reduction on copper-based catalysts toward multicarbon products. Adv Funct Mater, 2021, 31: 2102151
Peterson AA, Abild-Pedersen F, Studt F, et al. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ Sci, 2010, 3: 1311
Woldu AR, Huang Z, Zhao P, et al. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts. Coord Chem Rev, 2022, 454: 214340
Liu S, Zhang B, Zhang L, et al. Rational design strategies of Cu-based electrocatalysts for CO2 electroreduction to C2 products. J Energy Chem, 2022, 71: 63–82
Fu W, Wang Y, Tian W, et al. Non-metal single-phosphorus-atom catalysis of hydrogen evolution. Angew Chem Int Ed, 2020, 59: 23791–23799
Kuhl KP, Cave ER, Abram DN, et al. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ Sci, 2012, 5: 7050
Zheng Y, Vasileff A, Zhou X, et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J Am Chem Soc, 2019, 141: 7646–7659
Zhu Y, Cui X, Liu H, et al. Tandem catalysis in electrochemical CO2 reduction reaction. Nano Res, 2021, 14: 4471–4486
Li Q, Chen W, Xiao H, et al. Fe isolated single atoms on S, N codoped carbon by copolymer pyrolysis strategy for highly efficient oxygen reduction reaction. Adv Mater, 2018, 30: 1800588
Albo J, Irabien A. Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. J Catal, 2016, 343: 232–239
Albo J, Alvarez-Guerra M, Castaño P, et al. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chem, 2015, 17: 2304–2324
Jia Y, Hsu HS, Huang WC, et al. Probing the roles of indium oxides on copper catalysts for enhanced selectivity during CO2-to-CO electrochemical reduction. Nano Lett, 2023, 23: 2262–2268
Han L, Song S, Liu M, et al. Stable and efficient single-atom Zn catalyst for CO2 reduction to CH4. J Am Chem Soc, 2020, 142: 12563–12567
Zhao J, Chen Z, Zhao J. Metal-free graphdiyne doped with sp-hybridized boron and nitrogen atoms at acetylenic sites for high-efficiency electroreduction of CO2 to CH4 and C2H4. J Mater Chem A, 2019, 7: 4026–4035
Liu X, Wang Z, Tian Y, et al. Graphdiyne-supported single iron atom: A promising electrocatalyst for carbon dioxide electroreduction into methane and ethanol. J Phys Chem C, 2020, 124: 3722–3730
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (92061109) and the Natural Science Basic Research Program of Shaanxi (2021JCW-20 and 2022KJXX-18).
Author information
Authors and Affiliations
Contributions
Author contributions Yu Q supervised the project and organized the collaboration. Li X wrote the manuscript and Yu X and Yu Q revised the manuscript. All authors discussed the review, edited and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Xiaojiao Li is pursuing a Master degree at the School of Materials Science and Engineering, Shaanxi University of Technology, under the supervision of Prof. Qi Yu. Her research focuses on theoretical calculations of single-atom catalysts.
Qi Yu is a professor at Shaanxi Key Laboratory of Catalysis, Shaanxi University of Technology. She obtained her BSc, MSc and PhD degrees from Jilin University, followed by visiting scholar at Tsinghua University and the National University of Singapore for several years. Now she is the deputy director of Shaanxi Laboratory of Catalysis and director of Institute of Graphene at Shaanxi University of Technology. Her current research interests focus on theoretical calculations of single-atom catalysts.
Rights and permissions
About this article
Cite this article
Li, X., Yu, X. & Yu, Q. Research progress on electrochemical CO2 reduction for Cu-based single-atom catalysts. Sci. China Mater. 66, 3765–3781 (2023). https://doi.org/10.1007/s40843-023-2597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2597-8