Abstract
Gasoline particulate filters are one potential technology path to address the EU6 particulate number regulation for gasoline-powered vehicles. While the passive and active soot regeneration with diesel particulate filters is well understood, almost no data exist under gasoline engine conditions where there is essentially no NO2 and O2 available for soot oxidation under normal stoichiometric λ = 1 operation. The aim of this paper is to provide information and data to address these gasoline engine-specific conditions. The operation and conditions for a particulate filter in a gasoline direct injection engine environment are discussed utilizing results obtained in a broad range of laboratory and vehicle experiments. Since the accumulation of sufficient soot mass quantities proofed to be challenging on a vehicle, the present study is using Printex® U as surrogate soot, representing the least reactive fraction of particulate matter originating from the combustion. Conditions which enable the oxidation of soot under normal driving operation are identified. Conditions enabling passive soot oxidation in a gasoline engine exhaust system are proposed as well as potential worst case conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, passenger cars powered by modern diesel engines are all equipped with diesel particulate filters and their particulate mass (PM) and number (PN) emissions are well below the regulated limit of 4.5 mg/km and 6 × 1011 #/km, respectively, as defined by the European EU5 and EU6 regulations. Gasoline engines based on port fuel injection typically have particle number emissions which are also below this limit [1]. Gasoline direct injection engines (GDI), however, were found to exceed this limit, in many cases significantly. PN emissions in the range of 8 × 1011 to 40 × 1011 #/km are reported [1–4]. The market share of GDI engines is expected to increase dramatically over the next years due to their superior fuel economy and lower CO2 emissions [5]. This trend has raised significant concern since particulates from combustion engines are considered as carcinogenic by the World Health Organization (WHO) [6, 7], and the emissions of particulates occur along the roadside in urban areas with direct impact on the people who live and work there [8]. One solution to address the concern over the particulate emissions of GDI-powered vehicles is the use of a particulate filter. Examples for concepts based on this approach demonstrating the general technical feasibility have been reported recently [9–12].
The management of the soot accumulated within a particulate filter during operation has been subject to significant development work in the case of diesel engines, i.e., [13]. Key steps in the soot management are (a) the monitoring of the mass of soot accumulated and (b) the control of the mass of soot within defined limits. The latter is essential to avoid excessive buildup of pressure drop or the risk of runaway soot oxidation conditions which could result in a damage of components [13]. While the situation is understood for diesel applications, many unknowns exist for gasoline engines. The mass-based particulate matter emissions of direct injection gasoline engines are typically well below those of diesel engines; however, conditions exist under which soot can be accumulated. Examples are repeated cold starts at very low temperatures with only short trips in between or extended driving at very high load without any fuel cut, i.e., motoring of the engine, during which oxygen would be provided to the exhaust. Based on the low rates of emissions observed, current assumptions and limited data available suggest that under severe conditions particulate matter quantities in the range of 2–5 g might accumulate. Particulate matter from internal combustion engines typically contains volatile components as well as elemental carbon (EC). Wang et al. showed that for GDI engines, the volatile components can represent as much as 65–70 % of the total particulate matter [24]. In general, the composition and morphology of the particulate matter depends on the conditions during its generation as well as the exposure during operation. The main concern from an application perspective is the elemental carbon fraction, since it represents the least reactive components and cannot be evaporated during operation. Therefore, the present paper is focused on the elemental carbon fraction and, for simplicity, will we frequently reference it as “soot”. More work is needed to better quantify the particulate mass that can be accumulated and its composition, but this is outside the scope of the present paper. What we want to discuss in this contribution is the situation in which soot has been accumulated within a gasoline particulate filter, independent of how this was achieved, and address the questions related to the potential for passive soot oxidation mechanisms under gasoline engine conditions.
Passive soot oxidation refers to conditions under which soot is oxidized during normal engine operation, i.e., without an active change in the engine controls to create conditions more favorable for soot oxidation. In diesel applications, passive soot oxidation occurs at appreciable rates by oxidizing soot with NO2 present in the exhaust gas at temperatures in the range of 300–450 °C, which can be achieved under some medium load conditions. Although there is excess oxygen in diesel exhaust, the typical exhaust gas temperatures of 150–550 °C do not allow for high enough reactions rates to compensate for the rate at which soot is emitted by the diesel engine. Due to the limited availability of NO2 and the low temperatures in diesel passenger car applications, passive soot oxidation is often insufficient, requiring periodic active regeneration steps in which the exhaust gas temperature is elevated to 550–650 °C by means of modified engine operation, accelerating the rate at which the excess oxygen reacts with soot.
In gasoline applications, the exhaust gas temperatures are typically higher compared to diesel. However, due to the high efficiency of the upstream or integrated three-way catalyst (TWC), essentially no NO2 is available for the passive soot oxidation known from diesel applications. In addition, in normal stoichiometric λ = 1 operation, there also is no appreciable oxygen available as oxidant. Oxygen is available only during fuel cuts (i.e., motoring, where the engine pumps air from the intake to the exhaust). This is shown in Fig. 1, providing examples of the transient exhaust gas temperature and oxygen concentration in city and highway driving with a DI gasoline engine-powered passenger car. Temperatures are shown for two positions in the exhaust line, close-coupled to the turbocharger (T4) and in an underfloor position (T5), representing the locations currently considered for gasoline particulate filters [9]. The city driving is characterized by frequent short fuel cuts and temperatures that exceed those with diesel engines under similar driving profiles, but which are still well below what would be considered high enough for appreciable soot oxidation in diesel systems. The highway driving shows higher temperatures but less frequent and often fairly short fuel cuts.
In this paper, we will discuss that, despite their typically short duration of only a few seconds, fuel cuts represent a mechanism for passive soot oxidation in gasoline applications, even in city driving with relatively low exhaust temperatures. Fuel cuts also represent a condition for a potential worst case scenario that can lead to high temperatures inside the filter if high soot loads are present and the fuel cut occurs during high load operation.
2 Experimental
2.1 Thermo-Gravimetric Analysis of Soot Oxidation
Thermo-gravimetric characterization was performed using a NETZSCH STA 409 C/CD. The majority of experiments were run using Printex U as surrogate soot with high elemental carbon content. In addition, some reference measurements have been performed with particulate matter collected on a GDI engine. The sample mass of soot was typically between 13 and 14 mg, placed in an alumina crucible. Experiments were performed using synthetic air at a gas flow rate of 100 ml/min. Operation was either isothermal or at constant heating rate of 5 K/min. The isothermal measurement comprised an initial drying step in nitrogen atmosphere, a rapid heating to the target temperature, and then a 600-min hold at the target temperature, followed by a final burn out to determine any inorganic residue. The duration of the hold at the target temperature was selected to allow for a sufficient change in sample mass even at low temperatures.
2.2 Laboratory Reactor Experiments
Transient soot oxidation experiments were performed using an adiabatic laboratory reactor. The reactor is designed to operate with 2 in. (50.8 mm) diameter particulate filter samples. Experiments have been performed with particulate filter samples of 4.5–6 in. (114.3–152.4 mm) length. The properties of the filter technologies used are summarized in Table 1. Both are advanced products developed specifically for gasoline particulate filter applications [9]. The reactor is connected to different gas lines, enabling operation under different model exhaust gas conditions. The feed gas flow rate and composition is controlled by mass flow controllers. In our experiments, the reactor was operated with nitrogen, simulating engine operation at stoichiometric combustion conditions (λ = 1), and compressed air, simulating a fuel cut during motoring. The flow rates during the fuel cut were scaled down to represent operation of a full size 4.66 in. diameter particulate filter mounted to a typical four cylinder gasoline engine operating at 17 and 50 kg/h during the fuel cut. The temperature of the feed gas can be heated using an electrical heater. The maximum temperature that could be achieved was 700 °C, which is reasonably representative of the exhaust temperature directly downstream of a turbocharger during a rapid acceleration. Prior to the soot oxidation experiments, the filter samples were loaded with a predefined mass of Printex U soot utilizing a dedicated separate soot loading rig.
The reactor exhaust was connected to a Horiba emission bench, measuring O2, CO, and CO2 to monitor the soot oxidation process. In addition, up to nine thermocouples (K-type, 0.5 mm) were inserted into the filter from the outlet channels. This enabled the measurement of transient filter temperatures at different axial and radial locations.
Figure 2 shows an example of a typical simulated fuel cut experiment, representative of gasoline engine operation. A typical experiment starts with the heating of the soot-loaded sample under constant nitrogen flow (no soot oxidation) to the target temperature of the experiment (note, heat up phase not shown in Fig. 2). This operation under nitrogen simulates the normal stoichiometric engine operation. Then, a fuel cut is simulated by switching the feed gas to synthetic air for a defined period of time (e.g., 5–120 s). This can be seen in the example provided in Fig. 2 at t ∼ 600 s. The mass flow during the fuel cut is reduced in the example shown, copying the conditions observed in one of our test vehicles. Other experiments have been performed with constant mass flow. The oxidation of soot during this fuel cut or oxygen pulse and the resultant exothermic heat release can be seen from the increase in local filter temperatures. The electric heater remains on for the entire experiment.
2.3 Vehicle Experiments
For the vehicle experiments, modern EU5-certified GDI vehicles (C segment, four cylinder) were used. The vehicles were operated with the basic engine controls and calibration developed for operation with TWC only. No strategies or controls of a gasoline particulate filter (GPF) were available or added.
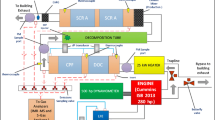
The vehicles were equipped with on-board data acquisition system, monitoring a number of relevant engine parameters available from the ECU and obtained via the OBD interface as well as data from additional sensors installed on the vehicle. This included the measurement of the intake air mass flow, several pressures measured along the exhaust system, additional oxygen measurements via lambda probes as well as temperatures at different locations of the exhaust and inside the GPF. A schematic of the instrumentation is provided in Fig. 3.
Operation of the vehicles was done on public roads by driving under different statistically defined mission profiles such as (slow) urban, mixed, or highway. Examples of the operating statistics of the different driving profiles are provided in Table 2. Examples of transient readings for some of the cycles were already shown in Fig. 1.
In the experiments to study the soot oxidation under transient vehicle operation, the canned GPFs were initially loaded externally with a predefined mass of soot (Printex U) using a laboratory soot loader. The soot-loaded filter was then installed onto the vehicle, either in a close-coupled or an underfloor position. The vehicle with the soot-loaded GPF installed was then operated over a predefined distance according to the respective driving profile. Typical operation was over one or two shifts per day, comprising only few cold starts. To determine the amount of soot oxidized, the vehicle was intermittently stopped and the filter was removed from the vehicle for weighing. The filter was weighed hot at a defined temperature >150 °C to minimize the impact of moisture absorbed onto the soot or catalyst coating. After the weighing, the filter was reinstalled onto the vehicle and the mileage accumulation continued. The distance between two measurements was dependent on the driving profile and exhaust conditions.
For the experiments discussed in this paper, no information was available for the transient particulate matter raw emissions coming from the engine. Measurements performed with the test vehicles on chassis dyno over the New European Drive Cycle (NEDC) suggest raw emissions in the range of 0.5–1 mg/km.
2.4 Modeling
To facilitate the analysis and interpretation of the results obtained as well as to enable projections for soot oxidation under conditions outside the experimentally covered parameter space, detailed mathematical modeling was applied. For the simulations, the commercial software AxiSuite was used [15], representing a widely used tool validated broadly across the industry and academia involved in mobile exhaust gas treatment. A detailed description of the implemented mathematical model and numerical methods can be found for example in references [15, 16]. In brief, the simulations reported in this paper utilized the AxiTrap software module representing a dynamic, one, or multidimensional filter model, considering for the conservation of mass, energy, and momentum in the inlet and outlet channels as well as the porous wall phase. All relevant mass, heat, and momentum transport phenomena are described based on state of the art correlations. The specific filter technologies are defined using the relevant physical material properties.
With respect to reactions, the present work is focused on the oxidation of soot only. The soot oxidation kinetics were calibrated to the experimental data using the generic rate expression implemented in AxiSuite. Details will be discussed in the results section. Gas reactions, although well represented by state of the art kinetic rate expressions in AxiSuite, were in most cases not considered since no transient gas phase concentrations were available during the vehicle operation.
3 Results
3.1 Soot Oxidation Kinetics
The focus of this paper is aimed at providing an insight into the conditions that enable oxidation of soot accumulated in a gasoline particulate filter. As mentioned above, soot in the context of this work is defined as the elemental carbon portion of particulate matter emitted by the engine. The understanding of the soot oxidation kinetics at the prevalent conditions, especially with respect to temperatures, is essential. In general, the oxidation of soot with oxygen can be described by the following simple rate equation:
Here, m s represents the soot mass, p O2 the oxygen partial pressure, t the time, k the reaction rate constant, A the frequency factor, E a the activation energy, R the gas constant and T the temperature. n and r describe the reaction order in soot and oxygen, respectively.
Past work on the oxidation of soot was focused on conditions relevant to diesel applications evaluating temperatures in the range of >500 °C to allow for sufficient rates enabling filter regeneration within a few minutes. Examples are provided in references [17–20]. Typically, n = 1 and for diesel soot activation energies of E a = 125–155 kJ/mol are commonly used. For the present work, this has been expanded to temperatures as low as 300 °C, to also capture the soot oxidation by O2 during normal gasoline vehicle operation, including urban driving. In addition, the objective was to obtain kinetic data for the soot used in our laboratory reactor and vehicle experiments.
The low particulate mass emissions of GDI engines make it very challenging to collect gasoline soot on a vehicle. The focus of the present work was the evaluation of conditions that enable soot oxidation in a gasoline environment rather than the detailed impact of the soot composition. Therefore, the present work was performed primarily using Printex U (Degussa) as surrogate soot, known to be representative of diesel soot with high elemental carbon (EC) fraction [21, 22]. Figure 4 shows the comparison of the thermo-gravimetric analysis (TGA) analysis at constant heating rate (5 K/min) in air for Printex U in comparison and a small sample of soot collected on a modern GDI engine. The GDI soot was collected primarily during cold starts and some limited engine operation under certification cycle conditions. The comparison demonstrates that Printex U is indeed a reasonable surrogate showing a similar behavior at the low temperatures of special interest. Choi et al. also found Printex U to behave reasonably similar to GDI soot [23], Choi et al. At higher temperatures, Printex U behaves somewhat less reactive, representing a kind of “worst” case soot. This difference is potentially due to a lower elemental carbon (EC) content of the GDI soot based on the fact that its origin was primarily cold starts (note, Printex U has >98 % EC). Based on this, all further experiments were performed with Printex U.
To study the soot oxidation in air in the temperature range of interest between 300 and 550 °C, isothermal TGA measurements have been performed. Results are shown in Fig. 5a. Figure 5b shows the extracted reaction rates. All data show excellent fit to the rate expression provided above (not shown). Also shown in Fig. 5b is the apparent activation energy for the entire data set (solid line) as well as for the two ends of the experimentally covered temperature range (dashed lines). For the latter, only the two values at the two ends were used, which is not sufficient for a reliable quantification but does allow to get directional results. Using all data, we observe an apparent activation energy of 109 kJ/mol, which is below the value typically observed for diesel applications. However, we can observe that the data are not well represented by one single activation energy and if we consider the high temperature data only (500–550 °C), we obtain a value of 152 kJ/mol, which is consistent with the value known from diesel applications. For the low temperature range, the data suggest a significantly lower activation energy, here as low as 60 kJ/mol between 300 and 350 °C. These observations are consistent with observations made by Koltsakis et al. [14], who report 153 kJ/mol for temperatures above 550 °C and 67 kJ/mol for low temperatures down to 400 °C. To address this observation, these authors proposed a two-step kinetic rate expression considering for a low and a high temperature path, which has been implemented in AxiSuite. For the modeling work and the simulation of the vehicle data reported in the later sections, the two-step kinetic rate expression implemented in AxiSuite has been calibrated to the values found in the TGA studies.
To evaluate a potential impact of the filter material (cordierite) or a three-way catalyst on the soot oxidation, additional TGA experiments have been performed at 450 °C. For these experiments, filter material of an uncoated and a TWC-coated GPF (both cordierite) were ground to powder and mixed uniformly with the soot at a 1:1 weight ratio. This ratio is somehow arbitrary but, in combination with the filter being ground to powder, represents a condition with more contact area between soot and filter material compared to the real application, where the contact area is limited to the geometric filter surface. The results showed no significant effect of the filter and catalyst material, which is likely explained by the fact that there is limited soot-solid contact area to facilitate a direct catalysis. Since the operation was in air, any potential benefit from the oxygen storage material of the TWC on the soot oxidation in a close to λ = 1 operation would not be detected either.
3.2 Laboratory Reactor Experiments Simulating Fuel Cuts
The soot oxidation under highly transient, short fuel cuts as frequently observed in vehicle operation was studied in dedicated laboratory experiments, following the procedure described above with an example shown in Fig. 2. Key experimental variables evaluated were the duration of the oxygen pulse (fuel cut), the soot load, the temperature, the flow rate, the oxygen concentration, and the filter technology. In addition, experiments were performed with uncoated as well as TWC-coated filters.
In Fig. 6, results are shown for simulated fuel cut experiments in which the duration of the oxygen pulse was varied between 10 and 50 s. All data are for the uncoated DuraTrap GC 200/8 technology. Results are shown for two different initial soot loads, 2 and 5 g/l, and two initial temperatures, 650 and 700 °C, both representing temperatures common for a high engine load operation prior to the fuel cut. With respect to the soot loads, it is worth mentioning that the 2 g/l represents the anticipated maximum in use loading level, whereas the 5 g/l represent an extreme case that is unlikely to occur.
The results shown in Fig. 6 generally follow the expected trend. Higher initial soot load, higher initial temperature, and longer periods of oxygen supply result in higher exothermic heat release. The detailed analysis of the data, however, shows that in these examples, the increase in temperature and the oxidation rate are limited by two different parameters. Starting with the data for a soot load of 2 g/l, we observe only a very moderate influence of the initial filter temperature. The offset between the two data sets is more or less maintained independently of the duration of the oxygen pulse. For both data sets, we can also observe that the maximum temperature does initially increase as the period with oxygen supply is extended. However, beyond a duration of 20–30 s, the maximum temperature levels off to about ∼80 K above the initial temperature. The weighing of the samples showed residual soot in the filter, and the plateau is not explained by depletion in soot but rather that the rate of heat release becomes balanced by the convective heat removal with the exhaust gas flow, as also shown for DPF in [13].
The data obtained with the extreme soot load of 5 g/l and the high temperature of 700 °C at first glance look qualitatively similar. We observe an initial lag period, followed by a steep increase in maximum temperature which then levels off to a plateau beyond 30 s of oxygen supply. In this case, the plateau is not determined by the balance between heat release and convective heat removal. Compared to 800 °C, the oxidation rates at 1000–1200 °C are roughly 20–100 times faster, respectively. Under these conditions, the plateau is a result of depletion in soot after a few seconds due to the high rates of oxidation. This can also be derived from the data shown in Fig. 7, where the maximum temperature rise obtained in experiments with the uncoated GC 200/8 filter is plotted vs. the mass of soot oxidized. The latter was determined gravimetrically by weighing the filter samples after the various fuel cut experiments. In case of the experiments in which 1100–1200 °C were reached, the entire 5 g/l of soot were oxidized. The data in Fig. 7 also show that for the 2 g/l experiments, about half of the initial soot load remained after a temperature rise of 80 K. Finally, the evaluation of the results for the extreme soot load of 5 g/l but the lower temperature of 650 °C show conditions somewhat in between those discussed so far. The initial soot oxidation rate is lower, and a longer period exists during which the reaction is quenched before it takes off based on self-acceleration as a result of the heat release. In this case, the plateau is not reached within the experiments shown but one can anticipate that this would be the case after roughly 50 s.
The effect a TWC coating has on the oxidation of soot under these fuel cut conditions at high initial temperatures is shown in Fig. 8. The washcoat applied to the GC HP 300/8 was formulated to provide the entire functionality of a close-coupled TWC in a European EU6 application. Shown in Fig. 8 is the peak temperature observed as a function of the initial soot load in the filter for conditions with 40 s oxygen pulse, an initial temperature of 700 °C, and a flow rate during the fuel cut of 3.1 kg/h. In all cases, the temperatures observed for the TWC-coated filter are higher compared to the uncoated filter. The density and heat capacity of the two uncoated filter technologies is comparable, and with the TWC coating, the GC HP filter actually has a higher specific heat capacity. Therefore, the higher temperatures cannot be attributed to differences in the capacity to absorb heat during the transient event. The explanation of the difference is provided by the data shown in Fig. 9. Transient temperatures along the centerline of the filter samples and exhaust concentrations measured for CO and CO2 are provided for 40 s oxygen pulse experiments. The absolute soot mass on the filters is comparable at roughly 0.52 g but it should be mentioned that the filters had different lengths. From the data shown in Fig. 9, one can observe that for the uncoated filter, the soot (i.e., carbon) is converted to CO and CO2 whereas only CO2 is observed as product for the coated filter. This phenomenon is well known for diesel particulate filters, where already low levels of precious metal catalyst results in any CO formed to be further oxidized to CO2. The higher heat of reaction for the total oxidation vs. the partial oxidation to CO results in a significantly higher amount of energy released per unit of carbon oxidized, leading to higher filter temperatures, which further accelerate the reaction.
With the TWC-coated filter sample, further simulated fuel cut experiments have been performed in which the initial temperature has been varied, covering the range from 475 to 700 °C. Other conditions were maintained constant. Experiments were performed at oxygen pulse durations of 30, 40, and 60 s. The soot load applied in these experiments was ∼1.5–2 g/l. In Fig. 10, the oxidation rates obtained from the integration of the CO2 emissions are plotted vs. 1/T. As reference, some of the TGA data are added as well, showing fair agreement in the measured rates between these two experimental methods. The results are reasonably independent of the duration of oxygen pulse. The observed apparent activation energy of 114 kJ/mol is somewhat on the low end of what was expected for this temperature range.
The results discussed so far demonstrated that at the conditions typical for gasoline applications, appreciable quantities of soot are oxidized during fuel cut events. For the high temperatures of 700 °C, it was shown that during a single oxygen pulse of 30 s (or longer), anywhere between 50 and 90 % of the soot is oxidized. Even at lower temperatures, measurable rates were observed. In realistic vehicle applications, we typically observe many fuel cuts. To assess the regeneration efficiency of such a multitude of fuel cuts, cyclic experiments were performed at intermediate temperatures of 500 and 600 °C. In these experiments, the filters were initially loaded with ∼2 g/l of soot. Then, the filters were heated to the target temperature in nitrogen followed by cyclic exposure to repeated oxygen pulses (i.e., fuel cuts). A total of 140 cycles consisting of 40 s synthetic air followed by 60 s nitrogen was applied. At the end of the experiments, the remaining soot mass was determined gravimetrically. The experiments were performed with two cordierite filter technologies listed in Table 1, a thinwall SiC filter with 300 cpsi cell density and an aluminum titanate (AT) DPF, also with 300 cpsi. All filters used in these experiments were uncoated. The two cordierite filters were used to determine any impact from cell density, 200 vs. 300 cpsi. The SiC and AT filter were used to assess the impact of material density (thermal inertia) and thermal conductivity on the passive soot oxidation during such transient conditions. Both SiC and AT filters had similar mass and volumetric heat capacity, significantly higher compared to the cordierite filters, and the thermal conductivity is high for SiC and low for AT and cordierite. At the higher temperature of 600 °C, all filters had similar high regeneration efficiency of 80–85 %. This efficiency is close to the maximum that can be achieved at these temperatures. Although designed to be adiabatic, there are some heat losses through the periphery of the small filters, resulting in lower temperatures close to the skin. At the lower temperature of 500 °C, which is of significant practical relevance, a clear differentiation was observed. The results showed that 30 % of the soot was regenerated for the two cordierite filters. For the AT filter, 24 % of soot was oxidized, whereas for the SiC filter technology only 18 % of the soot was oxidized. This demonstrates that under transient conditions at low to medium temperatures, the thermal inertia (i.e., mass) of the filter has a clear effect, and lower mass is preferable to maximize the utilization of the proposed passive regeneration mechanism. The comparison of the AT and SiC data, at similar thermal inertia and cell density, show that a low thermal conductivity is preferable, to prevent the axial and radial dissipation of heat.
3.3 Results and On-Road Experience from Vehicle Experiments
The objective of the first set of vehicle experiments was to explore the passive soot oxidation in a gasoline environment as identified in the laboratory experiments discussed above. Focus was on an underfloor installation of a GPF as least favorable filter position with respect to temperatures due to the heat losses. For this vehicle, the turbocharger is towards the front of the engine block and the distance between the turbocharger and filter was about 1400 mm. The first set of vehicle tests were performed during middle European winter conditions with low ambient temperatures between −7 and +3 °C. The filters tested were a GC 200/8 in 4.66 in. diameter by 4.5 in. length and a TWC-coated GC HP 300/8 in 4.66 in. diameter by 6 in. length. Prior to the tests, the filters were loaded to ∼3.3–3.6 g/l of soot (Printex U), resulting in a different initial soot mass of 4.2–4.6g, respectively.
In the first phase, both filters were tested with the vehicle operating in the slow highway mode with a maximum velocity of ∼100 km/h. The typical statistics of this operation were provided in Table 2, and examples for the temperature distribution and the oxygen concentration vs. the temperature at filter inlet are provided in Fig. 11a. The temperature at filter inlet is centered around 500 °C, with a significant time at temperatures well below 500 °C. Some short excursions up to 600 °C occur. From the oxygen concentration data, we can see that they are more or less bimodal (note: every data point corresponds to a measurement, the data acquisition frequency was 1 Hz). Most data are close to 0 % and some are at ∼20 %. The data at ∼0 % are obtained during normal λ = 1 operation, whereas the data at 20 % are from fuel cuts, i.e., short motoring phases during which the intake air is pumped through the engine into the exhaust. The latter are all between 250 and 550 °C, and none is at a higher temperature. In Fig. 12a, the results from the regular weighing of the filter are shown, providing the evolution of the soot mass with mileage (or time). It is obvious that for both filters, the soot mass declines with mileage. In both cases, roughly 50 % of the initial soot mass has been oxidized after 1000 km. It is worth noting that this occurs despite any soot that would be accumulated from the engine emissions, considering that the engine used was a normal EU5 engine with particle number emissions in the order of ∼2 × 1012 #/km. The data suggest that the coated GPF shows a steeper slope and faster oxidation of the soot. To explore this further, in Fig. 12 also shown are data from the detailed numerical simulation with AxiTrap. For the simulations, the transient exhaust temperature, flow, and oxygen concentration were used as boundary conditions. With respect to soot oxidation, only the reaction with oxygen was considered with the kinetics calibrated as discussed above. In the simulations, identical soot oxidation kinetics were used for both filters, i.e., no catalytic effect was considered. Yet they describe the experimental data reasonably well and also show the different slopes. This leads to the conclusion that in our tests, the apparent difference in oxidation rates is actually not due to the coating but rather to the differences in total soot mass. As it was shown above, the reaction rate is proportional to the soot mass (i.e., the surface area for reaction) which is initially higher for the experiment with coated filter.
In the second phase, the same vehicle was operated in a slow city driving mode. The same filters were used and loaded to similar initial soot loads prior to the test. The statistics for the slow city driving were provided in Table 2, and Fig. 11b shows again examples of the temperature distribution and the oxygen vs. temperature plot. One can see that the temperatures at the filter were primarily below 400 °C and never exceeded 500 °C. The oxygen concentration shows much more transients as a result of the frequent accelerations and decelerations, with many short phases of motoring. The results from the soot mass determination vs. mileage are shown in Fig. 12b. Despite the very low exhaust temperatures and the potential of additional soot emitted from the engine, a clear reduction in soot mass can be observed for both filters. The simulations also represent the data well. When comparing the data from city and highway driving, one needs to keep in mind that while similar mileage is shown in the two charts of Fig. 12, it takes about 10–12 h to drive 1000 km in this highway mode whereas the same distance takes 40–50 h in the city mode.
In a third campaign, a different vehicle was tested, again with a filter mounted in an underfloor position. Because of the installation of the turbocharger, the distance to the underfloor filter is shorter in this vehicle and about 700 mm. The ambient temperature during this campaign was at an average of ∼13 °C. The objective of this experiment was to gain additional insight in the soot oxidation during slow urban driving. The filter was again preloaded with 6.5 g of Printex U soot and installed on the vehicle. The driving profile was separated into three discrete phases. During the first phase, the vehicle was operated at an average velocity of ∼29 km/h and a maximum velocity of ∼50 km/h. The second phase had a slightly higher average velocity of ∼35 km/h, and somewhat faster accelerations up to 70 km/h were allowed. The last segment represented very slow operation with an average and maximum velocity of ∼20 and ∼30 km/h, respectively. The transient data from this experiment over a total distance of roughly 2600 km is shown in Fig. 13. The top diagrams show the entire experiment. The transient velocity and filter inlet temperature, averaged over 1000 s for illustration purposes, are shown in the top diagram together with the soot mass determined during the weighing steps and the soot mass evolution from the detailed simulation with AxiTrap. The agreement between the simulated and the experimentally determined soot mass is excellent. In the simulations, the kinetics described in the previous sections, considering the low temperature mechanism, have been used. The analysis of the experimental data from the vehicle experiments also yielded an apparent activation energy of 59 kJ/mol, which is practically identical to the value obtained by TGA (Fig. 5 low temperature region). For this vehicle, some information was available for the engine particulate emissions, which was considered in the simulations. The three phases of the experiment with different average and maximum velocity can be clearly differentiated. One can also see that for this vehicle, the exhaust gas temperature is even lower compared to the previous examples and averages around 350 °C. From the simulated soot mass data, one can identify individual events during which soot is oxidized at higher rate. Details with higher resolution and the data in 1 Hz are provided for two events in the diagrams at the bottom. In both examples, one can clearly correlate the reduction in soot mass with the presence of a fuel cut that lasts for a few seconds and during which the oxygen concentration ramps to ∼20 %.
In a fourth campaign, a TWC-coated filter (GC HP 300/8, ∅4.66 × 6 in.) was installed close-coupled directly at the turbocharger, without a flow through TWC in between. The objective of this testing was to explore the potential worst case soot oxidation in this configuration with a maximum in exhaust temperature. The filter was again loaded with soot (4.8 g) prior to the experiments. The vehicle was then carefully driven to a nearby highway, preventing any fuel cut during the transfer. On the highway, the vehicle was accelerated rapidly, resulting in a rapid increase in exhaust temperature. After a stable, high temperature of ∼700 °C upstream and ∼750 °C inside the GPF was reached, a fuel cut was introduced and maintained for roughly 40 s (at t ∼ 370 s in Fig. 14). The temperatures inside the filter as well as the oxygen concentration up- and downstream of the GPF are shown in Fig. 14. The maximum temperature reached within the filter was ∼1030 °C, which is in excellent agreement to the laboratory test data at similar conditions discussed previously and shown in Fig. 8 (the specific soot load in the vehicle experiments was ∼3 g/l). The experiment was repeated multiple times with filters which were regenerated and then loaded again with 4.4–4.8 g soot (not shown). Consistent results were obtained. From the difference in the oxygen traces in Fig. 14, one can observe the consumption of O2 due to the oxidation of the soot. The low levels at the outlet during the first ∼20 s of the fuel cut as well as the fairly flat temperature traces during this period suggest a limitation in oxygen. Simulations confirmed local depletion of oxygen inside the filter. Although no direct measurement of the soot mass oxidized was available, a second fuel cut, which occurred roughly 300 s after the one shown (at t ∼ 700 s in Fig. 14) under roughly the same conditions, showed no increase in filter temperature and no difference was observed in the oxygen concentration up- and downstream of the filter. This suggests that the majority of the soot had already been oxidized in the one single event, which is in line with the observations from the laboratory experiments at comparable conditions.
4 Discussion
Despite the general lack of oxygen during λ = 1 operation of a gasoline engine and the lack of NO2, as utilized in diesel particulate filters, the data presented in this paper offer an alternative path for passive soot oxidation in a gasoline particulate filter. The short but frequent fuel cut conditions during motoring, when air is pumped through the engine, offer opportunities during which soot accumulated can react with oxygen, regenerating the filter to some extent. The overall soot balance is determined by how far the rate of oxidation exceeds the rate at which soot is emitted by the engine. In the vehicle experiments discussed, including fairly severe conditions of underfloor installations during winter and slow city driving only, the net soot balance was always showing a reduction in soot load.
The rule of thumb, representing common experience in the field of diesel particulate filters, that “soot does not react with oxygen below 550 °C”, has to be revised for gasoline applications. In diesel applications, the raw emissions of the engine are significantly higher compared to gasoline engines. Therefore, higher oxidation rates are required to enable a net reduction in soot load. In addition, since active measures have to be taken in diesel applications, impacting fuel economy and gaseous emissions, there is a significant desire to regenerate soot-loaded particulate filters within a few minutes. In our gasoline vehicle experiments, soot was oxidized utilizing fuel cuts only, even at low exhaust temperatures below 400 °C. This was possible since the raw emissions from the engine were much lower compared to diesel, and the cumulative time used was on the order of hours rather than minutes.
The results have shown that low thermal mass and low thermal conductivity are an advantage to benefit from the transients during operation and maximize the passive soot oxidation. With respect to the effect of catalytic coating, the results at higher temperatures showed that the oxidation rates and temperatures are higher when a TWC was present. This was primarily due to the conversion of any CO that was formed as a partial oxidation product of soot and the associated additional heat release which indirectly accelerated the soot oxidation reactions. At lower temperatures, with a generally lower exothermic heat release due to the moderate rates, the effect of coating is expected to be smaller. In the vehicle experiments, no difference was observed between a coated and an uncoated filter.
The maximum temperature inside the filter during soot oxidation is determined by a number of factors. In our experiments, we have observed that at low temperatures, the maximum temperature is determined by the balance of heat released to the enthalpy that is removed from the filter via the exhaust gas flow. Once the exothermic heat release exceeds the capability of this convective heat removal, the peak temperature can be limited by the total amount of soot present as well as the rate at which oxygen is provided for the reaction. From a control perspective, the latter potentially offers an attractive path to manage extreme soot oxidation events, especially since in difference to diesel applications, it is easier to manage the oxygen flow in a gasoline environment.
5 Conclusions
Conditions enabling passive soot oxidation in gasoline particulate filter applications have been provided. A broad range of experimental data and methods has been used to establish a basic understanding, comprising laboratory experiments as well as vehicle demonstrations, supplemented by detailed numerical simulations. Good agreement was observed between the laboratory methods and the vehicle experiments. In the examples studied, the passive soot oxidation rates exceeded the rate at which the engines emitted soot. However, although a range of reasonably severe conditions was covered, the study can obviously not be entirely conclusive and will not cover all potential applications. It does, however, provide promise that practical solutions will likely be simpler compared to diesel applications.
References
Mamakos, A., Manfredi, U.: “Physical Characterization of Exhaust Particle Emissions from Late Technology Gasoline Vehicles”. JRC Scientific and Policy Reports JRC 72196, 2012. doi:10.2788/32371
Slavnich, D: “Caught in a Trap”. Engine Technol Int, March 2014, p. 42-46
Kim, Y., Kim, Y., Kang, J., Jun, S., Rew, S.H., Lee, D., S. Park: "Fuel Effect on Particle Emissions of a Direct Injection Engine," SAE Technical Paper 2013-01-1559, 2013. doi:10.4271/2013-01-1559
Chan, T., Meloche, E., Kubsh, J., Brezny, R., Rosenblatt, D., Rideout, G.: Impact of ambient temperature on gaseous and particle emissions from a direct injection gasoline vehicle and its implications on particle filtration. SAE Int J Fuels Lubr 6(2), 350–371 (2013). doi:10.4271/2013-01-0527
Schöppe, D., Zhang, H., Rösel, G,. Achleitner, E., Kapphan, F., Dupont, H.: Next generation engine management systems for gasoline direct injection. Proceedings 34. Internationales Wiener Motorensymposium, 2013
WHO Press Release No 213. IARC: Diesel engine exhaust carcinogenic, 12 June 2012
WHO Press Release No 221 IARC: Outdoor air pollution a leading environmental cause of cancer deaths, 17 October 2013
Pelucchi, C., Negri, E., Gallus, S., Boffetta, P., Tramacere, I., La Vecchia, C.: Long-term particulate matter exposure and mortality: a review of European epidemiological studies. BMC Public Health 9, 453 (2009)
Boger, T., Gunasekaran, N., Bhargava, R., Bischof, C.: Particulate filters for DI gasoline engines. MTZ 74(6), 452–458 (2013)
Kern, B., Spiess, S., Richter, J.: Comprehensive gasoline exhaust gas aftertreatment, an effective measure to minimize the contribution of modern direct injection engines to fine dust and soot emissions?, SAE Technical Paper 2014-01-1513, 2014. doi:10.4271/2014-01-1513
Greenwell, D.: “Optimisation of Three Way Filter (TWFTM) coatings and systems for Euro 6”. Proceedings of 2nd Int. Conference Advanced Emission Control Concepts for Gasoline Engines, 2013
Harth, K., Wassermann, K., Arnold, M., Siemund, S., Siani, A., Schmitz T., Neubauer T.: “Katalysierte Benzin Partikelfilter: Integrierte Lösungen für strenge Emissionsregulierungen“. Proceedings 34. Internationales Wiener Motorensymposium, 2013
Boger, T., Rose, D., Tilgner, I., Heibel, A.: Regeneration strategies for an enhanced thermal management of oxide diesel particulate filters. SAE Int J Fuels Lubr 1(1), 162–172 (2009). doi:10.4271/2008-01-0328
Kalogirou, M.E., M. Schejbal and G.C. Koltsakis, “Experimental procedures for efficient after-treatment model calibration”. Proceedings of CLEERS Workshop 2010
Axisuite software version 2013A, Axisuite user guide, Exothermia, 2013
Koltsakis, G., Haralampous, O., Depcik, C., Ragone, J.C.: Catalyzed diesel particulate filter modeling. Rev Chem Eng 29(1), 1–61 (2013)
Stanmore, B.R., Brilhac, J.F., Gilot, P.: The oxidation of soot: a review of experiments, mechanisms and models. Carbon 39, 2247–2268 (2001)
Messerer, A., Niessner, R., Pöschl, U.: Comprehensive kinetic characterization of the oxidation and gasification of model and real diesel soot by nitrogen oxides and oxygen under engine exhaust conditions: measurement, Langmuir-Hinshelwood, and Arrhenius parameters. Carbon 44, 307–324 (2006)
Dernaika, B., Uner, D.: A simplified approach to determine the activation energies of uncatalyzed and catalyzed combustion of soot. Appl Catal B Environ 40, 219–229 (2003)
Yezerets, A., Currier, N.W., Eadler, H.A., Suresh, A., Madden, P.F., Branigin, M.A.: Investigation of the oxidation behavior of diesel particulate matter. Catal Today 88, 17–25 (2003)
Locker, R., N: Gunasekaran and C. Sawyer, "Diesel particulate filter test methods," SAE Technical Paper 2002-01-1009, 2002, doi:10.4271/2002-01-1009
Setiabudi, A., Makkee, M., Moulijn, J.A.: The role of NO2 and O2 in the accelerated combustion of soot in diesel exhaust gases. Appl Catal B Environ 50, 185–194 (2004)
Choi, S., Seong H. J., Lee, K.O.: “Oxidation characteristics of soot from a gasoline direct-injection (GDI) engine”. Proceedings of DOE CLEERS Workshop, Dearborn (MI), 2014
Wang, C., Xu, H., Herreros, J.M., Lattimore T., Shuai, S.: “Fuel effect on particulate matter (PM) composition and soot oxidation in a GDI engine”. Proceedings 2014 Cambridge Particle Meeting
Acknowledgments
The authors would like to thank S. Berre and V. Miranda Da Costa for performing the TGA experiments used in this study and W. von Hagn and R. Wilhelm for their support with the laboratory reactor experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boger, T., Rose, D., Nicolin, P. et al. Oxidation of Soot (Printex® U) in Particulate Filters Operated on Gasoline Engines. Emiss. Control Sci. Technol. 1, 49–63 (2015). https://doi.org/10.1007/s40825-015-0011-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-015-0011-1