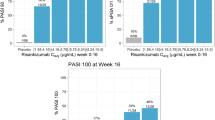
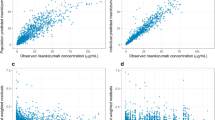
Abstract
Introduction
Risankizumab is an anti-IL23 monoclonal antibody approved for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis (PsA). This work characterizes the pharmacokinetics of risankizumab in PsA compared with psoriasis and evaluates the efficacy and safety exposure–response relationships in PsA.
Methods
The population pharmacokinetic analyses included data from 1527 participants that originated from one phase 1 healthy participant study, one phase 2 dose-ranging study in patients with PsA with an open-label extension study, and two pivotal phase 3 studies in patients with PsA, where the clinical regimen of risankizumab 150 mg administered subcutaneously (SC) at weeks 0, 4, and every 12 weeks thereafter was compared with placebo. Pharmacokinetics were analyzed using nonlinear mixed-effects modeling. Simulation analyses using the final model were conducted to evaluate the impact of covariates on exposure. Data from 1407 patients with PsA from the phase 3 studies were included in the exposure–response analyses. Graphical analyses were used to evaluate efficacy and safety exposure–response relationships, and logistic regression was conducted for further assessment of efficacy exposure–response relationships.
Results
Risankizumab pharmacokinetics were well described by a two-compartment model with first-order SC absorption and elimination. None of the evaluated covariates showed clinically relevant impact on exposure. On the basis of the final model, systemic clearance, steady-state volume of distribution, and terminal phase elimination half-life were estimated to be ~ 0.31 L/day, 11.1 L, and 26.3 days, respectively, for a typical 90 kg patient with PsA. Absolute SC bioavailability was estimated to be 83.5%. Exposure–response quartile analyses suggested that exposures associated with the clinical regimen maximized efficacy across the endpoints evaluated. No exposure dependency was observed for key safety endpoints.
Conclusions
Risankizumab exhibited linear and time-independent pharmacokinetics in patients with PsA and was comparable to patients with plaque psoriasis. Efficacy and safety exposure–response analyses support that the clinical regimen achieved robust efficacy with a favorable safety profile for patients with active PsA.
Clinical Trials: NCT02596217, NCT02719171, NCT02986373, NCT03671148, and NCT03675308.
Clinical Trials:
NCT02596217, NCT02719171, NCT02986373, NCT03671148, and NCT03675308.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Risankizumab exhibited linear pharmacokinetics in patients with active PsA with pharmacokinetic parameters consistent with those observed in plaque psoriasis. |
Under the clinical regimen (150 mg SC at weeks 0 and 4, and every 12 weeks thereafter), steady-state systemic concentrations of risankizumab were achieved by approximately week 16. |
None of the covariates evaluated in the population pharmacokinetic analyses resulted in a clinically relevant impact on risankizumab exposure. |
Exposure–response analyses for efficacy and safety indicated that this clinical regimen for risankizumab achieved robust efficacy responses with no apparent relationship between risankizumab exposure and key safety variables in patients with active PsA. |
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic inflammatory disease classified as a subtype of spondylarthritis and is associated with peripheral joint inflammation, enthesitis, dactylitis, and cutaneous manifestations. Left untreated, patients with PsA can have persistent inflammation, progressive joint damage, disability, and a reduced quality of life [1, 2]. For most patients, skin manifestations predate the arthritis [1].
Risankizumab (ABBV-066) is a humanized immunoglobulin G1 (IgG1) monoclonal antibody (mAb) that selectively binds with high affinity to the p19 subunit of the human cytokine interleukin-23 (IL-23) [3]. By blocking IL-23 from binding to its receptor, risankizumab inhibits IL-23 dependent cell signaling and the release of pro-inflammatory cytokines, and thus has potential to treat immune-mediated inflammatory diseases. Risankizumab is currently approved for the treatment of moderate to severe chronic plaque psoriasis [4] and PsA [5, 6], and is being developed for other inflammatory diseases, including Crohn’s disease [7, 8] and ulcerative colitis [8].
Risankizumab pharmacokinetics were well characterized in healthy participants and patients with plaque psoriasis, exhibiting typical IgG1 mAb linear pharmacokinetics with biexponential disposition and a long terminal elimination half-life (t1/2) [9, 10]. In plaque psoriasis, none of the covariates identified in the population pharmacokinetic analyses had a clinically meaningful impact on risankizumab efficacy. Efficacy and safety exposure–response analyses in patients with psoriasis using pooled data from phase 1–3 studies supported the approved clinical regimen of risankizumab 150 mg administered subcutaneously (SC) at weeks 0 and 4, and every 12 weeks (q12w) thereafter [9].
Following the initial development and approval in plaque psoriasis, risankizumab was also evaluated in phase 2 and pivotal phase 3 studies in patients with active PsA where risankizumab exhibited superior efficacy versus placebo in key disease outcomes and was well tolerated [5, 6, 11, 12]. Leveraging these data, population pharmacokinetic and exposure–response analyses were conducted for risankizumab in PsA. Results from the analyses reported herein demonstrated that the same clinical regimen (150 mg SC at weeks 0 and 4, and q12w thereafter) as approved in plaque psoriasis is appropriate for patients with active PsA and supported the approval for PsA.
Methods
Data Sources
Five clinical studies were utilized in the population pharmacokinetic analyses, namely one phase 1 (NCT02596217 [10], study 1) study in healthy participants, one phase 2 study (NCT02719171 [11], study 2) with an extension study (NCT02986373 [12], study 3), and two pivotal phase 3 studies (NCT03675308/KEEPsAKE 1 [5] and NCT03671148/KEEPsAKE 2 [6], study 4 and study 5, respectively). The latter four studies were all in patients with active PsA. The two phase 3 studies (studies 4 and 5) were utilized in the exposure–response analyses. Safety and efficacy data as well as detailed descriptions of these studies have been reported previously [5, 6]. Brief summaries of these studies with details pertinent to the analyses herein are provided in Supplementary Material (Supplementary Table S1).
All studies were conducted in accordance with Good Clinical Practice guidelines and the ethical principles that have their origin in the Declaration of Helsinki. The protocols and informed consent forms were approved by the ethics committee or institutional review boards at each site, and all participants provided written informed consent prior to any study-related procedures.
Pharmacokinetic Sampling and Analysis
A summary of blood sample collection times for analysis of risankizumab and anti-drug antibody (ADA) concentrations for each study is provided in Supplementary Table S1. Risankizumab plasma or serum concentrations were determined by a validated enzyme linked immunosorbent assay with a lower limit of quantitation (LLOQ) of 5, 10, and 4.34 ng/mL for the phase 1, 2, and 3 studies, respectively [13]. The presence of ADAs to risankizumab was assessed via a tiered approach using a validated electrochemiluminescence assay (screening, confirmatory, and titration analysis as appropriate) [14]. All samples positive for ADA were further characterized in a validated neutralizing antibody (NAb) assay [13, 14].
Population Pharmacokinetics Analyses
A population pharmacokinetic model developed using data from healthy participants and patients with moderate to severe chronic plaque psoriasis [10] was leveraged and refined using data from patients with active PsA.
Comparison of Risankizumab Pharmacokinetics in Psoriasis and Psoriatic Arthritis
To demonstrate the appropriateness of leveraging the psoriasis population pharmacokinetic model in PsA, graphical comparisons were carried out as follows:
-
The observed risankizumab concentrations in patients with plaque psoriasis treated with the approved clinical regimen of 150 mg SC at weeks 0 and 4, and q12w thereafter (referred to hereafter as “clinical regimen”) in phase 3 psoriasis studies were compared graphically with the observed risankizumab concentrations in patients with active PsA enrolled in phase 2 and 3 studies and treated with the same clinical regimen.
-
A simulation-based comparison using the parameter estimates from the previously developed psoriasis model [10] and the patient-specific information in PsA phase 2 and 3 studies was performed through simulations of 1000 replicates of the plasma concentration data for the PsA phase 2 and 3 studies based on the actual dose administered and sampling time. Median and 5th and 95th percentiles of the observed data were compared graphically with the 95% confidence intervals (CIs) of the corresponding percentiles of the simulated data.
Population Pharmacokinetic Model Development for Psoriatic Arthritis
The psoriasis population pharmacokinetic model (described as “starting model” hereafter) was fit to the data. This was a two-compartment model with first-order processes describing SC absorption and elimination, and with risankizumab clearance correlated with weight, baseline serum albumin, baseline serum creatinine, baseline high-sensitivity C-reactive protein (hsCRP), and relatively high ADA titers. Model parameters were estimated using the first-order conditional estimation method with interaction between interindividual variability (IIV) and residual variability [FOCE with η–ε INTERACTION].
For each of the covariate effects included in the starting model, the relationship was retained if the 95% CIs did not overlap with 0 and its removal from the model rendered a statistically significant worsening in the model fit (at α = 0.001; similar to the criteria used in the development of the starting model). The resulting “base model” was refined in a stepwise forward inclusion (α = 0.01) and backward elimination (α = 0.001) covariate search [15] as implemented in Perl Speaks NONMEM (PsN Version 4.8.1) to evaluate the statistical significance of other covariates that could be relevant to the PsA population. Covariates tested included: age, sex, race, baseline levels of alanine transaminase, aspartate transaminase, total bilirubin, creatinine clearance, baseline Disease Activity Score in 28 joints (DAS28 score), baseline Health Assessment Questionnaire-Disability Index (HAQ-DI) score, Psoriasis Area Severity Index (PASI) score, duration of disease, presence of axial spondylitis, number of prior biologic therapies, number of prior conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), concomitant use of methotrexate, and immunogenicity. The cutoff value identified in the previous analyses [10] for the ADA effect on risankizumab clearance was adjusted depending on the dilution factor used for the ADA assay in each of the studies.
After establishing the final model structure, the fixed and random effects parameter estimates were updated; goodness-of-fit plots, bootstrap analyses, and visual predictive checks (VPCs) were used to support model evaluation and selection. After identifying statistically significant covariates for risankizumab pharmacokinetic parameters, simulations were carried out for 10,000 virtual subjects, with IIV, without residual error to evaluate their impact on risankizumab steady-state concentration at the end of a dosing interval (Ctrough) and area under the concentration–time curve over a dosing interval (AUCtau).
Risankizumab exposures [maximum concentration (Cmax), AUCtau, and Ctrough] were simulated using the final population pharmacokinetic model for virtual subjects with active PsA who were administered the clinical regimen. Exposures were summarized over the first (weeks 0–4), second (weeks 4–16), and third (weeks 16–28, representing steady-state values) dosing intervals.
Exposure–Response Analyses for Efficacy and Safety
The exposure–response analyses for efficacy evaluated the following endpoints: proportions of patients with PsA achieving American College of Rheumatology (ACR) 20, ACR50, and ACR70 responses at week 24, PASI75 [for patients with ≥ 3% body surface area (BSA) psoriasis at baseline] at week 24, and minimal disease activity (MDA) evaluated at week 24, in the phase 3 studies (studies 4 and 5).
Since pharmacokinetic samples for phase 3 studies were collected predose in week 28 only, post-hoc individual pharmacokinetic parameter estimates were not used to simulate individual exposure predictions for patients enrolled in phase 3 studies because of the limited information available on an individual level. Given that risankizumab reaches steady state by week 16 for the approved clinical regimen [9], observed Ctrough at week 28 for patients who received risankizumab from the beginning of the phase 3 studies was assumed to be correlated with exposure levels at week 24, and therefore was used to assess relationships with efficacy endpoints evaluated at week 24. The correlation between observed concentrations at week 24 and week 28 for the phase 2 study was evaluated (correlation coefficient 0.961) to confirm the appropriateness of the assumption. The relationships between efficacy endpoints at week 24 and observed risankizumab Ctrough at week 28 for phase 3 studies were evaluated graphically using exposure-quartile plots. In these plots, patients who received placebo in period 1 (weeks 0–24) of either phase 3 study were binned together. Patients who received risankizumab were binned into quartiles according to their individual observed exposures, and vertical bars represented the percent of responders for a given efficacy endpoint per quartile for ACR20/50/70, PASI 90/100, and MDA responses.
For each of the efficacy endpoints at week 24, logistic regression models with treatment effect (yes/no) and exposure effect (observed Ctrough at week 28) were first evaluated and compared after accounting for stratification factors. An exposure and/or a treatment effect were included in the model only if proven to be statistically significant (α = 0.01). For patients who received placebo at the beginning of each study, concentrations were assumed to be zero for the exposure–response analyses. The logistic regression models were constructed as follows:
where P(Yi = 1) is the probability that the observation Y from subject i is equal to 1, with 1 indicating the event of interest occurred, α is the intercept parameter (reflecting placebo response), βCtrough is the slope for Ctrough at week 28, Ctrough,i is the observed risankizumab concentration in subject i. βTRT is the estimated magnitude of treatment effect, RZBi is an indicator of receiving risankizumab in subject i (yes = 1/no = 0). The βj are the coefficients for prespecified stratifying factors (and potential additional covariates) with respective values xj,i in subject i.
For the phase 3 studies, stratification factors used for randomizations and accounted for in the models included: number of prior biologic therapies (0 versus ≥ 1, study 5), number of csDMARDs (0 versus ≥ 1, studies 4 and 5), psoriasis surface area (PSA; ≥ 3% BSA versus < 3% BSA, studies 4 and 5) at baseline, presence of dactylitis (yes versus no, study 4), and presence of enthesitis (yes versus no, study 4) at baseline. Only efficacy endpoints exhibiting a statistically significant exposure (Ctrough) effect (p value < 0.01 for βCtrough) were evaluated further for identification of additional covariates. If a statistically significant exposure effect was observed for an endpoint, the potential effect of the demographic covariates of body weight, age, sex, and race was tested as well as the disease covariates of extent of disease at baseline (DAS28 and PASI), duration of PsA, with or without axial spondylitis at baseline and concomitant use of methotrexate.
Exposure–response analyses for safety evaluated the following safety variables graphically: any adverse event (AE), any serious AE (SAE), any infection, and any serious infection. Proportions of patients who experienced these safety events across period 1 (weeks 0–24) of phase 3 studies were determined among patients who received placebo and in each observed risankizumab exposure quartile at week 28.
Software
The population pharmacokinetic model was developed using nonlinear mixed-effects modeling based on NONMEM (Version 7.4.4) compiled with the GNU Fortran compiler (Version 7.5.5). R (Version 3.6.3) was used for the exposure–response analyses.
Results
Data
Data from patients with active PsA (N = 1460) and healthy participants (N = 67) who received risankizumab and had at least one post-treatment measurable concentration were included in the population pharmacokinetic analyses. Data from patients with active PsA enrolled in phase 3 studies [study 4 (N = 964) and study 5 (N = 443)], who received placebo or at least one dose of risankizumab, were included in the efficacy and safety exposure–response analyses. Summaries of demographic variables and other intrinsic factors are presented in Supplementary Table S2.
In total, 3875 risankizumab plasma concentration measurements from 1546 individuals were acquired across all studies. Data from 19 individuals were excluded owing to the lack of a valid dose or concentration record, and 214 measurements were made prior to the first risankizumab dose. Out of the remaining 3642 measurements, 11 (0.3%) measurements were below the LLOQ, which were also excluded (resulting in exclusion of no additional individual’s data), leaving a total of 3631 concentration measurements from 1527 individuals included in the analyses. The total number of individuals included in the dataset for studies 1, 2/3, 4, and 5 are 67, 177, 391, and 892, respectively, yielding 683, 1663, 391, and 894 pharmacokinetic observations, respectively.
Population Pharmacokinetic Analyses
Comparison of Risankizumab Pharmacokinetics in Psoriasis and Psoriatic Arthritis to Support the Use of the Psoriasis Population Pharmacokinetic Model
The similarity of risankizumab exposure levels between psoriasis and PsA patient populations was demonstrated by graphical comparison of the observed concentrations at week 28 (Fig. 1a) for the clinical regimen. Simulation-based comparisons (Fig. 1b and c) showed that the model based on patients with psoriasis adequately captured the observed data in patients with PsA. On the basis of these graphical comparisons, the previously developed psoriasis population pharmacokinetic model [10] was deemed appropriate for characterizing risankizumab pharmacokinetics in PsA.
Comparison of risankizumab pharmacokinetics in psoriasis and psoriatic arthritis to support the use of the psoriasis population pharmacokinetic model. Graphical comparisons of risankizumab exposure levels for psoriasis and PsA patient populations on the clinical regimen of 150 mg SC at weeks 0, 4, and q12w thereafter are shown for a week 28 predose observed risankizumab concentrations in patients with PsA and psoriasis, b simulated risankizumab concentrations as a function of time since first dose using psoriasis population pharmacokinetic model and observed concentration in patients with PsA in the phase 2 study, and c simulated and observed risankizumab predose concentrations at week 28 in the phase 3 studies. The shaded regions show the 95% CIs around median and 5th and 95th percentiles of simulated risankizumab concentrations. The gray dots denote the observed risankizumab concentration in the PsA studies, and the lines/dashed lines in (b) and the crosses in (c) show the median and 5th and 95th percentile of the observed risankizumab concentration in the PsA studies. PsA, psoriatic arthritis; SC, subcutaneous; q12w, every 12 weeks
Model Refinement Using Psoriatic Arthritis Data
The psoriasis population pharmacokinetic model was fit to the data from four phase 2 and 3 studies in patients with active PsA and one phase 1 study in healthy participants. Relationships for the effect of high ADA titers on clearance (CL) and weight on peripheral volume of distribution (V2) did not meet the criteria for retaining the covariates (described above in Population Pharmacokinetic Model Development for Psoriatic Arthritis) and was therefore removed. A single estimate for bioavailability was made [10], and the term for IIV on bioavailability was removed. This model served as a base model for the covariate search (retaining the effects of body weight on CL and central volume of distribution [V1], the effects of baseline serum creatinine, baseline serum albumin, and hsCRP on CL). The forward-inclusion backward-elimination covariate search identified only age as an additional statistically significant covariate on CL. The covariate–parameter relationships for CL and V1 using the final model are shown in Eqs. (2) and (3), respectively.
The effect of ADA on CL [evaluated as time invariant flag (yes/no), time variant flag (yes/no)], and NAb on CL [evaluated as time invariant flag (yes/no), time variant flag (yes/no)] were found not to be statistically significant. Furthermore, similar exposure levels at week 28 concentrations across patients and participants grouped by their ADA titer levels using different ADA titer thresholds confirmed the lack of an apparent relationship between ADA titers and CL (data on file at AbbVie).
The estimated pharmacokinetic parameter values and their associated variability for the final pharmacokinetic model are listed in Table 1. The computed η-shrinkages for CL, V1, and ka were 16.0%, 46.7%, and 79.2%, respectively. Bootstrap analyses confirmed robustness of model, with 998 out of 1000 runs converging successfully with all parameters in good agreement with the final model parameters and relatively narrow 95% CIs. On the basis of the final population pharmacokinetic model, risankizumab systemic CL, V1, V2, volume of distribution at steady state (Vss), and t1/2 were estimated to be approximately 0.31L per day, 6.8 L, 4.3 L, 11.1 L, and 26.3 days, respectively, for a typical 90 kg patient with PsA. Risankizumab absolute SC bioavailability (pooling drug substances utilized in earlier and later stages of development [10]) was estimated to be 83.5%.
Model Evaluations and Simulations
The goodness-of-fit for the final model, based on data from the phase 2 and 3 studies, was evaluated graphically and is shown in Supplementary Fig. S1. The plots of the population predicted and individual predicted concentrations versus the observed concentrations indicated that the model adequately described the data over the entire range of risankizumab systemic concentrations. The conditional weighted residuals did not show any trends when plotted against time or population predictions, indicating lack of bias in the model. VPCs for the risankizumab systemic concentration showed that the model described the central tendency and variability in the observed data adequately for different single and multiple dose regimens in phase 2 and 3 studies (for regimens, see Supplementary Table S1). VPCs for the clinical regimen for phase 2 and 3 studies are shown in Supplementary Fig. S2.
On the basis of the simulations, the impact of covariates included in the final model on risankizumab model-predicted, steady-state Ctrough and AUCtau (weeks 16–28 dosing interval) for the clinical regimen is depicted in Fig. 2. Body weight was found to have a modest impact on risankizumab exposure. Patients with PsA with relatively high body weight (> 75th percentile; 99 kg) and low body weight (< 25th percentile; 74 kg) were predicted to have ~ 20% lower and 30% higher exposures, respectively, compared with the reference group (74–99 kg). However, these modest exposure differences were not deemed clinically meaningful on the basis of the lack of exposure–response relationships for safety and efficacy over the exposure range associated with the clinical dosing regimen (see Discussion section). None of the other statistically significant covariates showed a meaningful impact on risankizumab exposures (Fig. 2). Simulated risankizumab exposures following administration of the clinical regimen in a virtual population are summarized in Table 2.
Simulated impact of statistically significant covariates identified in the population pharmacokinetic analyses on risankizumab exposures for patients with psoriatic arthritis. Points represent medians and error bars represent 95% confidence intervals of the normalized exposure ratios across 200 simulation replicates. The vertical black dashed line shows exposure ratio of 1 relative to the reference group, and the shaded area represents the 0.8–1.25 default equivalence boundaries. AUC, area under the concentration–time curve between weeks 16 and 28 (AUCtau); Ctrough, concentration after a dosing interval at week 28; hsCRP, high-sensitivity C-reactive protein
Exposure–Response Analyses for Efficacy
ACR
Exposure–response quartile plots for ACR20/50/70 responses at week 24 for the phase 3 studies are shown in Fig. 3a–c. For all ACR response endpoints, patients treated with risankizumab showed higher response rates compared with placebo at week 24. The upper exposure quartiles consistently showed numerically higher response rates compared with the lower exposure quartiles across all ACR response endpoints, including ACR20 (the primary endpoint for the pivotal phase 3 studies).
Exposure–response relationships for efficacy endpoints at week 24 from phase 3 studies. Values on the x-axis represent range of the observed phase 3 risankizumab Ctrough at week 28 for each quartile. Plots show %response (using nonresponder imputation) and n/N, where n represents number of responders and N represents total number of patients in each exposure-quartile bin. For ACR, PASI, and MDA analyses (at week 24), 52, 26, and 52 patients (N), respectively, from phase 3 studies were excluded from these analyses owing to missing Ctrough values at week 28. Of these 52 patients for ACR, 28 (53.8%), 19 (36.5%), and 9 (17.3%) were ACR20 (a), ACR50 (b), and ACR70 (c) responders, respectively. Of the 26 patients for the PASI endpoint, 11 (42.3%) and 9 (34.6%) were PASI 90 (d) and PASI 100 (e) responders, respectively. Of these 52 patients for the MDA endpoint, 14 (26.9%) were MDA (f) responders at week 24. ACR20/50/70, at least 20%/50%/70% improvement in American College of Rheumatology response criteria; Ctrough, concentration at the end of a dosing interval; PASI 90/100, at least 90%/100% improvement in Psoriasis Area and Severity Index relative to baseline; MDA, minimal disease activity
Logistic regression analyses that accounted for study stratification factors for ACR20/50/70 at week 24 revealed a statistically significant treatment effect (p < 0.01), but no statistically significant exposure–response relationship. Parameter estimates are provided in Table 3. Of the stratification factors, patients with no prior biologic treatment showed higher probability of achieving ACR20 and ACR50 at week 24, whereas patients with PSA of < 3% of their BSA at baseline had lower probability of achieving ACR20 and ACR50 responses at week 24.
PASI
The PASI 90/100 responses were evaluated only for patients with PSA ≥ 3% of BSA at baseline. Exposure–response quartile plots for PASI 90/100 responses at week 24 are presented in Fig. 3d, e. Similar to ACR responses, patients treated with risankizumab showed higher response rates for PASI 90/100 at week 24 compared with patients treated with placebo. For PASI 90, all exposure quartiles showed a similar response rate, whereas for PASI 100 the upper two quartiles showed numerically higher response rates as compared with the lower exposure quartiles. Like ACR responses, logistic regression analyses with models that accounted for study stratification factors for PASI 90/100 at week 24 identified a statistically significant treatment effect for risankizumab, but no statistically significant exposure–response relationships. Consistent with the ACR responses, these data indicate that the selected clinical regimen evaluated in phase 3 provided robust efficacy across the exposure range as assessed by the PASI 90/PASI 100 responses at week 24.
MDA
Exposure–response quartile plots exploring the impact of observed risankizumab exposure at week 28 on MDA response at week 24 (Fig. 3f) showed numerically higher response rates with increasing risankizumab exposure. Logistic regression analyses with a treatment effect model that accounted for stratification factors revealed risankizumab treatment effect to be significant (Table 3). Risankizumab exposure did not have a statistically significant influence on MDA response at week 24. Of the stratification factors, patients with no enthesitis at baseline showed lower probability of achieving MDA response at week 24.
Exposure–Response Analyses for Safety
The relationships between observed week 28 risankizumab Ctrough (from studies 4 and 5) and percentage of patients who experienced any AE, SAE, infection, or serious infection over the first 24 weeks (placebo-controlled period) are shown in Fig. 4. Rates of incidence of the safety variables of interest over the first 24 weeks evaluated were comparable to placebo, with no apparent relationship with risankizumab exposure over the entire exposure range observed with the clinical regimen.
Exposure–response quartile analyses for safety events of interest over weeks 0–24 in phase 3 studies. Values on the x-axis represent range of the observed phase 3 studies risankizumab Ctrough at week 28 for each quartile. Plots show %response (using nonresponder imputation) and n/N, where n represents number of responders and N represents total number of patients in each exposure-quartile bin. In this analysis, 52 patients (N) from the phase 3 studies who had missing Ctrough at week 28 were excluded. Of these 52 patients, 9 (17.3%), 2 (3.8%), 24 (46.2%), and 3 (5.8%) had a infections, b serious infections, c adverse events, and d serious adverse events, respectively, by week 24. Ctrough, concentration at the end of a dosing interval
Discussion
The analyses presented herein report the first assessment of risankizumab pharmacokinetics and exposure–response relationships in patients with active PsA. Results showed similarity in risankizumab pharmacokinetics between patients with plaque psoriasis and those with PsA. Furthermore, the same clinical regimen approved in chronic plaque psoriasis (150 mg SC at weeks 0 and 4, and q12w thereafter) provided robust efficacy across the different clinical endpoints in PsA with no exposure-dependent increase in safety risks for AE, SAE, infection, or serious infection when compared with placebo.
Risankizumab pharmacokinetics has been well characterized in healthy participants and patients with psoriasis [9, 10]. On the basis of the similarities between psoriasis and PsA, the population pharmacokinetic model previously developed for psoriasis [10] was refined and fit to data from patients with active PsA. This was supported by comparable risankizumab concentrations observed following the same dosing regimen (150 mg SC at weeks 0 and 4, and q12w thereafter) in both populations as well as the model simulation comparisons. The final population pharmacokinetic model in PsA is a two-compartment model with first-order SC absorption and first-order elimination. The parameters of the model were estimated with high precision (relative standard error ranging from approximately 5.1% to 29.3%). Model evaluation using goodness-of-fit plots, VPCs, and bootstrap analyses indicated that the model adequately described risankizumab pharmacokinetics. Parameter estimates in the PsA model were consistent with the original plaque psoriasis model [10], further confirming the comparability across both patient populations. Similar to patients with plaque psoriasis [10], risankizumab steady-state systemic concentrations were achieved by approximately week 16 in patients with active PsA following the administration of the same clinical regimen. The predicted mean steady-state (at the end of the third dosing interval, weeks 16–28) Cmax and Ctrough were 11.6 μg/mL and 1.88 μg/mL, respectively, also consistent with psoriasis patient findings [9].
Covariate analyses demonstrated that baseline albumin, baseline hsCRP, baseline serum creatinine, and age were statistically correlated with risankizumab CL in patients with PsA. However, none of these covariates had a clinically meaningful impact over the range of covariate values observed in patients with PsA. Similar to other IgG1 mAbs and consistent with the psoriasis pharmacokinetic model [10], body weight was statistically correlated with risankizumab CL. Despite the modest differences in exposures across the body weight range, exposure–response analyses for all efficacy endpoints evaluated have shown the absence of a statistically significant effect of exposure. The incidences of safety events were similar across the entire range of risankizumab exposure and comparable to patients treated with placebo. Therefore, the modest differences in exposures because of differences in body weight were deemed clinically irrelevant. Other intrinsic factors, including sex, race, liver function markers (total bilirubin, alanine transaminase, and aspartate transaminase), did not impact risankizumab exposures. PsA disease-related characteristics, including presence of axial spondylitis, baseline DAS28 score, baseline PASI score, duration of disease, prior use of biologic therapy (0 versus ≥ 1), prior use of csDMARDs (≤ 1 versus > 1), and use of methotrexate (yes/no), had no impact on risankizumab pharmacokinetics. Treatment-emergent anti-risankizumab antibodies [evaluated either as a flag (positive/negative) or as a titer], showed no statistically significant effects on risankizumab CL. None of the patients in the phase 3 studies had NAbs [9, 10].
As is often the case for late-stage patient trials, the pharmacokinetic data in the two pivotal phase 3 PsA studies were limited to the predose samples collected at week 28 to accommodate for immunogenicity sample collections at the trough timepoint. The collection time at week 28 for pharmacokinetics is different from that for the primary endpoints at week 24. Additionally, the limited amount of pharmacokinetic data made it challenging to use the population pharmacokinetic model to simulate individual exposures. Nevertheless, because risankizumab is known to reach steady state by week 16 and exposures at week 24 and week 28 in patients recruited in the phase 2 PsA study were found to be highly correlated, observed Ctrough at week 28, instead of model-simulated individual exposure predictions, were used to investigate exposure–efficacy relationships for endpoints evaluated at week 24.
Exposure–response analyses of risankizumab efficacy endpoints at week 24 were carried out using data from the two phase 3 studies in patients with active PsA. Since the primary efficacy endpoints during the double-blind treatment period in the phase 2 study were evaluated up to week 16, data from this study were not included in the analyses. With the phase 3 data, exposure–response analyses consistently showed that the clinical regimen provided robust efficacy across the entire exposure range compared with placebo for all endpoints evaluated at week 24. Exposure–response quartile analyses showed that patients with relatively lower risankizumab exposures had numerically lower response rates compared with higher exposures observed for the efficacy endpoints at week 24. This suggests that further lowering exposures at doses less than 150 mg might cause lower efficacy response rates for these endpoints.
Logistic regression analyses at week 24 demonstrated that patients treated with risankizumab showed statistically significant higher response rates compared with placebo for all evaluated endpoints. After accounting for a treatment effect, risankizumab systemic exposures showed no statistically significant exposure–response relationships for all endpoints within the range of exposures evaluated in phase 3 studies. This indicates that further increasing exposures at doses greater than 150 mg is unlikely to provide additional efficacy. Analyses of the key safety variables of interest indicated no apparent relationship between risankizumab exposure and any AE, SAE, infection, or serious infection over the first 24 weeks in the phase 3 studies of risankizumab in patients with active PsA.
Conclusions
Risankizumab exhibited linear pharmacokinetics in patients with active PsA that was consistent with pharmacokinetics observed in patients with plaque psoriasis. None of the covariates evaluated were found to have a clinically meaningful impact on risankizumab exposures. The exposure–response for efficacy and safety analyses indicates that the clinical regimen of risankizumab (150 mg SC at weeks 0 and 4, and q12w thereafter) achieved robust efficacy responses with no apparent relationship between risankizumab exposure and key safety variables in patients with active PsA.
References
Duarte GV, Faillace C, Freire-de-Carvalho J. Psoriatic arthritis. Best Pract Res Clin Rheumatol. 2012;26(1):147–56. https://doi.org/10.1016/j.berh.2012.01.003.
Gladman DD, Chandran V. Observational cohort studies: lessons learnt from the University of Toronto Psoriatic Arthritis Program. Rheumatol (Oxf). 2011;50(1):25–31. https://doi.org/10.1093/rheumatology/keq262.
Singh S, Kroe-Barrett RR, Canada KA, Zhu X, Sepulveda E, Wu H, et al. Selective targeting of the IL23 pathway: generation and characterization of a novel high-affinity humanized anti-IL23A antibody. MAbs. 2015;7(4):778–91. https://doi.org/10.1080/19420862.2015.1032491.
SKYRIZI® (risankizumab-rzaa) injection, for subcutaneous use. [United States package insert]. North Chicago, IL; AbbVie Inc. 2019.
Kristensen LE, Keiserman M, Papp K, McCasland L, White D, Lu W, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225–31. https://doi.org/10.1136/annrheumdis-2021-221019.
Ostor A, Van den Bosch F, Papp K, Asnal C, Blanco R, Aelion J, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis. 2022;81(3):351–8. https://doi.org/10.1136/annrheumdis-2021-221048.
AbbVie Inc. A long term extension trial of BI 655066/ABBV-066 (Risankizumab), in patients with moderately to severely active Crohn's Disease. ClincialTrials.gov identifier: NCT02513459. 2020. https://clinicaltrials.gov/ct2/show/NCT02513459. Accessed 26 Mar 2021.
AbbVie Inc. A Study to Evaluate the Effect of Intravenous (IV) Infusions of Risankizumab on Pharmacokinetics of Cytochome P450 Substrates in Adult Participants With Moderately to Severely Active Ulcerative Colitis or Crohn's Disease. ClincialTrials.gov identifier: NCT04254783. 2020. https://clinicaltrials.gov/ct2/show/NCT04254783. Accessed 26 Mar 2021.
Pang Y, Khatri A, Suleiman AA, Othman AA. Clinical pharmacokinetics and pharmacodynamics of risankizumab in psoriasis patients. Clin Pharmacokinet. 2020;59(3):311–26. https://doi.org/10.1007/s40262-019-00842-5.
Suleiman AA, Minocha M, Khatri A, Pang Y, Othman AA. Population pharmacokinetics of risankizumab in healthy volunteers and subjects with moderate to severe plaque psoriasis: integrated analyses of phase I–III clinical trials. Clin Pharmacokinet. 2019;58(10):1309–21. https://doi.org/10.1007/s40262-019-00759-z.
AbbVie, Inc. BI 655066/ABBV-066/Risankizumab compared to placebo in patients with active psoriatic arthritis [ClinicalTrials.gov identifier NCT002719171]. National Institutes of Health, ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT02719171. Accessed 4 March 2022.
AbbVie, Inc. A study to investigate safety with risankizumab in psoriatic arthritis subjects who have completed week 24 visit of study M16-002 [ClinicalTrials.gov identifier NCT02986373]. National Institutes of Health, ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT02986373. Accessed 4 March 2022.
Suleiman AA, Khatri A, Minocha M, Othman AA. Population pharmacokinetics of the interleukin-23 inhibitor risankizumab in subjects with psoriasis and Crohn’s disease: analyses of phase I and II trials. Clin Pharmacokinet. 2019;58(3):375–87. https://doi.org/10.1007/s40262-018-0704-z.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–60. https://doi.org/10.1056/NEJMoa1607017.
Svensson RJ, Jonsson EN. Efficient and relevant stepwise covariate model building for pharmacometrics. CPT Pharmacometr Syst Pharmacol. 2022. https://doi.org/10.1002/psp4.12838.
Acknowledgements
Funding
The studies and the journal’s Rapid Service Fee were supported by AbbVie. AbbVie provided financial support for the studies and participated in the study design, study conduct, analysis and interpretation of data, and the writing, review, and approval of this manuscript.
Medical Writing, Editorial and Other Assistance
The authors acknowledge the work of Siddartha Kondapally and Aneri Pandya, employees of AbbVie, in programming the datasets. The authors also thank Stormy Koeniger, PhD, an employee with AbbVie for medical writing support.
Authorship Contributions
N.T., R.D., W.L., Y.P., and A.A.S. contributed to the study concept and design. N.T. and A.G. analyzed the data. All authors contributed to the analyses and interpretation of the data and the writing of this manuscript.
Disclosures
Neha Thakre, Ronilda D’Cunha, Aline Goebel, Wei Liu, and Yinuo Pang are employees of AbbVie Inc and may hold stock or stock options. Ahmed A. Suleiman is a former AbbVie Employee and may hold AbbVie stock or options.
Compliance with Ethics Guidelines
The studies reported herein were conducted in accordance with the International Council for Harmonisation (ICH) guidelines, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. Approval was granted by institutional review boards and independent ethics committees at participating institutions. All participants provided written consent prior to participation or study-related procedures. All individual participants signed informed consent regarding publishing their data.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis datasets), as well as other information (e.g., protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Thakre, N., D’Cunha, R., Goebel, A. et al. Population Pharmacokinetics and Exposure–Response Analyses for Risankizumab in Patients with Active Psoriatic Arthritis. Rheumatol Ther 9, 1587–1603 (2022). https://doi.org/10.1007/s40744-022-00495-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00495-0