Abstract
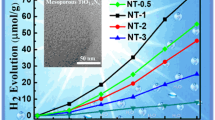
Semiconductor mesoporous single crystals have attracted considerable attention due to the merits of excellent light absorption ability and large surface area for photocatalysis capability. However, most of the materials have a relative large size (∼um), which can hardly suppress the recombination of photogenerated electrons and holes. In this work, we synthesized a series of nano mesoporous TiO2 mixed crystals (brookite and rutile) with high carrier transfer efficiency, improved mass transfer and diffusion abilities, which are crucial for the outstanding photocatalytic performance. Meanwhile, the key factors in the growth process and mechanism of the mesoporous TiO2 mixed crystals were addressed. With the assistance of Pt nanoparticles, H2 and H2O2 production can be simultaneously achieved and the efficiencies can reach up to 9.46 ± 0.56 and 3.29 ± 1.28 µmol mg−1 h−1, respectively. The H2 evolution rate is 2.8-fold higher than that of the reported TiO2 nanoparticle catalyst (3.34 µmol mg−1 h−1). The excellent photocatalytic efficiency can be attributed to the special mixed crystal structure of the mesoporous TiO2 crystals. This work provides new ideas and guidance for precise synthesis of nano mesoporous TiO2 mixed crystals and enhancing their water splitting performance with high value-added products.
摘要半
导体介孔单晶具有良好的光吸收能力和较大的比表面积, 因而受到广泛关注. 但多数材料的尺寸较大(∼um), 难以抑制光电子与空穴的复合. 在本工作中, 我们准确地合成了一系列载流子转移效率高、 传质和扩散能力强的纳米介孔板钛矿-金红石TiO2混晶. 同时, 详细研究了介孔TiO2混晶生长的关键因素和机理. 以Pt纳米颗粒作为助催化剂, 实现了全分解水产氢气和过氧化氢, 氢气和过氧化氢的生成速率分别达到9.46±0.56和3.29±1.28 μmol mg−1 h−1. 氢气产率是目 报道的TiO2(3.34 μmol mg−1 h−1)的2.8倍. 介孔TiO2晶体的纳米尺度和混合晶体结构使其具有优异的光催化效率. 本工作为纳米介孔TiO2混合晶体的精准合成, 以及光催化全分解水制备高附加值产品提供了新思路.
Similar content being viewed by others
References
Bagheri S, Mohd Hir ZA, Yousefi AT, et al. Progress on mesoporous titanium dioxide: synthesis, modification and applications. Microporous Mesoporous Mater, 2015, 218: 206–222
Dong Y, Fei X, Zhou Y. Synthesis and photocatalytic activity of mesoporous - (001) facets TiO2 single crystals. Appl Surf Sci, 2017, 403: 662–669
Alagarasi A, Rajalakshmi PU, Shanthi K, et al. Ordered mesoporous nanocrystalline titania: a promising new class of photocatalyic materials. Catal Today, 2018, 309: 202–211
Zou M, Xiong F, Ganeshraja AS, et al. Visible light photocatalysts (Fe, N):TiO2 from ammonothermally processed, solvothermal self-assembly derived Fe-TiO2 mesoporous microspheres. Mater Chem Phys, 2017, 195: 259–267
Zhou Y, Fang W, Deng Y, et al. Enhanced photoreduction of Cr(VI) and photooxidation of NO over TiO2−x mesoporous single crystals. RSC Adv, 2017, 7: 55927–55934
Fan Z, Meng F, Gong J, et al. One-step hydrothermal synthesis of mesoporous Ce-doped anatase TiO2 nanoparticles with enhanced photocatalytic activity. J Mater Sci-Mater Electron, 2016, 27: 11866–11872
Lan K, Liu Y, Zhang W, et al. Uniform ordered two-dimensional mesoporous TiO2 nanosheets from hydrothermal-induced solvent-confined monomicelle assembly. J Am Chem Soc, 2018, 140: 4135–4143
Jiao Y, Chen X, He F, et al. Simple preparation of uniformly distributed mesoporous Cr/TiO2 microspheres for low-temperature catalytic combustion of chlorobenzene. Chem Eng J, 2019, 372: 107–117
Lu T, Wang Y, Wang Y, et al. Synthesis of mesoporous anatase TiO2 sphere with high surface area and enhanced photocatalytic activity. J Mater Sci Tech, 2017, 33: 300–304
Wang L, Xie Y, Liu W, et al. Synthesis of mesoporous core-shell TiO2 microstructures with coexposed {001}/{101} facets: enhanced intrinsic photocatalytic performance. Environ Sci Pollut Res, 2018, 25: 31250–31261
Jitputti J, Pavasupree S, Suzuki Y, et al. Synthesis and photocatalytic activity for water-splitting reaction of nanocrystalline mesoporous titania prepared by hydrothermal method. J Solid State Chem, 2007, 180: 1743–1749
Xie C, Yang S, Shi J, et al. Highly crystallized C-doped mesoporous anatase TiO2 with visible light photocatalytic activity. Catalysts, 2016, 6: 117
Mohamed MA, Wan Salleh WN, Jaafar J, et al. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO2: Biotemplate assisted sol-gel synthesis and photocatalytic activity. Appl Surf Sci, 2017, 393: 46–59
Wu T, Kang X, Kadi MW, et al. Enhanced photocatalytic hydrogen generation of mesoporous rutile TiO2 single crystal with wholly exposed {111} facets. Chin J Catal, 2015, 36: 2103–2108
Xie C, Yang S, Li B, et al. C-doped mesoporous anatase TiO2 comprising 10 nm crystallites. J Colloid Interface Sci, 2016, 476: 1–8
Yang Y, Li Y, Wang J, et al. Graphene-TiO2 mesoporous spheres assembled by anatase and rutile nanowires for efficient NO photooxidation. J Alloys Compd, 2017, 699: 47–56
Zhang W, Zhou Y, Dong C, et al. Carbon-dot-modified TiO2−x mesoporous single crystals with enhanced photocatalytic activity for degradation of phenol. Res Chem Intermed, 2018, 44: 4797–4807
Zheng X, Kuang Q, Yan K, et al. Mesoporous TiO2 single crystals: facile shape-, size-, and phase-controlled growth and efficient photocatalytic performance. ACS Appl Mater Interfaces, 2013, 5: 11249–11257
Fang WQ, Huo Z, Liu P, et al. Fluorine-doped porous single-crystal rutile TiO2 nanorods for enhancing photoelectrochemical water splitting. Chem Eur J, 2014, 20: 11439–11444
Zhou Y, Yi Q, Xing M, et al. Graphene modified mesoporous titania single crystals with controlled and selective photoredox surfaces. Chem Commun, 2016, 52: 1689–1692
Dörr TS, Deilmann L, Haselmann G, et al. Ordered mesoporous TiO2 gyroids: effects of pore architecture and Nb-doping on photocatalytic hydrogen evolution under UV and visible irradiation. Adv Energy Mater, 2018, 8: 1802566
Zhang W, He H, Tian Y, et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem Sci, 2019, 10: 1664–1670
Lu J, Wang Y, Huang J, et al. In situ synthesis of mesoporous C-doped TiO2single crystal with oxygen vacancy and its enhanced sunlight photocatalytic properties. Dyes Pigments, 2017, 144: 203–211
Sreethawong T, Laehsalee S, Chavadej S. Comparative investigation of mesoporous- and non-mesoporous-assembled TiO2 nanocrystals for photocatalytic H2 production over N-doped TiO2 under visible light irradiation. Int J Hydrogen Energy, 2008, 33: 5947–5957
Zhao Z, Xing Y, Li H, et al. Constructing CdS/Cd/doped TiO2 Z-scheme type visible light photocatalyst for H2 production. Sci China Mater, 2018, 61: 851–860
Likodimos V, Han C, Pelaez M, et al. Anion-doped TiO2 nanocatalysts for water purification under visible light. Ind Eng Chem Res, 2013, 52: 13957–13964
Wang Y, Zhu Y, Yang X, et al. Plasmonic Au decorated single-crystal-like TiO2-NaYF4 mesoporous microspheres for enhanced broadband photocatalysis. Chin J Chem, 2017, 35: 949–956
Pan J, Liu G, Lu GQM, et al. On the true photoreactivity order of {001}, {010}, and {101} facets of anatase TiO2 crystals. Angew Chem Int Ed, 2011, 50: 2133–2137
Fan Y, Hu G, Yu S, et al. Recent advances in TiO2 nanoarrays/ graphene for water treatment and energy conversion/storage. Sci China Mater, 2019, 62: 325–340
Ran J, Zhang J, Yu J, et al. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem Soc Rev, 2014, 43: 7787–7812
Li D, Zhou H, Honma I. Design and synthesis of self-ordered mesoporous nanocomposite through controlled in-situ crystallization. Nat Mater, 2004, 3: 65–72
Kondo JN, Domen K. Crystallization of mesoporous metal oxides. Chem Mater, 2008, 20: 835–847
Jiao W, Xie Y, Chen R, et al. Synthesis of mesoporous single crystal rutile TiO2 with improved photocatalytic and photoelectrochemical activities. Chem Commun, 2013, 49: 11770
Vequizo JJM, Matsunaga H, Ishiku T, et al. Trapping-induced enhancement of photocatalytic activity on brookite TiO2 powders: comparison with anatase and rutile TiO2 powders. ACS Catal, 2017, 7: 2644–2651
Moss B, Lim KK, Beltram A, et al. Comparing photoelectrochemical water oxidation, recombination kinetics and charge trapping in the three polymorphs of TiO2. Sci Rep, 2017, 7: 2938
Di Paola A, Bellardita M, Palmisano L. Brookite, the least known TiO2 photocatalyst. Catalysts, 2013, 3: 36–73
Monai M, Montini T, Fornasiero P. Brookite: Nothing new under the sun? Catalysts, 2017, 7: 304
Nobre FX, Gil Pessoa Junior WA, Ruiz YL, et al. Facile synthesis of nTiO2 phase mixture: characterization and catalytic performance. Mater Res Bull, 2019, 109: 60–71
Yang Z, Wang B, Cui H, et al. Synthesis of crystal-controlled TiO2 nanorods by a hydrothermal method: rutile and brookite as highly active photocatalysts. J Phys Chem C, 2015, 119: 16905–16912
Allen NS, Mahdjoub N, Vishnyakov V, et al. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym Degradation Stability, 2018, 150: 31–36
Xu H, Zhang L. Controllable one-pot synthesis and enhanced photocatalytic activity of mixed-phase TiO2 nanocrystals with tunable brookite/rutile ratios. J Phys Chem C, 2009, 113: 1785–1790
Lin J, Zhao L, Heo YU, et al. Mesoporous anatase single crystals for efficient Co(2+/3+)-based dye-sensitized solar cells. Nano Energy, 2015, 11: 557–567
Zhang H, Banfield JF. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. J Phys Chem B, 2000, 104: 3481–3487
Mamaghani AH, Haghighat F, Lee CS. Systematic variation of preparation time, temperature, and pressure in hydrothermal synthesis of macro-/mesoporous TiO2 for photocatalytic air treatment. J Photochem Photobiol A-Chem, 2019, 378: 156–170
Tompsett GA, Bowmaker GA, Cooney RP, et al. The Raman spectrum of brookite, TiO2 (Pbca, Z = 8). J Raman Spectrosc, 1995, 26: 57–62
Cao Y, Li X, Bian Z, et al. Highly photocatalytic activity of brookite/rutile TiO2 nanocrystals with semi-embedded structure. Appl Catal B-Environ, 2016, 180: 551–558
Bhattacharyya D, Kumar P, Smith YR, et al. Plasmonic-enhanced electrochemical detection of volatile biomarkers with gold functionalized TiO2 nanotube arrays. J Mater Sci Tech, 2018, 34: 905–913
Byrappa K, Adschiri T. Hydrothermal technology for nanotechnology. Prog Cryst Growth Charact Mater, 2007, 53: 117–166
Rossetti Jr. GA, Watson DJ, Newnham RE, et al. Kinetics of the hydrothermal crystallization of the perovskite lead titanate. J Cryst Growth, 1992, 116: 251–259
Liu S, Zhou J, Liu R, et al. Preparation of nano ZrO2 by integrated technique of high gravity field and microwave field. J Synth Cryst, 2014, 43: 79–86
Zhou W, Liu X, Cui J, et al. Control synthesis of rutile TiO2 microspheres, nanoflowers, nanotrees and nanobelts via acid-hydrothermal method and their optical properties. CrystEngComm, 2011, 13: 4557
Li Y, Zhang M, Guo M, et al. Hydrothermal growth of well-aligned TiO2 nanorod arrays: Dependence of morphology upon hydrothermal reaction conditions. Rare Met, 2010, 29: 286–291
Meng A, Zhang L, Cheng B, et al. Dual cocatalysts in TiO2 photocatalysis. Adv Mater, 2019, 31: 1807660
Xiao F, Zhou W, Sun B, et al. Engineering oxygen vacancy on rutile TiO2 for efficient electron-hole separation and high solar-driven photocatalytic hydrogen evolution. Sci China Mater, 2018, 61: 822–830
Testino A, Bellobono IR, Buscaglia V, et al. Optimizing the photocatalytic properties of hydrothermal TiO2 by the control of phase composition and particle morphology. a systematic approach. J Am Chem Soc, 2007, 129: 3564–3575
Li C, Yang W, Li Q. TiO2-based photocatalysts prepared by oxidation of TiN nanoparticles and their photocatalytic activities under visible light illumination. J Mater Sci Tech, 2018, 34: 969–975
Zhang W, Zhang H, Xu J, et al. 3D flower-like heterostructured TiO2@Ni(OH)2 microspheres for solar photocatalytic hydrogen production. Chin J Catal, 2019, 40: 320–325
Kinsinger NM, Wong A, Li D, et al. Nucleation and crystal growth of nanocrystalline anatase and rutile phase TiO2 from a water-soluble precursor. Cryst Growth Des, 2010, 10: 5254–5261
Tang Y, Zhou P, Chao Y, et al. Face-to-face engineering of ultra-thin Pd nanosheets on amorphous carbon nitride for efficient photocatalytic hydrogen production. Sci China Mater, 2019, 62: 351–358
Cao S, Chan TS, Lu YR, et al. Photocatalytic pure water splitting with high efficiency and value by Pt/porous brookite TiO2 nanoflutes. Nano Energy, 2020, 67: 104287
Nakabayashi Y, Nishikawa M, Saito N, et al. Significance of hydroxyl radical in photoinduced oxygen evolution in water on monoclinic bismuth vanadate. J Phys Chem C, 2017, 121: 25624–25631
Seabold JA, Choi KS. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem Mater, 2011, 23: 1105–1112
Kumaravel V, Mathew S, Bartlett J, et al. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl Catal B-Environ, 2019, 244: 1021–1064
Zhang W, Hu Y, Yan C, et al. Surface plasmon resonance enhanced direct Z-scheme TiO2/ZnTe/Au nanocorncob heterojunctions for efficient photocatalytic overall water splitting. Nanoscale, 2019, 11: 9053–9060
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21703046), and the Ministry of Science and Technology of China (2016YFA0200902).
Author information
Authors and Affiliations
Contributions
Author contributions Wang L designed and modified the samples and performed the experiments; Piao L and Cao S provided pivotal advice; Wang L wrote the paper with support from Piao L and Cao S. All authors contributed to the general discussion.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Lichao Wang is currently a master student at the School of Chemical Engineering and Technology, Tianjin University. He joined the National Center for Nanoscience and Technology as a joint student. His research interests focus on the design and fabrication of photocatalysts for H2 production.
Shuang Cao received her PhD degree from the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences under the supervision of Prof. Yong Chen and Prof. Wen-Fu Fu in 2016. She joined the National Center for Nanoscience and Technology as a research associate in 2016. Her research focuses on designing and optimizing photocatalysts for H2 production.
Zhi Ma is an associate professor of the School of Chemical Engineering and Technology, Tianjin University. He received his PhD degree from Tianjin University in 1998. He worked in Tianjin University after his master’s degree in 1990. His research interest focuses on the application and exploitation aspects of nanometer catalyst.
Lingyu Piao received her PhD degree from Tianjin University in 2002. She did her postdoctoral research at Peking University and Marie Curie University, respectively from 2002 to 2005. She joined the National Center for Nanoscience and Technology in 2005. Her research interest focuses on the design and synthesis of nano functional materials and their applications in energy, environmental photocatalysis and biology.
Supplementary Information
Rights and permissions
About this article
Cite this article
Wang, L., Xiao, Z., Liu, Y. et al. Mesoporous TiO2 mixed crystals for photocatalytic pure water splitting. Sci. China Mater. 63, 758–768 (2020). https://doi.org/10.1007/s40843-019-1253-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-019-1253-8