Abstract
Background
The association between anti-dementia drugs and arrhythmia is uncertain. In addition, the effects of certain drug combinations are not yet well known.
Objective
We investigated the association between anti-dementia drugs and arrhythmia. Furthermore, we investigated the effects of anti-dementia drugs both alone and in combination on the likelihood of arrhythmia in patients with dementia.
Methods
We examined the Japanese Adverse Drug Event Report database (JADER) from April 2004 to May 2022 for dementia drug users aged ≥ 60 years. We calculated the unadjusted reported odds ratio (ROR) and adjusted ROR for confounding factors. Furthermore, we examined the association of various combinations of anti-dementia drugs with the development of arrhythmias.
Results
There were 6718 arrhythmia cases identified out of 333,702 reported cases. The unadjusted ROR results were as follows: donepezil alone (ROR 4.39, 95% confidence interval [CI] 3.89–4.95), rivastigmine alone (2.10, 1.53–2.87), galantamine alone (3.87, 3.04–4.94), memantine alone (2.25, 1.59–3.20), and combination of choline esterase inhibitor and memantine (2.56, 1.84–3.57). In a multivariate analysis, the RORs remained significant.
Conclusions
Regardless of whether anti-dementia drugs were used alone or in combination, attention should be paid to the occurrence of arrhythmias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We investigated the association between anti-dementia drugs and irregular heartbeat using the Japanese Adverse Drug Event Report database. |
Regardless of drug mechanism, anti-dementia drugs included many reports of arrhythmic adverse events. |
1 Introduction
Dementia is a syndrome of cognitive decline beyond what is assumed to be age-related physiological ageing [1]. There are several types of dementia, including Alzheimer's disease, frontotemporal dementia, vascular dementia, and Lewy body dementia, with approximately 50 million patients affected worldwide, a value predicted to exceed 130 million by 2050 [2,3,4]. In general, elderly patients have a more frequent occurrence of drug-related adverse events (AEs) than younger patients [5] . Increased drug sensitivity due to age-related changes in pharmacokinetics is one cause of side effects in elderly patients [5].
Currently, there are four anti-dementia drugs among two drug classes approved for use in Japan, of which donepezil, galantamine, and rivastigmine function as cholinesterase inhibitors (ChE-Is) and memantine is classified as an N-methyl-d-aspartate (NMDA) receptor antagonist [1]. Cholinesterase inhibitors increase acetylcholine levels in the brain by inhibiting acetylcholine breakdown at the synapse. N-methyl-d-aspartate receptor antagonists selectively act on NMDA receptors and inhibit channel function, thereby inhibiting the progression of glutamatergic neuronal damage [4]. Usually, one of the ChE-Is is selected and prescribed, and ChE-Is are rarely used in combination with each other. There are no specific restrictions on the use of memantine in combination with ChE-I, but in Japan, the indication listed in the package insert is to be given to patients with moderate or severe dementia. In that case, the combination therapy would be prescribed for more severe dementia than the ChE-I monotherapy.
Cholinesterase inhibitors are associated with a significantly higher incidence of gastrointestinal side effects [6, 7]. Uncommon side effects of ChE-Is include arrhythmias such as tachycardia and bradycardia [8]. Conversely, memantine’s effects on the heart are not well characterized [9]. In addition, ways in which each ChE-I affects arrhythmia risk and how various combination therapies of ChE-Is and memantine affect the likelihood of arrhythmia development are unknown. Furthermore, previous clinical studies lacked information concerning elderly patients, (aged > 85 years), as they were either excluded or accounted for only a small number of the sample size.
The Japanese Adverse Drug Event Report database (JADER) is a voluntary reporting subsystem maintained by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan to evaluate the post-marketing safety of approved drugs. The data are reported by medical professionals and pharmaceutical companies and are used for pharmacovigilance. The reported odds ratio (ROR) is used in pharmacovigilance research as a signal detection index [10,10,11,12,14]. It is the proportion of spontaneous reports on a drug associated with a specific AE divided by the proportion of AEs corresponding to all except the drug of interest.
We analyzed the associations between anti-dementia drugs and arrhythmias in single or combination use. Since the frequency of arrhythmia adverse events caused by anti-dementia drugs is unknown and is assumed to be rare, we aimed to detect the signals using JADER, which is a spontaneous reporting system but the largest database of side effects in Japan.
2 Methods
2.1 Data Sources
The present study was conducted using JADER (http://www.pmda.go.jp), which is anonymized by the regulatory authority prior to publication. The database consists of four data tables: demographic information (demo202204.csv), drug information (drug202204.csv), AE information (reac202204.csv), and primary diseases (hist202204.csv) [15]. Each data table is linked by an identification number. The analysis used all assigned codes (suspected drug, interacting drug, concomitant drug) for each drug. We searched the JADER database from April 2004 to March 2022.
2.2 Data Preprocessing
Ages were registered in 10-year increments. Considering the age at which anti-dementia drugs are mainly prescribed in the present study, we included patients aged ≥ 60 years. We extracted cases involving ChE-Is donepezil, galantamine, and rivastigmine. We also extracted cases of memantine use as an NMDA receptor antagonist, a type of anti-dementia drug with a mechanism of action different from that of a ChE-I. Furthermore, we extracted cases involving the combined use of ChE-Is and NMDA receptor antagonists to study the associations of this combination with arrhythmia. Sex and age were excluded in cases with unclear or missing data. We also excluded cases with combined use of ChE-Is from our analysis.
2.3 Definition of AEs
We used the preferred term (PT) defined by the Medical Dictionary for Regulatory Activities (MedDRA®) version 25.0. We used 112 PTs for arrhythmia (SMQ code: 20000049, Appendix1-1) and 39 PTs for chronic kidney disease (CKD) (SMQ code: 20000213, Appendix1-2). The SMQ contains two categories of “broad” and “narrow.” We used “narrow” to specifically search for AEs because of the noise from increasing the number of PTs selected by using “broad”.
2.4 Signal Detection
We used RORs calculated using a contingency table generally used in pharmacovigilance studies [16]. The requirements for signal detection were ≥ 2 cases of a specific AEs for a particular drug, and the lower limit of the 95% confidence interval (CI) for the estimated ROR was > 1 [16]. We calculated the adjusted RORs using multivariate logistic regression analyses [17,17,19, 20] and controlling for age, sex, presence or absence of CKD, antiarrhythmic drug use, and anti-dementia drugs, as confounding factors. In addition, three models were used in the multivariate analysis: (1) considering ChE-I, memantine, and combination therapy as separate treatments; (2) accounting for the interaction term between ChE-I and memantine; and (3) considering memantine, ChE-I, and memantine combination therapy as separate treatments, each ChE-I separately. In analyses 1 and 3, the population was divided so that there were no overlapping cases. Furthermore, a subgroup analysis was performed to consider the influence of the use of antiarrhythmic drugs (Appendix 2) on arrhythmias.
2.5 Statistical Analyses
All statistical analyses were performed using the JMP Pro 15.2.0 software program (SAS Institute Inc., Cary, NC, USA). A p value of < 0.05 was considered statistically significant.
2.6 Ethical Statement
The present study was observational research using an anonymized database and did not require ethical approval. All studies were conducted in accordance with the tenets of the Declaration of Helsinki.
3 Results
3.1 Characteristics
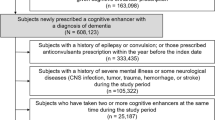
The JADER database contained 758,542 reports from April 2004 to March 2022 (Fig. 1). After removing cases with ages < 60 years as well as those containing missing data on the age, sex, primary disease; and those with the combination of anti-dementia drugs, there were 6718 arrhythmia cases identified out of 333,702 reported cases (SMQ code: 20000049). Adverse events were most frequently reported among patients aged 70–79 years, as were arrhythmias. Cases of arrhythmias presenting with comorbid CKD accounted for approximately 10% of all cases. There were very few reported cases/non-cases of patients aged ≥ 100 years. The number of reported cases of arrhythmia for each anti-dementia drug was n = 322 for donepezil (donepezil alone 302, with memantine 20), n = 43 for rivastigmine (rivastigmine alone 41, with memantine 2), n = 86 for galantamine (galantamine alone 71, with memantine 15), and n = 70 for memantine (memantine alone 33, with ChE-Is 37). Memantine was used in combination with ChE-Is in about half of the cases (Table 1).
3.2 Calculation of Unadjusted and Adjusted RORs
Univariate analysis (unadjusted RORs in Table 2), total use of ChE-I, including monotherapy and combination therapy, indicated significant ROR (ROR 3.83, 95% CI 3.47–4.22), and donepezil (4.39, 3.89–4.95), rivastigmine (2.10, 1.53–2.87), galantamine (3.87, 3.04–4.94), and memantine (2.25, 1.59–3.20) were also significant, compared to the non-use of each drug. Simultaneous administration of ChE-I and memantine showed a significant ROR (2.56, 1.84–3.57) compared with not receiving combination therapy. For each ChE-I and memantine combination, RORs were detected for combinations of donepezil and memantine (2.18, 1.39–3.42), and galantamine and memantine (4.94, 2.70–8.41), although the combination of rivastigmine and memantine was not significant.
In a multivariate analysis (adjusted ROR1, 2, and 3 in Table 2), adjusted RORs were calculated considering age, sex, CKD, use of antiarrhythmic drug and the choice of anti-dementia medications (monotherapy and combination). In adjusted ROR1, use of ChE-I and memantine in monotherapy and combination therapy were significant signals. In adjusted ROR2 in Table 2, the interaction was a sub-multiplicative (ROR for ChE-I*memantine 0.57, 0.44–0.73). An additive interaction was also calculated from the reported proportions, and its value was − 0.047, indicating a sub-additive interaction.
In the case of memantine use, the ROR was almost unchanged with the ChE-I combination, but the ROR was lower with the ChE-I combination than with ChE-I alone. However, as seen in adjusted ROR1, a signal was also detected with combination therapy compared to no anti-dementia drug use. In adjusted ROR3, all medications, such as donepezil (3.71, 3.29–4.20), rivastigmine (1.77, 1.29–2.43), and galantamine (3.33, 2.60–4.25) and memantine alone (1.92, 1.35–2.73), and the ChE-I and memantine combination (2.23, 1.60–3.12) were signals detected, compared to non-use.
There was a trend towards higher adjusted RORs with increasing age (with reference to individuals in the 60–69 age group), except for 100 years. The likelihood of arrhythmia was approximately 7% higher in females than males when adjusting for possible confounders (ROR 1.02, 95% CI 1.02–1.12) The presence of CKD had similarly significant higher reporting odds for both unadjusted and adjusted analyses (ROR 1.72, 95% CI 1.58–1.86, and ROR 1.67, 95% CI 1.54–1.81, respectively) compared to cases in which CKD was not reported as a comorbidity.
3.3 Subgroup Analyses (with or Without Antiarrhythmic Drugs)
Significant signals were detected for anti-dementia drugs, even after accounting for the use of antiarrhythmic drugs. However, the use of antiarrhythmics is a small fraction of the total AE reports, and proarrhythmic effects are also known to be present when antiarrhythmic drugs are used. On the other hand, antiarrhythmic drugs may partially suppress arrhythmias associated with anti-dementia drugs. Therefore, we stratified the cases by the presence or absence of antiarrhythmic drugs and performed a subgroup analysis to examine whether anti-dementia drug-related arrhythmias are sufficiently signaled in each stratum. The patient background for the subgroup analysis is shown in Table 3. In each group with reported arrhythmic adverse events (case group), the proportion of anti-dementia drug use in the no antiarrhythmic drug use group was 8.37%, which was higher than the value of 5.00% in the antiarrhythmic drug use group.
In the group without antiarrhythmic drug use, the ChE-I drugs donepezil, rivastigmine, and galantamine and the NMDA receptor antagonist memantine as well as the combination of ChE-Is and memantine showed significance for both unadjusted and adjusted RORs (Table 4). In the group with the use of antiarrhythmic drugs, the adjusted and unadjusted and adjusted RORs for ChE-I, donepezil, rivastigmine, and galantamine were all significant. However, neither memantine nor the combination of ChE-Is and memantine was significant (Table 4).
Finally, when RORs were calculated separately for side effects related to QT prolongation syndrome/Torsades de Pointes (TdP) and other side effects, ROR signals were detected for each anti-dementia drug in both side effect groups (Appendix 3).
4 Discussion
In clinical practice, memantine and ChE-I are used alone or in combination in patients with Alzheimer’s disease [1]. The present study, which analyzed the JADER database containing 333,702 reports, indicated the association of anti-dementia drugs such as ChE-Is (donepezil, rivastigmine, galantamine) with arrhythmias. Cholinesterase inhibitors alone, memantine alone, and their use in combination had significant associations with arrhythmias.
An association between bradycardia and ChE-I was previously reported in cohort studies [21, 22]. QT prolongation occurred more frequently in patients taking donepezil than in elderly patients without donepezil in a single-center cohort study [23]. The JADER database study reported an association between donepezil and QT prolongation [12]. However, to our knowledge, no previous reports have detected an increase in reports of arrhythmias with memantine use. Arrhythmias not associated with QT prolongation were also shown for the first time to be associated with all anti-dementia drugs. Furthermore, we have shown the robustness of the signal detection under various conditions of age, sex, presence of CKD and antiarrhythmic drug use.
Cholinesterase inhibitors can affect the heart through increased levels of acetylcholine, considering that ChE is abundantly distributed in the heart [24]. In addition, given that ChE-Is act in a concentration-dependent manner, older female patients may have a lower renal function than younger patients or males [5, 7]. Consequently, there may be a high risk of developing side effects of ChE-Is in woman, elderly and CKD. Women are more at risk than men for certain types of arrhythmias. For example, prescribing drugs associated with QT prolongation, patient-related risk factors are known as female sex, age > 65 years, uncorrected electrolyte disturbances [25]. Elderly patients and patients with CKD are more likely to have increased drug blood levels due to lower renal function [26].
Memantine has inhibitory effects on NMDA receptor activation, but its influence on the heart is not yet well known [9]. In vivo studies and studies in healthy adults have reported that memantine alone did not induce bradycardic AEs and carried no risk of QT prolongation and TdP [27, 28]. However, case reports and pharmacovigilance studies have described an association between memantine and cardiovascular AEs [9, 29, 30].
There may be an interaction between ChE-I and memantine in combination for arrhythmia, possibly based on drug-drug interactions, but the pharmacological significance of this interaction is unknown. Usually, according to the package insert, the two drugs are not used together by chance, but only when the dementia is more severe. Studies dealing with severe dementia are known to be fraught with difficulties [31], and in the present study, there is the possibility of an impact on ROR due to interactions stemming from reporting bias, problems with non-adherence, and an increase in other side effects.
Considering the impact of antiarrhythmic drug use, the use of each anti-dementia drug alone may directly prevent the patients from maintaining sinus rhythm, since the arrhythmia occurred in patients who were not on arrhythmic drugs, i.e., did not have arrhythmia which required treatment. The fact that anti-dementia drugs further increased arrhythmias in antiarrhythmic drug users may have augmented the proarrhythmic effects of the anti-dementia drugs or may have been involved in an independent mechanism, but there is room for further basic investigation. Of note, some of the RORs for anti-dementia drug use with arrhythmia treatment have no signal detected, which may be due to the proarrhythmic effects of antiarrhythmic drugs, or a selection bias that patients treated for arrhythmias are more prone to arrhythmias in nature, resulting in a lower reported odds ratio as a result of more arrhythmias even in the absence of anti-dementia drugs. There is evidence showing that antiarrhythmic drugs increase AEs and proarrhythmic events, and some antiarrhythmics may also increase mortality [32]. At the very least, care should be taken in the combination use of ChE-I and antiarrhythmic drugs.
Based on the present findings, continuous follow-up with side-effect monitoring may be needed for patients receiving anti-dementia drugs.
4.1 Limitations
Several limitations associated with the present study warrant mention. First, the JADER database is made up of spontaneous reports from healthcare professionals and pharmaceutical companies. It may be affected by issues of over-reporting, under-reporting, missing data, and a lack of denominators [33]. Under-reporting of AEs is more likely with severe dementia, such as in cases involving the combination of anti-dementia medications, than with mild dementia. Data on dementia subtypes were not available for the present study. Furthermore, the present study considered CKD a comorbidity. There are other baseline characteristics that need to be controlled for, such as heart disease, neurological disease, and thyroid disease [26]. Dose, route of administration, and severity of arrhythmia and dementia might also be included, but considering the many missing entries in the database, meaningful results could not be obtained on the information. Additionally, there is the possibility of reporting duplicates by health care professionals and pharmaceutical companies, as the JADER database makes it difficult to remove duplicates. Second, the findings of spontaneous reporting data are hypotheses, and risk quantification cannot be performed [33]. Appropriate cohort studies and randomized trials are necessary to test the hypothesis [34]. Third, the JADER database does not provide detailed laboratory data, so we cannot accurately assess renal function. Finally, the present study did not investigate the period from drug administration to AEs, as there were many missing data in the reported database.
5 Conclusions
Cholinesterase inhibitors and memantine may affect arrhythmias. We may need to confirm side effects, whether drugs are administered alone or in combination.
References
Dementia Clinical Practice Development Committee JSoN. Clinical practice guideline for dementia 2017. 2017.
Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015—the global impact of dementia. London: Alzheimer’s Disease International; 2015.
Jin B, Liu H. Comparative efficacy and safety of therapy for the behavioral and psychological symptoms of dementia: a systemic review and Bayesian network meta-analysis. J Neurol. 2019;266(10):2363–75.
Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70.
Akishita M. Guidelines for medical treatment and its safety in the elderly Nippon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics 2007;44(1):31-34.
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50(1):136–45.
Rogers SL, Doody RS, Mohs RC, Friedhoff LT, Group atDS. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158(9):1021–31.
Howes LG. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Saf. 2014;37(6):391–5.
Gallini A, Sommet A, Montastruc JL. Does memantine induce bradycardia? A study in the French PharmacoVigilance Database. Pharmacoepidemiol Drug Saf. 2008;17(9):877–81.
Sato K, Mano T, Iwata A, Toda T. Subtype-dependent reporting of stroke with SGLT2 inhibitors: implications from a Japanese Pharmacovigilance Study. J Clin Pharmacol. 2020;60(5):629–35.
Inoue M, Matsumoto K, Tanaka M, Yoshida Y, Satake R, Goto F, et al. Analysis of chemotherapy-induced peripheral neuropathy using the Japanese Adverse Drug Event Report database. Sci Rep. 2021;11(1):11324.
Sasaoka S, Matsui T, Hane Y, Abe J, Ueda N, Motooka Y, et al. Time-to-onset analysis of drug-induced long QT syndrome based on a spontaneous reporting system for adverse drug events. PLoS ONE. 2016;11(10): e0164309.
Katsuhara Y, Ogawa T. Acute renal failure, ketoacidosis, and urogenital tract infections with SGLT2 inhibitors: signal detection using a Japanese Spontaneous Reporting Database. Clin Drug Investig. 2020;40(7):645–52.
Hosohata K, Inada A, Oyama S, Niinomi I, Wakabayashi T, Iwanaga K. Adverse cutaneous drug reactions associated with old- and new-generation antiepileptic drugs using the Japanese Pharmacovigilance Database. Clin Drug Investig. 2019;39(4):363–8.
Nomura K, Takahashi K, Hinomura Y, Kawaguchi G, Matsushita Y, Marui H, et al. Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des Dev Ther. 2015;9:3031–41.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Van Puijenbroek EP, Egberts AC, Meyboom RH, Leufkens HG. Signalling possible drug-drug interactions in a spontaneous reporting system: delay of withdrawal bleeding during concomitant use of oral contraceptives and itraconazole. Br J Clin Pharmacol. 1999;47(6):689–93.
van Puijenbroek EP, Egberts AC, Heerdink ER, Leufkens HG. Detecting drug–drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2000;56(9–10):733–8.
Suzuki Y, Suzuki H, Umetsu R, Uranishi H, Abe J, Nishibata Y, et al. Analysis of the interaction between clopidogrel, aspirin, and proton pump inhibitors using the FDA adverse event reporting system database. Biol Pharm Bull. 2015;38(5):680–6.
Noguchi Y, Tachi T, Teramachi T. Review of Statistical Methodologies for Detecting Drug–Drug Interactions Using Spontaneous Reporting Systems Frontiers in Pharmacology 2019;10:1319.
Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand S-LT, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–73.
Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs New England Healthcare System. J Am Geriatr Soc. 2009;57(11):1997–2003.
Kuwahata S, Takenaka T, Motoya T, Masuda K, Yonezawa H, Shinchi S, et al. Effect of QT prolongation in patients taking cholinesterase inhibitors (donepezil) for Alzheimer’s disease. Circ Rep. 2021;3(3):115–21.
Kho J, Ioannou A, Mandal AKJ, Missouris CG. Donepezil induces ventricular arrhythmias by delayed repolarisation. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(3):559–60.
Khatib R, Sabir FRN, Omari C, Pepper C, Tayebjee MH. Managing drug-induced QT prolongation in clinical practice. Postgrad Med J. 2021;97(1149):452–8.
Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai K, et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ J. 2021;85(7):1104–244.
Kambayashi R, Goto A, Hagiwara-Nagasawa M, Izumi-Nakaseko H, Shinozaki M, Kawai S, et al. Analysis of clinically-reported, memantine-induced cardiovascular adverse responses using the halothane-anesthetized dogs: reverse translational study. J Pharmacol Sci. 2022;148(4):343–50.
Park J-W, Kim K-A, Park J-Y. Effect of memantine on QT/QTc interval in a Healthy Korean Population. Clin Pharmacol Drug Dev. 2021;10(10):1209–15.
Takehara H, Suzuki Y, Someya T. QT prolongation associated with memantine in Alzheimer’s disease. Psychiatry Clin Neurosci. 2015;69(4):239–40.
Kajitani K, Yanagimoto K, Monji A, Maruyama T. Memantine exacerbates corrected QT interval prolongation in Alzheimer’s disease: a case report from an unintentional rechallenge. J Am Geriatr Soc. 2016;64(1):232–3.
Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, Thiébaut R, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098–109.
Valembois L, Audureau E, Takeda A, Jarzebowski W, Belmin J, Lafuente-Lafuente C. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049.
Michel C, Scosyrev E, Petrin M, Schmouder R. Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles? Clin Drug Investig. 2017;37(5):415–22.
VIII CfIOoMSWG. Practical aspects of signal detection in pharmacovigilance. 2010.
Acknowledgements
The authors thank the pharmaceutical department staff for their support of our research activities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was received.
Meiji Pharmaceutical University
Conflict of interest
Shotaro Kobayashi, Norio Sugama, Hiroyuki Nagano, Masahiro Takahashi, and Akifumi Kushiyama declare that they have no conflicts of interest.
Data availability
The datasets generated during and/or analyzed during the current study are available in the OPENICPSR repository (https://www.openicpsr.org/openicpsr/).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
SK, NS, HN, AM, MT, and AK contributed to the concept and planning of the work. SK and AK analyzed and interpreted the data. SK, NS, HN, AM, MT, and AK reviewed the manuscript critically. All authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kobayashi, S., Sugama, N., Nagano, H. et al. Analysis of Adverse Events of Cholinesterase Inhibitors and NMDA Receptor Antagonists on Arrhythmias Using the Japanese Adverse Drug Event Report Database. Drugs - Real World Outcomes 10, 321–329 (2023). https://doi.org/10.1007/s40801-023-00362-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-023-00362-6