Abstract
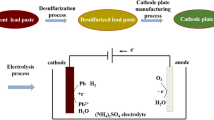
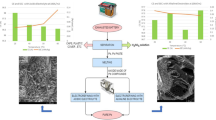
The recovery of lead from spent lead acid battery paste (SLP) is not only related to the sustainable development of the lead industry, but also to the sustainable evolution environment. An innovative process is proposed for the recovery of high purity metallic lead from spent lead acid battery paste (SLP) by electrodeposition at 333–353 K in choline chloride-urea deep eutectic solvent (ChCl-urea DES). The electrochemical behavior of SLP on low carbon steel (LCS) electrode has been investigated by cyclic voltammetry and chronoamperometry measurement. Results imply that Pb(IV) could be reduced preferentially to Pb(II) by ChCl-urea DES, which possesses a strong reducibility. Then the reduction of Pb(II) is a diffusion-controlled quasi-reversible process and the diffusion coefficient increases gradually from 2.70 × 10–8 cm2/s at 333 K to 6.79 × 10–8 cm2/s at 353 K. The initial nucleation stage of lead on LCS electrode is a three-dimensional instantaneous nucleation process. In addition, the effects of temperature on the current efficiency and specific energy consumption during the recovery of lead have been discussed at 2.5 V for 1 h. The current efficiency is about 90.73% and the specific energy consumption is as low as 712.83 kW h/t at 353 K. Rod-shaped and fern-like lead powders can be obtained with a face-centered cubic structure and the predominately oriented crystallite varies from (200) at 333 K to (111) plane at 343 K and 353 K. This new system can efficiently shorten the production flow, lower the requirement for equipment, and avoiding emission of lead effluent and harmful gases. This article provides a green extraction method of lead and a theoretical guidance for the electrochemical extraction of valuable metals from spent battery materials in close neutral electrolyte.
Graphical Abstract









Similar content being viewed by others
References
Yu WH, Zhang PY, Yang JK, Li MY, Hu YC, Liang S, Wang JX, Li SY, Xiao KK, Hou HJ, Hu JP, Kumar RV (2019) A low-emission strategy to recover lead compound products directly from spent lead-acid battery paste: key issue of impurities removal. J Clean Prod 210:1534–1544
Ciro E, Lupi C, Mondal A, Pilone D (2021) Novel lead battery recycling process combining pyrometallurgical anode preparation and electrorefining. J Sustain Metall 7:1727–1735
Zuev D (2018) Introduction: towards an understanding of e-bike mobility in China. In: Urban mobility in modern China. Palgrave Macmillan, Cham
Tian X, Gong Y, Wu YF, Agyeiwaa A, Zuo TY (2014) Management of used lead acid battery in China: secondary lead industry progress, policies and problems. Resour Conser Recyl 93:75–84
Zan X, Zhang D (2022) Analysis on the optimal recycling path of Chinese lead-acid battery under the extended producer responsibility system. Sustainability 14:4950
Li Y, Yang SH, Taskinen P, He J, Liao FW, Zhu RB, Chen YM, Tang CB, Wang YJ, Jokilaakso A (2019) Novel recycling process for lead-acid battery paste without SO2 generation-reaction mechanism and industrial pilot campaign. J Clean Prod 217:162–171
Liang J, Mao JS (2015) Lead anthropogenic transfer and transformation in China. Trans Nonferr Metal Soc 25:1262–1270
Chen K, Huang L, Yan BZ, Li HB, Sun H, Bi J (2014) Effect of lead pollution control on environmental and childhood blood lead level in Nantong, China: an interventional study. Environ Sci Technol 48:12930–12936
Ministry of Natural Resources of the People’s Republic of China (2020) Ministry of natural resources. Geological Publishing House, Beijing
Zhang W, Yang JK, Wu X, Hu YC, Yu WH, Wang JX, Dong JX, Li MY, Liang S, Hu JP, Kumar RV (2016) A critical review on secondary lead recycling technology and its prospect. Renew Sustain Energy Rev 61:108–122
Li MY, Yang JK, Liang S, Hou HJ, Hu JP, Liu BC, Kumar RV (2018) Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. J Power Sources 436:226853
Pan JQ, Sun YZ, Li W, Knight J, Manthiram A (2013) A green lead hydrometallurgical process based on a hydrogen-lead oxide fuel cell. Nat Commun 4:2178
Zhu ZL, Liu H, Chen JSY, Kong H, Xu L, Hua ZS, Zhao Z (2020) Electrochemical behavior and electrolytic preparation of lead in eutectic NaCl-KCl melts. Trans Nonferr Met Soc 30:2568–2576
Li Y, Yang SH, Taskinen P, He J, Chen YM, Tang C, Jokilaakso A (2019) Recycling of spent lead-acid battery for lead extraction with sulfur conservation. JOM 72:3186–3194
Rabah MA, Barakat MA (2001) Energy saving and pollution control for short rotary furnace in secondary lead smelters. Renew Energy 23:561–577
Tian X, Wu YF, Hou P, Liang S, Qu S, Xu M, Zuo TY (2017) Environmental impact and economic assessment of secondary lead production: comparison of main spent lead-acid battery recycling processes in China. J Clean Prod 144:142–148
Liu WF, Deng XB, Zhang DC, Yang TZ, Chen L (2018) A clean process of lead recovery from spent lead paste based on hydrothermal reduction. Trans Nonferr Metal Soc 28:2360–2367
Pan JQ, Zhang C, Sun YZ, Wang ZH, Yang YS (2012) A new process of lead recovery from waste lead-acid batteries by electrolysis of alkaline lead oxide solution. Electrochem Commun 19:70–72
Xing P, Wang C, Wang L (2019) Hydrometallurgical recovery of lead from spent lead-acid battery paste via leaching and electrowinning in chloride solution. Hydrometallurgy 189:105134
Zhang X (2017) A clean and highly efficient leaching-electrodeposition lead recovery route in HClO4 solution. Int J Electrochem Sci 12:6966–6979
Pan JQ, Zhang X, Sun YZ, Song S, Li W, Wan PY (2016) Preparation of high purity lead oxide from spent lead acid batteries via desulfurization and recrystallization in sodium hydroxide. Ind Eng Chem Res 55:2059–2068
Liu K, Yang JK, Liang S, Hou HJ, Chen Y, Wang JX, Liu BC, Hu JP, Wang J (2018) An emission-free vacuum chlorinating process for simultaneous sulfur fixation and lead recovery from spent lead-acid batteries. Environ Sci Technol 52:2235–2241
Liu K, Liang S, Wang JX, Hou HJ, Yang JK, Hu JP (2018) Synthesis of the PbS dendritic nanostructure recovered from a spent lead-acid battery via an integrated vacuum chlorinating and hydrothermal process. ACS Sustain Chem Eng 6:17333–17339
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147
Paiva A, Craveiro R, Aroso I, Martins RLM, Reis RL, Duarte ARC (2014) Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain Chem Eng 2:1063–1071
Abbott AP, Capper G, Davies DL, Mckenzie KJ, Obi SU (2006) Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data 51:1280–1282
Ru JJ, Hua YX, Xu CY, Li J, Li Y, Wang D, Qi CC, Jie YF (2015) Morphology-controlled preparation of lead powders by electrodeposition from different PbO-containing choline chloride-urea deep eutectic solvent. Appl Surf Sci 335:153–159
Tan S, Bedoya FE, Hallett JP, Kelsall GH (2021) Evaluation of N, N, N-dimethylbutylammonium methanesulfonate ionic liquid for electrochemical recovery of lead from lead-acid batteries. Electrochim Acta 376:137893
Liao YS, Chen PY, Sun IW (2016) Electrochemical study and recovery of Pb using 1:2 choline chloride/urea deep eutectic solvent: a variety of Pb species PbSO4, PbO2, and PbO exhibits the analogous thermodynamic behavior. Electrochim Acta 214:265–275
Wang SB, Zhang ZT, Lu ZG, Xu ZH (2020) A novel method for screening deep eutectic solvent to recycle cathode of Li-ion batteries. Green Chem 14:1–10
Jagadeeswara RC, Venkatesan KA, Nagarajan K, Srinivasan TG (2009) Electrochemical behavior of europium (III) in N-butyl-N-methylpyrrolidinium bis (trifluoromethylsulfonyl) imide. Electrochim Acta 54:4718–4725
Bard AJ, Faulkner LR (2001) Fundamentals and applications. In: Electrochemical methods. Wiley, New York
Katayama Y, Fukui R, Miura T (2013) Electrodeposition of lead from 1-butyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)amide ionic liquid. J Electrochem Soc 160:D251–D255
Liu A, Shi Z, Reddy RG (2017) Electrodeposition of Pb from PbO in urea and 1-butyl-3-methylimidazolium chloride deep eutectic solutions. Electrochim Acta 251:176–186
Wong SM, Abrantes LM (2006) Lead electrodeposition from very alkaline media. Electrochim Acta 51:619–626
Gu YY, Zhou QH, Yang TZ, Liu W, Zhang DC (2011) Lead electrodeposition from alkaline solutions containing xylitol. Trans Nonferr Met Soc China 21:1407–1413
Cao XZ, Xu LL, Shi YY, Wang YW, Xue XX (2019) Electrochemical behavior and electrodeposition of cobalt from choline chloride-urea deep eutectic solvent. Electrochim Acta 295:550–557
Scharifker B, Hiills G (1983) Theoretical and experimental studies of multiple nucleation. Electrochim Acta 28:879–889
Yue D, Jia Y, Yao Y, Sun J, Jing Y (2012) Structure and electrochemical behavior of ionic liquid analogue based on choline chloride and urea. Electrochim Acta 65:30–36
Ru J, Hua Y, Wang D, Xu C, Li J, Li Y, Zhou Z, Gong K (2015) tableof in situ electrochemical reduction of solid PbO to lead in ChCl-EG deep eutectic solvent. Electrochim Acta 186:455–464
Haerens K, Matthijs E, Binnemans K, Van der Bruggen B (2009) Electrochemical decomposition of choline chloride based ionic liquid analogues. Green Chem 11:1357–1365
Popov KI, Pavlovic MG (1993) Electrodeposition of metal powders with controlled particle grain size and morphology. In: Modern aspects of electrochemistry. Springer, New York
Acknowledgements
The authors appreciate the financial support from the Yunnan Fundamental Research Projects (Grant No. 202101AT070425), National Natural Science Foundation of China (51604136), and Kunming University of Science and Technology Analysis and Test Fund (2019M20192202080).
Author information
Authors and Affiliations
Contributions
XG contributed to writing-original draft. JR contributed to methodology. YH contributed to conceptualization. WZ contributed to data curation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Hojong Kim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geng, X., Ru, Jj., Hua, Yx. et al. The Recovery of Lead from Spent Lead Acid Battery Paste by Electrodeposition in Deep Eutectic Solvent. J. Sustain. Metall. 8, 1257–1268 (2022). https://doi.org/10.1007/s40831-022-00563-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00563-3