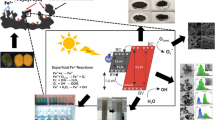
Abstract
Materials based on iron oxide are still the most widely used red pigments. Although the synthetic iron oxide pigment industry has matured, there have been continuous efforts for more circular economy-based methods for the production of pigments. In this study, hematite has been extracted by a hydrothermal method from mill-scale steel slag and the brown–red pigment has been prepared. The extraction was done using sulfuric acid as a leaching agent; the addition of hydrogen peroxide favors the transformation of Fe2+ into Fe3+. The total conversion in the process was tested using potassium dichromate. The pH was stabilized by ammonia, in the process, the brown precipitate was formed in the solution, then the precipitate was filtered using Whatman filter paper and dried to be a powder. The extracted powder was annealed in an air atmosphere at temperatures ranging from 700 to 1000 °C. XRD, SEM with EDS, UV–VIS-NIR, and CIE-L*a*b* were used for examining the annealing temperature effect on the coloring property of the extracted powder pigment. The results show that by adjusting the annealing temperature, a color chart ranging from brown to red can be obtained, which means, an increase in temperature causes the change in particle size and this change influenced the pigment to be changed from a light-red-brown to a dark brown. In the end, the findings presented in this study provide new pathways for the global supply of red pigment using the circular economy approach.
Graphical Abstract








Similar content being viewed by others
References
Schwarz M, Veverka M, Michalková E et al (2012) Utilization of industrial waste for ferrite pigments production. Versita Chem Pap 66:248–258. https://doi.org/10.2478/s11696-012-0154-2
Martín MI, López FA, Torralb JM (2012) Production of sponge iron powder by reduction of rolling mill scale. Ironmak Steelmak 39:155–162. https://doi.org/10.1179/1743281211Y.0000000078
Gaballah NM, Zikry AF, Khalifa MG et al (2013) Production of Iron from Mill Scale Industrial Waste via Hydrogen. Open J Inorg Non-Met Mater 3:23–28. https://doi.org/10.4236/ojinm.2013.33005
Bertelsmann-Scott T (2020) The circular economy: including Africa in Europe’s Circle, no. September. https://saiia.org.za/research/the-circular-economy-including-africa-in-europes-circle/
Mehta N, Dino GA, Passarella I et al (2020) Assessment of the possible reuse of extractive waste coming from abandoned mine sites: case study in Gorno, Italy. Sustain 12:1–22. https://doi.org/10.3390/su12062471
Mehta N, Dino GA, Ajmone-Marsan F et al (2018) Extractive waste management: a risk analysis approach. Sci Total Environ 622–623:900–912. https://doi.org/10.1016/j.scitotenv.2017.11.260
African Development Bank takes steps to accelerate the circular economy in Africa. https://www.afdb.org/en/news-and-events/african-development-bank-takes-steps-accelerate-circular-economy-africa-40033
Schoeman Y, Oberholster P, Somerset V (2021) A decision-support framework for industrial waste management in the iron and steel industry: a case study in Southern Africa. Case Stud Chem Environ Eng 3:100097. https://doi.org/10.1016/j.cscee.2021.100097
Prim SR, Folgueras MV, de Lima MA et al (2011) Synthesis and characterization of hematite pigment obtained from a steel waste industry. J Hazard Mater 192:1307–1313. https://doi.org/10.1016/j.jhazmat.2011.06.034
Hashimoto H, Inada H, Okazaki Y et al (2020) Bright yellowish-red pigment based on hematite/alumina composites with a unique porous disk-like structure. ACS Omega 5:4330–4337. https://doi.org/10.1021/acsomega.9b04297
Fenga F, Lia C, Jianb J et al (2019) Boosting hematite photoelectrochemical water splitting by decoration of TiO2 at the grain boundaries. Chem Eng J 368:959–967. https://doi.org/10.1016/j.cej.2019.03.005
Ma T, Zheng L, Zhao Y et al (2019) Highly porous double-shelled hollow hematite nanoparticles for gas sensing. ACS Appl Nano Mater 2:2347–2357. https://doi.org/10.1021/acsanm.9b00228
Miri A, Khatami M, Sarani M (2020) Biosynthesis, magnetic and cytotoxic studies of hematite nanoparticles. J Inorg Organomet Polym Mater 30:767–774. https://doi.org/10.1007/s10904-019-01245-6
Russell A (1999) Norganic pigments industry worldwide. Mater Technol 14:227–230. https://doi.org/10.1080/10667857.1999.11752843
Thejus PK, Krishnapriya KV, Nishanth KG (2021) A cost-effective intense blue color inorganic pigment for multifunctional cool roof and anti-corrosive coatings. Sol Energy Mater Sol Cells 219:110778. https://doi.org/10.1016/j.solmat.2020.110778
Kuskova YV, Kuskov VB (2017) Development of technology for the production of natural red iron oxide pigments. Inz Miner 1:217–220
Liu M, Ma G, Zhang X et al (2020) Preparation of black ceramic tiles using waste copper slag and stainless steel slag of electric arc furnace. Materials 13:1–12. https://doi.org/10.3390/ma13030776
Leskelä T, Leskelä M (1984) Preparation of yellow and red iron oxide pigments from iron(II) sulfate by alkali precipitation. Thermochim Acta. https://doi.org/10.1016/0040-6031(84)87056-2
M Gislev, M. Grohol (2018) European Commission, Report on Critical Raw Materials in the Circular Economy. https://doi.org/10.2873/331561
Castagnotto E, Locardib F, Slimani S et al (2021) Characterization of the Caput Mortuum purple hematite pigment and synthesis of a modern analog. Dye Pigm 185:108881. https://doi.org/10.1016/j.dyepig.2020.108881
Akinwekomi V, Maree JP, Zvinowanda C et al (2017) Synthesis of magnetite from iron-rich mine water using sodium carbonate. J Environ Chem Eng 5:2699–2707. https://doi.org/10.1016/j.jece.2017.05.025
Quadros F, Wolfart K, Müller M (2017) Sustainable innovative method to synthesize different shades of iron oxide pigments. Dye Pigm 137:403–409. https://doi.org/10.1016/j.dyepig.2016.10.024
Ryan MJ, Kney AD, Carley TL (2017) A study of selective precipitation techniques used to recover refined iron oxide pigments for the production of paint from a synthetic acid mine drainage solution. Appl Geochem 79:27–35. https://doi.org/10.1016/j.apgeochem.2017.01.019
Li X, Wang C, Zeng Y et al (2016) Bacteria assisted preparation of nano α-Fe 2O3 red pigment powders from waste ferrous sulfate. J Hazard Mater 317:563–569. https://doi.org/10.1016/j.jhazmat.2016.06.021
Lu Y, Xu J, Wang W et al (2020) Synthesis of iron-red hybrid pigments from oil shale semi-coke waste. Adv Powder Technol 31:2276–2284. https://doi.org/10.1016/j.apt.2020.03.020
Shena L, Qiao Y, Guo Y et al (2010) Preparation of nanometer-sized black iron oxide pigment by recycling of blast furnace flue dust. J Hazard Mater 177:495–500. https://doi.org/10.1016/j.jhazmat.2009.12.060
Du M, Du Y, Chen Z et al (2017) Synthesis and characterization of black ceramic pigments by recycling of two hazardous wastes. Appl Phys A Mater Sci Process. https://doi.org/10.1007/s00339-017-1181-1
Liu B, Zhang S, Pan D et al (2016) Synthesis and characterization of micaceous iron oxide pigment from oily cold rolling mill sludge. Procedia Environ Sci 31:653–661. https://doi.org/10.1016/j.proenv.2016.02.121
Akinwekomi V, Maree JP, Masindi V et al (2020) Beneficiation of acid mine drainage (AMD): a viable option for the synthesis of goethite, hematite, magnetite, and gypsum—gearing towards a circular economy concept. Miner Eng 148:1–9. https://doi.org/10.1016/j.mineng.2020.106204
Klupp Taylor RN, Seifrt F, Zhuromskyy O et al (2011) Painting by numbers: nanoparticle-based colorants in the post-empirical age. Adv Mater 23:2554–2570. https://doi.org/10.1002/adma.201100541
Costa TC, Soares PA, Campos CEM et al (2019) Industrial steel waste as an iron source to promote heterogeneous and homogeneous oxidation/reduction reactions. J Clean Prod 211:804–817. https://doi.org/10.1016/j.jclepro.2018.11.201
Ali A, Ahmed IS (2019) Sol-gel auto combustion fabrication and optical properties of cobalt orthosilicate: Utilization as a coloring agent in polymer and ceramic. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2019.121888
Lassoued A, Dkhil B, Gadri A et al (2017) Control of the shape and size of iron oxide (α- Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys 7:3007–3015. https://doi.org/10.1016/j.rinp.2017.07.066
Lassoued A, Lassoued MS, Dkhil B et al (2018) Synthesis, photoluminescence, and magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys E Low-Dimens Syst Nanostruct 101:212–219. https://doi.org/10.1016/j.physe.2018.04.009
Ozdemir O, Banerjee SK (1984) High temperature stability of maghemite. Geophys Res Lett 11:161–164
XiangXuLi BHW (2008) Effects of calcination temperature on the performance of nano-sized iron oxide catalysts for ethylbenzene dehydrogenation. Akad Kiado 94:175–182. https://doi.org/10.1007/s11144-008-5269-7
Phu ND, Ngo DT, Hoang LH et al (2011) Crystallization process and magnetic properties of amorphous iron oxide nanoparticles. J Phys D Appl Phys 44:1–15. https://doi.org/10.1088/0022-3727/44/34/345002
Nguyen DP, Tran QT, Trinh XS et al (2012) Crystallization and magnetic properties of amorphous iron-chromium oxide nanoparticles synthesized by sonochemistry. Adv Nat Sci Nanosci Nanotechnol 3:1–5. https://doi.org/10.1088/2043-6262/3/1/015017
Tamura K, Kunoh T, Nagaoka N et al (2020) High-quality inorganic red pigment prepared by aluminum deposition on biogenous iron oxide sheaths. ACS Appl Bio Mater. https://doi.org/10.1021/acsabm.0c00476
Kabir MH, Ali MM, Kaiyum MA, Rahman MS (2019) Effect of annealing temperature on structural morphological and optical properties of spray pyrolyzed Al-doped ZnO thin films. J Phys Commun. https://doi.org/10.1088/2399-6528/ab496f
Dehbi A, Dehmani Y, Oma H et al (2020) Hematite iron oxide nanoparticles (α-Fe2O3): Synthesis and modeling adsorption of malachite green. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103394
Lassoued A, Lassoued MS, Dkhil B et al (2018) Synthesis, structural, morphological, optical, and magnetic characterization of iron oxide (α-Fe2O3) nanoparticles by precipitation method: effect of varying the nature of precursor. Phys E Low-Dimens Syst Nanostruct 97:328–334. https://doi.org/10.1016/j.physe.2017.12.004
Opuchovic O, Kareiva A (2015) Historical hematite pigment: Synthesis by an aqueous sol-gel method, characterization, and application for the coloration of ceramic glazes. Ceram Int 41:4504–4513. https://doi.org/10.1016/j.ceramint.2014.11.145
Khoiroh LM, Mardiana D, Sabarudin A et al (2013) Synthesis of hematite pigments (alpha-Fe2O3) by thermal transformations of FeOOH. J Pure Appl Chem Res 2:27–34
Ahmed NM, Selim MM (2005) Enhancement of properties of red iron oxide—aluminium oxide solid solutions anticorrosive pigments. Pigm Resin Technol 34:256–264. https://doi.org/10.1108/03699420510620265
Lua Y, Donga W, Wang W et al (2019) A comparative study of different natural palygorskite clays for fabricating cost-efficient and eco-friendly iron-red composite pigments. Appl Clay Sci 167:50–59. https://doi.org/10.1016/j.clay.2018.10.008
Acknowledgements
This work was supported by the Emerging Technology Institute, the Estonian Research Council (ETAG) through the PSG 466 (R.E. Rojas-Hernandez), and the ETAG through PRG643 (I. Hussainova). The authors also acknowledge Thomas C. Alex from NML, India for DT-TGA, DLS, and ICP-MS characterization support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eticha, Z.G., Rojas-Hernandez, R.E., Olu, F.E. et al. Effect of Annealing Temperature of Brownish-Red Pigment Based on Iron Oxide Extracted by Hydrothermal Route from Mill-Scale Steel Slag. J. Sustain. Metall. 8, 218–227 (2022). https://doi.org/10.1007/s40831-021-00470-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00470-z