Abstract
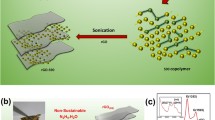
Graphene-like N,S-codoped bio-carbon nanosheets (GNSCS) were prepared by a facile and environment-friendly NaCl non-aqueous ionic liquid route to house sulfur for lithium-sulfur battery. The natural nori powder was calcined at 900°C for 3 h under Ar, in which NaCl non-aqueous ionic liquid can exfoliate carbon aggregates into nanosheets. The structural characterization of GNSCS by a series of techniques demonstrates the graphene-like feature. When evaluated as the matrix for sulfur cathode, GNSCS/S exhibits more prominent cycling stability and rate capability. A discharge capacity of 548 mA h g−1 at a current density of 1.6 A g−1 after 400 cycles was delivered with a capacity fade rate of only 0.13% per cycle and an initial Coulombic efficiency (CE) as high as 99.7%. When increasing the areal sulfur loading up to 3 mg cm−2, the discharge capacity can still be retained at 647 mA h g−1 after more than 100 cycles with a low capacity degradation of only ~0.30% per cycle. The features of N/S dual-doping and the graphene-like structure are propitious to the electron transportation, lithium-ion diffusion and more active sites for chemically adsorbing polysulfides. It is anticipated that other functional biochar carbon can also be attained via the low-cost, sustainable and green method.
摘要
本论文通过结构设计利用简单方法成功制备了一种二维N,S共掺杂类石墨烯纳米片复合结构, 即利用NaCl非离子液体的剥离作用 使生物质剥离得到二维片层类石墨烯结构. 这种新的非离子液体剥离技术较其他的碳材料剥离技术具有环境友好性、 低成本、 安全无毒 性等优势, 有利于实现量化制备锂硫电池电极材料. 该材料采用大自然中广泛存在的紫菜作为原料, 其内部富含的氨基酸为原位掺杂N,S元素提供了可能性. 二维结构的纳米片能够提供有效的导电性和电解液浸润性的网络结构, 同时还能够有效地降低电池在充放电循环过 程中导致的体积膨胀效应, 最终实现一种高机械性能、 优异电化学活性的电极在锂硫电池储能领域中的应用.
Similar content being viewed by others
References
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science, 2011, 334: 928–935
Manthiram A, Chung SH, Zu C. Lithium-sulfur batteries: progress and prospects. Adv Mater, 2015, 27: 1980–2006
Evers S, Nazar LF. New approaches for high energy density lithium–sulfur battery cathodes. Acc Chem Res, 2012, 46: 1135–1143
He J, Chen Y, Manthiram A. MOF-derived cobalt sulfide grown on 3D graphene foam as an efficient sulfur host for long-life lithiumsulfur batteries. iScience, 2018, 4: 36–43
He J, Chen Y, Manthiram A. Vertical Co9S8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li–S batteries. Energy Environ Sci, 2018, doi:10.1039/C8EE00893K
Wei Seh Z, Li W, Cha JJ, et al. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat Commun, 2013, 4: 1331–1337
Zhang C, Lv W, Zhang W, et al. Reduction of graphene oxide by hydrogen sulfide: a promising strategy for pollutant control and as an electrode for Li-S batteries. Adv Energy Mater, 2014, 4: 1301565
Lv W, Li Z, Zhou G, et al. Tailoring microstructure of graphenebased membrane by controlled removal of trapped water inspired by the phase diagram. Adv Funct Mater, 2014, 24: 3456–3463
Wang L, Zhao Y, Thomas ML, et al. In situ synthesis of bipyramidal sulfur with 3D carbon nanotube framework for lithiumsulfur batteries. Adv Funct Mater, 2014, 24: 2248–2252
Miao L, Wang W, Yuan K, et al. A lithium–sulfur cathode with high sulfur loading and high capacity per area: a binder-free carbon fiber cloth–sulfur material. Chem Commun, 2014, 50: 13231–13234
Mi K, Jiang Y, Feng J, et al. Hierarchical carbon nanotubes with a thick microporous wall and inner channel as efficient scaffolds for lithium-sulfur batteries. Adv Funct Mater, 2016, 26: 1571–1579
Mi K, Chen S, Xi B, et al. Sole chemical confinement of polysulfides on nonporous nitrogen/oxygen dual-doped carbon at the kilogram scale for lithium-sulfur batteries. Adv Funct Mater, 2017, 27: 1604265
Zhang C, Wu HB, Yuan C, et al. Confining sulfur in double-shelled hollow carbon spheres for lithium-sulfur batteries. Angew Chem, 2012, 124: 9730–9733
Jayaprakash N, Shen J, Moganty SS, et al. Porous hollow carbon@ sulfur composites for high-power lithium-sulfur batteries. Angew Chem, 2011, 123: 6026–6030
Zhao XS, Su F, Yan Q, et al. Templating methods for preparation of porous structures. J Mater Chem, 2006, 16: 637–648
He J, Lv W, Chen Y, et al. Direct impregnation of SeS2 into a MOFderived 3D nanoporous Co–N–C architecture towards superior rechargeable lithium batteries. J Mater Chem A, 2018, 6: 10466–10473
Imtiaz S, Zhang J, Zafar ZA, et al. Biomass-derived nanostructured porous carbons for lithium-sulfur batteries. Sci China Mater, 2016, 59: 389–407
Li J, Qin F, Zhang L, et al. Mesoporous carbon from biomass: onepot synthesis and application for Li–S batteries. J Mater Chem A, 2014, 2: 13916–13922
Gu X, Wang Y, Lai C, et al. Microporous bamboo biochar for lithium-sulfur batteries. Nano Res, 2015, 8: 129–139
Yu M, Li R, Tong Y, et al. A graphene wrapped hair-derived carbon/sulfur composite for lithium–sulfur batteries. J Mater Chem A, 2015, 3: 9609–9615
Dupont J. From molten salts to ionic liquids: A “nano” journey. Acc Chem Res, 2011, 44: 1223–1231
Zhang J, Qiu Y, Huang M, et al. Accelerated formation of strontium silicate by solid-state reaction in NaCl–H2O(v) system at lower temperature. Appl Surf Sci, 2015, 347: 57–63
Zhang J, Huang M, Yanagisawa K, et al. NaCl–H2O-assisted preparation of SrTiO3 nanoparticles by solid state reaction at low temperature. Ceramics Int, 2015, 41: 5439–5444
Liu F, Peng H, You C, et al. High-performance doped carbon catalyst derived from Nori biomass with melamine promoter. Electrochim Acta, 2014, 138: 353–359
Ferrari AC, Meyer JC, Scardaci V, et al. Raman spectrum of graphene and graphene layers. Phys Rev Lett, 2006, 97: 187401
Ferrari AC, Basko DM. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat Nanotechnol, 2013, 8: 235–246
Ren W, Saito R, Gao L, et al. Edge phonon state of mono-and fewlayer graphene nanoribbons observed by surface and interference co-enhanced Raman spectroscopy. Phys Rev B, 2010, 81: 035412
Sangwichien C, Aranovich GL, Donohue MD. Density functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surfs A-Physicochem Eng Aspects, 2002, 206: 313–320
Fang R, Zhao S, Pei S, et al. Toward more reliable lithium–sulfur batteries: an all-graphene cathode structure. ACS Nano, 2017, 10: 8676–8682
Zhang Z, Li Z, Hao F, et al. 3D interconnected porous carbon aerogels as sulfur immobilizers for sulfur impregnation for lithium-sulfur batteries with high rate capability and cycling stability. Adv Funct Mater, 2014, 24: 2500–2509
Li GC, Li GR, Ye SH, et al. A polyaniline-coated sulfur/carbon composite with an enhanced high-rate capability as a cathode material for lithium/sulfur batteries. Adv Energy Mater, 2012, 2: 1238–1245
Sun D, Yang J, Yan X. Hierarchically porous and nitrogen, sulfurcodoped graphene-like microspheres as a high capacity anode for lithium ion batteries. Chem Commun, 2015, 51: 2134–2137
Yang J, Ju Z, Jiang Y, et al. Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassiumion storage. Adv Mater, 2018, 30: 1700104
Kiciński W, Szala M, Bystrzejewski M. Sulfur-doped porous carbons: Synthesis and applications. Carbon, 2014, 68: 1–32
Gu W, Sevilla M, Magasinski A, et al. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: a case study for pseudocapacitance detection. Energy Environ Sci, 2013, 6: 2465–2476
Pang Q, Tang J, Huang H, et al. A nitrogen and sulfur dual-doped carbon derived from polyrhodanine@cellulose for advanced lithium-sulfur batteries. Adv Mater, 2015, 27: 6021–6028
Hou TZ, Chen X, Peng HJ, et al. Design principles for heteroatomdoped nanocarbon to achieve strong anchoring of polysulfides for lithium-sulfur batteries. Small, 2016, 12: 3283–3291
Qiu Y, Li W, Zhao W, et al. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett, 2014, 14: 4821–4827
Wang X, Weng Q, Liu X, et al. Atomistic origins of high rate capability and capacity of N-doped graphene for lithium storage. Nano Lett, 2014, 14: 1164–1171
Sun F, Wang J, Chen H, et al. High efficiency immobilization of sulfur on nitrogen-enriched mesoporous carbons for Li–S batteries. ACS Appl Mater Interfaces, 2013, 5: 5630–5638
Song J, Xu T, Gordin ML, et al. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Adv Funct Mater, 2014, 24: 1243–1250
Zhou G, Paek E, Hwang GS, et al. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat Commun, 2015, 6: 7760–7771
Guo J, Xu Y, Wang C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries. Nano Lett, 2011, 11: 4288–4294
Zhang FF, Huang G, Wang XX, et al. Sulfur-impregnated coreshell hierarchical porous carbon for lithium-sulfur batteries. Chem Eur J, 2014, 20: 17523–17529
Fang R, Zhao S, Sun Z, et al. More reliable lithium-sulfur batteries: status, solutions and prospects. Adv Mater, 2017, 29: 1606823
Zheng G, Yang Y, Cha JJ, et al. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett, 2011, 11: 4462–4467
Li L, Chen L, Mukherjee S, et al. Phosphorene as a polysulfide immobilizer and catalyst in high-performance lithium-sulfur batteries. Adv Mater, 2017, 29: 1602734
Zhao J, Zou X, Zhu Y, et al. Electrochemical intercalation of potassium into graphite. Adv Funct Mater, 2016, 26: 8103–8110
Zhou G, Pei S, Li L, et al. A graphene-pure-sulfur sandwich structure for ultrafast, long-life lithium-sulfur batteries. Adv Mater, 2014, 26: 625–631
Li L, Wu ZP, Sun H, et al. A foldable lithium–sulfur battery. ACS Nano, 2015, 9: 11342–11350
Han J, Xi B, Feng Z, et al. Sulfur–hydrazine hydrate-based chemical synthesis of sulfur@graphene composite for lithium–sulfur batteries. Inorg Chem Front, 2018, 5: 785–792
Yang K, Gao Q, Tan Y, et al. Microporous carbon derived from Apricot shell as cathode material for lithium–sulfur battery. Microporous Mesoporous Mater, 2015, 204: 235–241
Zhang S, Zheng M, Lin Z, et al. Activated carbon with ultrahigh specific surface area synthesized from natural plant material for lithium–sulfur batteries. J Mater Chem A, 2014, 2: 15889–15896
Wei S, Zhang H, Huang Y, et al. Pig bone derived hierarchical porous carbon and its enhanced cycling performance of lithium–sulfur batteries. Energy Environ Sci, 2011, 4: 736–740
Park MS, Yu JS, Kim KJ, et al. Porous carbon spheres as a functional conducting framework for use in lithium–sulfur batteries. RSC Adv, 2013, 3: 11774–11781
Zhang B, Xiao M, Wang S, et al. Novel hierarchically porous carbon materials obtained from natural biopolymer as host matrixes for lithium–sulfur battery applications. ACS Appl Mater Interfaces, 2014, 6: 13174–13182
Wang H, Chen Z, Liu HK, et al. A facile synthesis approach to micro–macroporous carbon from cotton and its application in the lithium–sulfur battery. RSC Adv, 2014, 4: 65074–65080
Xin S, Gu L, Zhao NH, et al. Smaller sulfur molecules promise better lithium–sulfur batteries. J Am Chem Soc, 2012, 134: 18510–18513
Zhao S, Li C, Wang W, et al. A novel porous nanocomposite of sulfur/carbon obtained from fish scales for lithium–sulfur batteries. J Mater Chem A, 2013, 1: 3334–3339
Acknowledgements
The authors gratefully acknowledge the financial supports provided by the National Natural Science Foundation of China (21601108 and U1764258), Young Scholars Program of Shandong University (2017WLJH15), the Fundamental Research Funds of Shandong University (2016JC033 and 2016GN010), and the Taishan Scholar Project of Shandong Province (ts201511004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Man Huang got her Master degree from Jiangsu University of Science and Technology. Now, she is a PhD student under the supervision of Prof. Shenglin Xiong and Prof. Yitai Qian at the School of Chemistry and Chemical Engineering, Shandong University, China. Her research interests mainly focus on the design and fabrication of novel carbon nanostructures for energy conversion and storage.
Shenglin Xiong received his PhD degree in inorganic chemistry from the University of Science & Technology of China in 2007. He worked at National University of Singapore from 2009 to 2011 as a Research Fellow. He is now a professor at the School of Chemistry and Chemical Engineering, Shandong University. His research interests include the design and development of micro/nanostructured composite materials and their applications in energy storage and conversion.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Huang, M., Yang, J., Xi, B. et al. Enhancing kinetics of Li-S batteries by graphene-like N,S-codoped biochar fabricated in NaCl non-aqueous ionic liquid. Sci. China Mater. 62, 455–464 (2019). https://doi.org/10.1007/s40843-018-9331-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-018-9331-x