Abstract
Introduction
In patients with rheumatoid arthritis (RA), attaining remission or low disease activity (LDA), as recommended by the treat-to-target approach, has shown to yield improvement in symptoms and quality of life. However, limited evidence from real-world settings is available to support the premise that better disease control is associated with lower healthcare costs. This study fills in evidence gaps regarding the cost of care by RA disease activity (DA) states and by therapy.
Methods
This retrospective cohort study linked medical and prescription claims from Optum Clinformatics Data Mart to electronic health record data from Illumination Health over 1/1/2010–3/31/2020. Mean annual costs for payers and patients were examined, stratifying on DA state and baseline use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologics, and targeted synthetic (ts)DMARDs. Subgroup analysis examining within-person change in costs pre- and post-initiation of new therapy was also performed. Descriptive statistics, means, and boot-strapped confidence intervals were analyzed by DA state and by RA therapy. Furthermore, multivariate negative binomial regression analysis adjusting for key baseline characteristics was conducted.
Results
Of 2339 eligible patients, 19% were in remission, 40% in LDA, 29% in moderate DA (MDA), and 12% in high DA (HDA) at baseline. Mean annual costs during follow-up were substantially less for patients in remission ($40,072) versus those in MDA ($56,536) and HDA ($59,217). For patients in remission, csDMARD use was associated with the lowest mean annual cost ($25,575), tsDMARD was highest ($75,512), and tumor necrosis factor inhibitor (TNFi) ($69,846) and non-TNFi ($57,507) were intermediate. Among new TNFi (n = 137) and non-TNFi initiators (n = 107), 31% and 26% attained LDA/remission, respectively, and the time to achieve remission/LDA was numerically shorter in TNFi vs. non-TNFi initiators. For those on biologics, mean annual within-person medical and inpatient costs were lower after achieving LDA/remission, although pharmacy costs were higher.
Conclusions
Cost of care increased with increasing DA state, with patients in remission having the lowest costs. Optimizing DA has the potential for substantial savings in healthcare costs, although may be partially offset by the high cost of targeted RA therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Real-world evidence available to support the premise that better rheumatoid arthritis (RA) disease control is associated with lower medical healthcare costs is limited. |
In this retrospective cohort study that linked administrative claims from Optum Clinformatics Data Mart to disease activity measures in electronic health record data from Illumination Health, we aimed to describe the attainment of specific disease activity state by RA therapy and quantify the associated costs and healthcare utilization. |
What was learned from the study? |
The cost of care increased with increasing disease activity. Patients in remission incurred one-third less in total medical costs compared with patients in high disease activity (HDA) and 29% less compared with those in moderate disease activity (MDA). Similarly, patients in remission had 51% and 56% lower inpatient costs compared to those with MDA and HDA, respectively. |
For patients who transitioned from M/HDA to remission or low disease activity (LDA), the mean annual outpatient and inpatient costs were approximately $5000 to $8000 lower, depending on the therapy received. |
Results from this analysis support the treat-to-target approach that encourages aggressive and appropriate treatment to achieve improved disease control. Reducing disease activity has the potential for substantial savings in healthcare costs; however, these savings may be partially offset by increased pharmacy costs. |
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder affecting about 1–2% of the North American population [1, 2]. Based on data from 2004 to 2014, the prevalence of RA in the U.S. is relatively low, estimated at 0.41–0.54%, which translates to approximately 1.3 million adults [3]. However, its associated cost of care is high, with an estimated annual cost of $19.3 billion (in 2005 dollars) in the U.S. [4]. Current management guidelines from the American College of Rheumatology recommend a treat-to-target (T2T) approach that starts with conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, and endorses advancing to biologic DMARDs (bDMARDs; e.g., tumor necrosis factor inhibitors [TNFis]) and targeted synthetic DMARDs (tsDMARDs; e.g., Janus kinase inhibitors [JAKis]) if patients do not achieve low disease activity (LDA) or remission. These therapies have been shown to dramatically improve symptoms and quality of life in a large proportion of RA patients, with achieving disease remission, or at least LDA being the goal of the treatment [5]. The possibility that better disease control, as encouraged by a T2T approach, presents an opportunity to not only improve clinical outcomes but also to potentially lower healthcare costs [6].
A limited amount of real-world evidence is available to support the premise that better RA disease control is associated with lower medical healthcare costs. In a retrospective analysis linking a large U.S. RA registry to fee-for-service Medicare claims data, healthcare utilization and cost savings of achieving remission or LDA in comparison to moderate or high disease activity (M/HDA) was quantified. Annual medical costs were lower by more than $3000 for patients in remission versus for patients with MDA [7]. As a component of reduced healthcare utilization, the incidence of hospitalizations was lowest for patients in remission and highest for patients with HDA [7]. In a separate analysis using the same RA cohort, patients with M/HDA had statistically higher rates of hospitalizations (37.3 per 100 patient-years) and joint surgeries (20.8 per 100 patient-years) compared with patients in sustained remission (13.5 per 100 patient-years for hospitalizations and 9.8 per 100 patient-years for joint surgeries) [8]. Rates for patients with LDA/MDA were intermediate between remission and HDA. This analysis provided supportive data consistent with the hypothesis that LDA may reduce RA-related costs. However, the high multimorbidity profile and older age of the cohorts linked to Medicare data has uncertain generalizability to younger and more typical RA patient cohorts. Moreover, the cost analysis did not evaluate whether the costs associated with an individual patient decreased if they initiated a new RA therapy and subsequently attained a lower disease activity state. Therefore, our objective was to describe the frequency of attainment of specific disease states by RA therapy and quantify the associated costs in a typical RA population with commercial health insurance receiving care in routine community practice settings.
Methods
Study Overview
This was a retrospective observational cohort study using medical and prescription claims from the Optum Clinformatics Data Mart (CDM) linked to rheumatology electronic health records (EHR) in the Illumination Health data warehouse. The Illumination Health repository includes data from a national rheumatology practice-based research network (Bendcare, part of the OneFlorida+ Clinical Research Network Consortium [9], one of the eight Clinical Research Networks that comprise PCORnet [10]). Data residing in the Illumination Health data warehouse are curated from more than 700 community rheumatology providers practicing throughout the United States. In aggregate, it contains longitudinal patient-level health plan claims data in addition to disease activity scores and other clinical measures for RA. The study period spanned January 1, 2010, through March 31, 2020, or as the intersection of the claims and EHR data (described in subsequent sections) allowed.
Cohort Selection
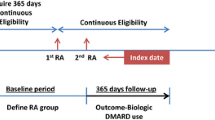
Adults (i.e., ≥ 18 years old) with a diagnosis of RA as identified by at least two or more rheumatologists’ diagnosis codes for RA using International Classification of Diseases (ICD)–9 codes (714.0, 714.2, and 714.81) and ICD-10 codes (M05.*, M06.*, ignoring M06.1 and M06.4) between January 1, 2010, and December 31, 2019, were eligible for the study. They were also required to have at least 6 months of continuous coverage with pharmacy and medical benefits in Optum CDM and at least two or more rheumatologist visits in the EHR data with a valid RA disease activity measurement in the Illumination Health data warehouse. Although there are several metrics by which clinical RA disease activity can be measured [11], the Clinical Disease Activity Index (CDAI) was preferred, as it incorporates data from both patients and rheumatology providers. Given that the CDAI is typically only assessed at clinic visits, and based on some quality metrics, may only be checked once or twice a year [12], CDAI values in the Illumination Health data warehouse were considered valid for up to 18 months after each recorded measurement before censoring occurred. Lastly, patients had to have at least one filled/administered medication claim for an RA medication, defined as the index drug, and they had to be continuously eligible in the health plan for at least 6 months following the index date (Optum CDM). Thus, patients were required to have data from both sources to be eligible for inclusion: CDAI and RA diagnoses from the EHR, and RA diagnosis, health coverage and pharmacy claims data from Optum CDM. Patients were excluded if they had any diagnosis of other autoimmune and connective tissue diseases (e.g., psoriatic arthritis, ankylosing spondylitis, and other spondyloarthropathies, systemic lupus erythematosus, scleroderma, dermatomyositis, polymyositis, and primary systemic vasculitis) in the year prior to the index date, and were censored if there was a diagnosis for these during follow-up. The study schema is described in Supplementary Fig. 1. The study protocol was approved by the Advarra Institutional Review Board (Pro00043329) and was conducted in accordance with the Health Insurance Portability and Accountability Act compliance requirements.
Index Date and Follow-up Periods
To establish a baseline period for covariate assessment, the start of follow-up was anchored at the index date, defined as the calendar date that the patient met all inclusion/exclusion criteria detailed above. Data in the 12 months prior to the index date were used to establish baseline demographic and clinical characteristics, including disease activity state and costs, with certain covariates (e.g., number of prior b/cs/tsDMARDs used) assessed using all available prior data, to a maximum of 36 months. Patients were censored at the time of loss of enrollment in the health plan, the end of the study period (March 31, 2020), or a gap of more than 18 months since the most recent CDAI value.
Data Sources
The study used data from the Optum CDM and linked it to the Illumination Health Real-World Evidence Platform. Optum CDM is derived from a database of administrative health claims for members of a large national managed care company affiliated with Optum. The database includes approximately 17–19 million annual covered lives. The CDM data comprise both commercial and Medicare Advantage health plan data, including individuals over the age of 65 years.
The Illumination Health platform is an EHR-based repository of real-world rheumatology data from community rheumatology practices representing more than 700 community rheumatology providers utilizing one of two commonly used EHR systems. The rheumatology practices are spread across the US, and the study population included in the study represents RA patients with commercial health insurance. It captures clinical elements, ICD-10 diagnoses, procedures, current and past medications, and laboratory results. Patient-generated data were also included, including patient-reported outcomes captured both in office and out-of-office via mobile technologies (tablet app and smartphone apps). Follow-up required concurrent enrollment in the commercial health plan that temporally overlapped with the first and last visit in the EHR data.
Linking Data Sources
Linking of Optum CDM and Illumination Health data elements was enabled by third-party software designed for this purpose (Datavant). Data were tokenized and de-identified, consistent with Expert Determination Certifier recommendations. Records across data components (e.g., medical claims, pharmacy claims, and EHR) were then linked using the unique token for each patient before the transfer of the data file to the analytic research team. Patients in the Illumination Health data warehouse were linked to the Optum database, after which the cohort selection criteria were applied. In addition to requiring overlapping enrollment in the provider’s practice and the health plan, an additional step to confirm the validity of the linkage was applied. This step required at least two RA diagnosis codes, at least one of which had to occur on the exact same calendar date in both datasets for the linkage to be considered valid.
Exposure Variables
RA disease activity as the main exposure variable was identified in the EHR data and was assessed using the CDAI and classified as remission (CDAI ≤ 2.8), low (LDA, CDAI > 2.8 and ≤ 10), moderate (MDA, CDAI > 10 and ≤ 22), and high (HDA, CDAI > 22) [13, 14]. Exposure variables included the most recent therapy prescribed to the patients for RA, prior to the index date. The therapy groups were as follows: csDMARDs: methotrexate, sulfasalazine, hydroxychloroquine, and leflunomide as monotherapy or in combination with other csDMARDs; TNFis: etanercept, adalimumab, certolizumab, golimumab, and infliximab as monotherapy or in combination with csDMARDs; non-TNFis: abatacept, rituximab, sarilumab, and tocilizumab as monotherapy or in combination with csDMARDs; tsDMARDs: tofacitinib, upadacitinib, and baricitinib (i.e., JAKi) as monotherapy or in combination with csDMARDs; and “none” was defined as none of the above treatments prescribed in the baseline period, even though they met the RA cohort inclusion criteria. National drug code and healthcare common procedure coding system codes were used to identify the medications prescribed to these patients in the claims data.
Outcome Assessment
The primary outcome was healthcare costs for different disease activity states. Costs were obtained directly from the health plan and were standardized prior to being made available to the study team to avoid disclosure of proprietary information. This procedure was done by the health plan prior to analysis to establish standard costs that reflect the allowed payments across all provider services. For example, professional service rates were assigned using a resource-based relative value scale approach.
Costs reflected those paid by the payer and the patient, and included the estimated paid amount, patient co-insurance, copayments, and deductibles. Costs were grouped as medical costs (outpatient physician visits, diagnostic and laboratory services), inpatient costs (relating to hospitalization), and pharmacy costs, which included both filled prescription medications and intravenous (IV) infusions (which typically are categorized as medical costs, but were reclassified so as to group RA medications that bill under the medical benefit as drug-related costs). Total costs represented the sum of these costs. To account for inflation, the study team used the cost factors table provided by the health plan [15]. The annual cost factor was multiplied by the cost data for each specific year and type of service to normalize to 2020 costs. The secondary outcomes include the mean time (in days) to achieve remission or LDA and the mean time patients remained in remission or LDA in a subgroup with M/HDA at the index date.
Covariates
Covariates were assessed at the index date and were used to characterize patients overall and by the disease states described previously. Covariates of interest included demographics, clinical characteristics, and comorbidities thought to potentially influence treatment response (e.g., diabetes mellitus, chronic obstructive pulmonary disease (COPD), fibromyalgia, and depression) and those common to RA (e.g., cardiovascular disease and osteoporosis). RA-related medications including nonsteroidal anti-inflammatory drugs, opioids, and glucocorticoids were also described.
Statistical Analysis
Overview
The primary analysis described the proportion of patients in remission or LDA, including those who started in a higher disease category and achieved remission or LDA over time. The study also assessed costs, overall and by baseline RA therapy groups. Standardized mean differences (SMDs) were used to compare exposure groups, with SMDs > 0.10 generally considered as being clinically relevant [16]. Because disease activity varies over time, disease activity as measured by the CDAI was updated during follow-up daily, as often as recorded in the EHR by the rheumatology provider. Costs were attributed to the CDAI disease activity category (remission, low, moderate, and high) associated with each person-day of follow-up, yielding time-based cost intervals. For example, an RA patient examined by a rheumatology provider and recorded in the EHR as being in moderate disease activity would have all costs for that day, and all subsequent costs until the next CDAI measurement, accrue to the moderate disease activity category. If at a subsequent rheumatologist visit they moved to low disease activity, then the subsequent costs would be attributed to the low disease activity category. The CDAI was updated, and healthcare costs were accrued, on a person-day basis.
Subgroup Analyses
An analysis of a subgroup of patients who were in M/HDA at the index date and who subsequently started a new RA therapy described the mean time in days to achieve remission or LDA. Among those who achieved LDA or remission, we also assessed the mean time in days in remission or LDA before censoring. Finally, because the associations between cost and disease activity did not provide direct evidence that costs for any given individual might be reduced if they attained LDA, we calculated a within-person difference in costs by comparing costs in the 6 months prior to initiating a new therapy versus the costs 4–10 months post initiation. The 4- to 10-month time frame was selected given that the onset of action of most RA therapies yields a near-maximal benefit by approximately 4 months [17,18,19].
Analytic Methods
Descriptive statistics were used to depict the demographic and clinical characteristics of the patients by disease activity and therapy group. Frequencies and percentages were reported for categorical variables. Means and standard deviations, and medians and percentiles were provided for continuous variables. Given the skewness of the data, log transformation was considered, but given the nontrivial frequency of zero costs, we bootstrapped confidence intervals. Two hundred resamples using replacement created a synthetic sample of the same size as the original dataset. Confidence intervals were estimated using the percentile intervals of the mean value of the 200 repetitions. Multivariable adjustment was done to control for confounding by age, sex, and comorbidities (ischemic heart disease, diabetes mellitus, osteoporosis, COPD, depression, and fibromyalgia), selected based on clinical knowledge and avoiding factors that might be causally related to RA disease activity or function, which might lead to overadjustment. Adjusted costs were modeled using negative binomial regression, with remission as the referent category. All analyses were performed using R Statistical Software (version 4.1.0) [20].
Results
There were 36,498 patients with ≥ 1 RA diagnosis code. After considering overlapping time periods where patients had data from both data sources, there were 2339 patients that met the inclusion criteria for the final study cohort (Fig. 1). A total of 344 patients were excluded because they lacked ≥ 6 months of follow-up in the health plan data.
At baseline (Table 1), 440 patients were in remission, 936 in LDA, 676 in MDA, and 287 in HDA. Patients in the HDA cohort were slightly younger (63 years compared with 69, 67, and 66 years for remission, LDA, and MDA cohorts, respectively) and all cohorts had predominantly female and Caucasian patients. Across disease activity categories, approximately one-fourth to one-half of the patients were younger than age 65 years, but all the study population (including those above age 65) had commercial health insurance. Approximately half of patients were on csDMARDs (49.9–55.5% based on disease activity category); 274 patients did not have any DMARD use in the baseline period despite meeting all inclusion criteria and were excluded from the therapy analyses. Patients in HDA were twice as likely to be on steroids and opioids compared with those in remission. The mean follow-up duration for patients in the study was 520 days.
Cost of Care by RA Disease Activity and Therapy Group
The overall mean annual cost of RA care was significantly higher for patients with MDA ($56,536) or HDA ($59,217) versus those in remission ($40,072) or with LDA ($48,027). Both total costs and each cost subtype were lowest among those in remission and increased with higher disease activity. For example, the mean pharmacy totals, including prescriptions and IV infusions, gradually increased with higher disease activity ($20,045 for patients in remission to $24,636 for patients with HDA) (Table 2, Supplementary Fig. 2). Medical costs also were lowest for patients in remission ($16,032) compared with patients in LDA ($19,792), MDA ($24,838), or HDA ($25,414).
After adjusting for key baseline characteristics, the mean annual total costs for patients in LDA, MDA, and HDA were higher by $5183, $10,080, and $13,304, respectively, than for patients in remission (Table 2).
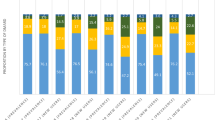
Comparing baseline therapy groups, annual mean costs during follow-up period were lowest for patients on csDMARDs ($34,683), highest for patients on tsDMARDs ($93,771), and similar among TNFis and non-TNFis ($72,301 and $68,420, respectively; Table 3). Figure 2 graphically describes medical and inpatient costs, excluding pharmacy-related costs, and showed that regardless of which RA therapy the patients was receiving, those in remission or LDA had lower costs than those in M/HDA. For patients in remission, csDMARD use was associated with the lowest mean total annual cost ($25,575), tsDMARD was highest ($75,512), and TNFi ($69,846) and non-TNFi ($57,507) were intermediate.
Mean annual medical and inpatient costs by index RA therapy and follow-up stage disease activity. Note: the above costs do not include pharmacy costs for outpatient prescription drugs, nor those given by IV infusion and billed as medical procedures. csDMARD conventional synthetic disease-modifying antirheumatic drug, TNFi tumor necrosis factor inhibitor, RA rheumatoid arthritis, tsDMARD targeted synthetic disease-modifying antirheumatic drug
Subgroup Analysis of RA Patients Initiating a New Therapy
The within-person cost analysis comparing costs when patients were in M/HDA prior to initiation of a new therapy versus 4–10 months after initiation is shown in Table 4. There were too few patients initiating tsDMARDs to be included in this analysis; similarly, there were too few patients initiating non-TNFis, so TNFi and non-TNFi initiators were combined into a single bDMARD category. Pharmacy total costs for the bDMARD and csDMARD patients were appreciably higher in the follow-up period, as expected when people switch to more expensive treatments (increased by $18,742 for individuals already on biologics, and $38,999 for those who had been on csDMARDs). Both medical costs without infusion and inpatient costs were appreciably lower in the post-initiation period than those in the baseline period.
The mean time to achieve remission/LDA was similar for TNFi and tsDMARD initiators (123 vs. 126 days), while the mean time for non-TNFi initiators to reach remission/LDA was slightly longer (149 days). The mean time in remission/LDA for patients receiving TNFis was numerically longer compared with that for those on non-TNFis and tsDMARDs (399 vs. 227 vs. 175 days).
Change in RA Disease Activity
The majority of patients remained in the same disease activity stage from baseline to end of the follow-up period (60–67% of patients in the on-diagonal cells), however 25% went from LDA at baseline to remission and 18% went from MDA at baseline to LDA during follow-up (Supplementary Table 1). Additionally, 177 patients (37% of all those ending in remission) achieved remission by end of follow-up, 123 (25%) patients from LDA, 46 (10%) from MDA, and 8 (2%) from HDA (Supplementary Table 1). Comparable results were shown when assessing disease activity change by therapy group (Supplementary Table 2), with most remaining in the same disease state as baseline regardless of the therapy. Similar proportions across each therapy group went from M/HDA to LDA. Only 10% or fewer patients started in HDA and stayed there by the end of follow-up. The proportion of patients who started in remission and LDA and remained in remission and LDA by the end of follow-up was appreciably higher, across therapies.
Discussion
In this analysis of RA patients receiving care in community rheumatology practice settings, lower disease activity, particularly remission, was associated with lower healthcare costs. Patients in remission incurred one-third lower medical costs of care compared with patients in HDA and 29% less medical costs compared with those in MDA. Similarly, patients in remission had 51% and 56% lower inpatient costs than patients in MDA and HDA, respectively. For individual patients who started in M/HDA who subsequently attained LDA or remission, annual outpatient and inpatient medical costs were approximately $5000–$8000 lower, depending on the therapy patients received.
The clinical benefits of attaining remission in RA have been well described. For example, the study of etanercept and methotrexate in combination or as monotherapy (SEAM)-RA trial recruited 371 RA patients receiving methotrexate and etanercept that were in sustained remission by the Simplified Disease Activity Index (SDAI ≤ 3.3) and randomized them to continue both therapies or discontinue either methotrexate or etanercept [21]. In the 253 patients that sustained remission through a 24-week open-label period and met trial inclusion criteria, the benefits on clinically assessed RA and patient-reported outcomes were most appreciable for those that remained in remission, which was approximately two-fold more likely among patients who remained on etanercept monotherapy rather than on methotrexate monotherapy [22]. Several other clinical trials and observational studies have likewise demonstrated the clinical benefits of remaining in remission [23,24,25,26]. A retrospective study compared the long-term clinical, functional, and cost outcomes in 471 Dutch patients with early RA treated according to T2T strategy and found that achieving early remission was associated with better clinical and functional outcomes, and lower healthcare costs. Patients in remission at 2, 3, or 6 months had significantly lower medication costs per patient over the first 2 and 3 years of treatment, but no notable differences in total healthcare resource costs (hospital admissions and consultations) were observed [26]. In another retrospective cohort study of 335 U.S. RA patients, using data from Optum EHR linked to claims from commercial and Medicare advantage health plans, Bergman et al., compared all-cause and RA-related costs and resource use over a 1-year period in patients who achieved DAS28 or RAPID3-based remission vs. those who did not achieve remission, and found that the annual all-cause total costs were significantly less in the remission cohort vs. non-remission cohort ($30,427 vs. $38,645), while the RA-related medical costs were numerically lower in the remission cohort vs. non-remission cohort ($8594 vs. $10,002) [24]. An observational analysis of 1084 Canadian RA patients assessed healthcare service utilization costs using data from the Alberta Biologics Pharmacosurveillance Program linked with provincial physician billing claims, outpatient visits, and hospitalizations in patients using biologics for RA. That study reported the mean annual healthcare service utilization cost savings of $2391 and $2104 for those in sustained remission (remission for ≥ 1 year) and those with non-sustained LDA (LDA for < 1 year) relative to the persistent M/HDA group [23]. Finally, in a longitudinal and cross-sectional analysis of 356 RA patients, Radner et al., compared direct and indirect costs in patients with different levels of disease activity (remission, LDA, and M/HDA) classified according to the SDAI scores and found significant differences in direct and indirect costs across disease activity levels with higher costs in patients with M/HDA (mean annual total directs costs in remission vs. LDA vs. M/HDA: €1368 vs. €1643 vs. €2340) [25]. Results from this analysis extend the evidence base showing that medical costs for patients in remission are lower than medical costs for those in higher disease activity states, an outcome that may be particularly compelling information for U.S. health plans, payers, and stakeholders with interest in value-based care in RA. Indeed, there are few U.S.-based studies that have been able to examine disease activity in relation to cost data paid by payers or health plans [7, 24]. The analysis conducted by Bergman et al. included only 335 patients (n = 125 who ever went into remission), all non-remission disease activity categories were aggregated together (perhaps due to sample size), disease activity was not considered in a time-varying fashion, and the DAS28-based definition of remission used was more permissive than CDAI-based definition of remission in the present study [24]. In another prior analysis of U.S. RA registry data linked to fee-for-service Medicare claims, patients were older (mean age approximately 70 years) and had a higher comorbidity profile than those in the current analysis [7]. While the largest cost driver in RA patients, biologic and targeted therapy use [27], was not reported on a person-time basis in that analysis, the annual costs for patients in remission were $11,146 per year, a crude difference of $9300 (1.83-fold) compared with costs for those in HDA. This was comparable with our observed difference in the ratio of costs for those in HDA compared with remission, which was 1.48-fold ($59,217/$40,072) different in this cohort. Finally, the Medicare-based analysis did not quantify the cost savings for individual patients who initiated a new RA therapy while in MDA or HDA to assess cost savings for those who achieved remission or LDA, as evaluated in the present study.
Many published cost analyses in RA have not been able to account for the influence of disease activity. Results from a systematic literature review of four studies found that among patients receiving bDMARDs, the total direct medical costs were $36,053 annually, and the costs attributable to RA itself comprised more than half that amount [28]. Our findings further describe that the costs related to not only treatment but also disease activity are appreciably less expensive for patients in remission than for patients in M/HDA. This finding was observed regardless of the background therapy (i.e., csDMARDs or b/tsDMARDs) received by patients. Although not an explicit focus of our analysis, prior work has also shown that failure to respond to RA treatment is associated with higher cost of care [29, 30], which should motivate health plans and other payers to authorize use of the most effective medications as quickly as possible so as to maximize the likelihood patients attain remission, or at least, LDA. To the extent that precision medicine in RA may allow more rational treatment selection regarding the choice of initial biologic or targeted therapy for individual RA patients [31], cost savings would be expected.
The strengths of our work include representation of a large number of RA patients treated in numerous and diverse community practice settings, with an analysis of a unique data source in which disease activity data as measured by rheumatology providers was linked with cost data from health plans. Indeed, although substantial economic literature exists in RA, few U.S. studies have been able to combine clinical disease activity measures with health plan cost data. Nevertheless, results of our study should be interpreted in light of its design. Disease activity as measured by the CDAI was updated only at rheumatology provider visits, which typically occur only a few times per year and likely resulted in some misclassification of disease activity. The effect of such misclassification likely would have attenuated differences between disease activity categories, yielding a conservative estimate. Additionally, the number of person-years in some exposure groups such as tsDMARDs were small, leading to wide confidence intervals. This analysis included but did not focus on comorbidities, which have been shown to relate to costs in RA patients [32]. Thus, these results may or may not generalize to younger patients with few comorbidities. Finally, drug costs were standardized by the health plan and do not reflect rebates or fees (e.g., specialty pharmacy), information that is typically considered proprietary information by health plans and makes it difficult to compare with costs measured in other datasets (e.g., Medicare or other commercial health plans).
Conclusions
RA patients in lower disease activity, particularly remission, had lower healthcare costs across the spectrum of treatment options. In addition to the clinical benefit of better disease control, results from this analysis support T2T guidelines that encourage aggressive and appropriate treatment to attain better disease control. Our findings provide insight into the opportunity for cost savings for payers and other stakeholders where financial incentives might be better aligned along with removal of administrative barriers such as burdensome prior authorization requirements, to encourage appropriate treatment escalation for patients who are not in LDA or remission.
References
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–22.
Jacobs P, Bissonnette R, Guenther LC. Socioeconomic burden of immune-mediated inflammatory diseases–focusing on work productivity and disability. J Rheumatol Suppl. 2011;88:55–61.
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37:1551–7.
Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26:77–90.
Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1108–23.
Ostor AJ, Sawant R, Qi CZ, Wu A, Nagy O, Betts KA. Value of remission in patients with rheumatoid arthritis: a targeted review. Adv Ther. 2022;39(1):75–93.
Curtis JR, Chen L, Greenberg JD, Harrold L, Kilgore ML, Kremer JM, et al. The clinical status and economic savings associated with remission among patients with rheumatoid arthritis: leveraging linked registry and claims data for synergistic insights. Pharmacoepidemiol Drug Saf. 2017;26:310–9.
Boytsov N, Harrold LR, Mason MA, Gaich CL, Zhang X, Larmore CJ, et al. Increased healthcare resource utilization in higher disease activity levels in initiators of TNF inhibitors among US rheumatoid arthritis patients. Curr Med Res Opin. 2016;32:1959–67.
The OneFlorida+ Clinical Research Network Consortium. https://onefloridaconsortium.org/about/. Accessed 16 Mar 2022.
PCORnet. The National Patient-Centered Clinical Research Network. https://pcornet.org/network/. Accessed 16 Mar 2022.
England BR, Tiong BK, Bergman MJ, Curtis JR, Kazi S, Mikuls TR, et al. 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res (Hoboken). 2019;71:1540–55.
Rheumatoid arthritis (RA): Periodic assessment of disease activity (2019). Version 4. https://qpp.Cms.Gov/docs/qpp_quality_measure_specifications/cqm-measures/2019_measure_177_mipscqm.Pdf. Accessed 15 Dec 2021.
Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100-108.
Mack ME, Hsia E, Aletaha D. Comparative assessment of the different American College of Rheumatology/European League Against Rheumatism remission definitions for rheumatoid arthritis for their use as clinical trial end points. Arthritis Rheumatol. 2017;69:518–28.
OptumInsight. Clinformatics DataMart. User Manual (2020). Eden Prairie, MN, USA
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Chen M, Peng D, Zhang Z, Zuo G, Zhao G. Efficacy of etanercept for treating the active rheumatoid arthritis: an updated meta-analysis. Int J Rheum Dis. 2016;19:1132–42.
Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ. 2016;353: i1777.
Singh JA, Hossain A, Mudano AS, Tanjong Ghogomu E, Suarez-Almazor ME, Buchbinder R, et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;5:CD012657.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. https://www.R-project.org/. Accessed 16 Mar 2022.
Curtis JR, Trivedi M, Haraoui B, Emery P, Park GS, Collier DH, et al. Defining and characterizing sustained remission in patients with rheumatoid arthritis. Clin Rheumatol. 2018;37:885–93.
Curtis JR, Emery P, Karis E, Haraoui B, Bykerk V, Yen PK, et al. Etanercept or methotrexate withdrawal in rheumatoid arthritis patients in sustained remission. Arthritis Rheumatol. 2021;73:759–68.
Barnabe C, Thanh NX, Ohinmaa A, Homik J, Barr SG, Martin L, et al. Healthcare service utilisation costs are reduced when rheumatoid arthritis patients achieve sustained remission. Ann Rheum Dis. 2013;72:1664–8.
Bergman M, Zhou L, Patel P, Sawant R, Clewell J, Tundia N. Healthcare costs of not achieving remission in patients with rheumatoid arthritis in the United States: A retrospective cohort study. Adv Ther. 2021;38:2558–70.
Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther. 2014;16:R56.
Ten Klooster PM, Oude Voshaar MAH, Fakhouri W, de la Torre I, Nicolay C, van de Laar M. Long-term clinical, functional, and cost outcomes for early rheumatoid arthritis patients who did or did not achieve early remission in a real-world treat-to-target strategy. Clin Rheumatol. 2019;38:2727–36.
Hsieh P-H, Wu O, Geue C, McIntosh E, McInnes IB, Siebert S. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis. 2020;79:771–7.
Hresko A, Lin T-C, Solomon DH. Medical care costs associated with rheumatoid arthritis in the US: A systematic literature review and meta-analysis. Arthritis Care Res (Hoboken). 2018;70:1431–8.
Curtis JR, Schabert VF, Harrison DJ, Yeaw J, Korn JR, Quach C, et al. Estimating effectiveness and cost of biologics for rheumatoid arthritis: application of a validated algorithm to commercial insurance claims. Clin Ther. 2014;36:996–1004.
Grabner M, Boytsov NN, Huang Q, Zhang X, Yan T, Curtis JR. Costs associated with failure to respond to treatment among patients with rheumatoid arthritis initiating TNFi therapy: a retrospective claims analysis. Arthritis Res Ther. 2017;19:92.
Cohen S, Wells AF, Curtis JR, Dhar R, Mellors T, Zhang L, et al. A molecular signature response classifier to predict inadequate response to tumor necrosis factor-α inhibitors: the NETWORK-004 prospective observational study. Rheumatol Ther. 2021;8:1159–76.
An J, Nyarko E, Hamad MA. Prevalence of comorbidities and their associations with health-related quality of life and healthcare expenditures in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38:2717–26.
Acknowledgements
Funding
The study was sponsored by Amgen Inc. Amgen also funded the Rapid Service Fee for this publication.
Editorial Assistance
Lakshmi Narendra Bodduluru, PhD, of Cactus Life Sciences (part of Cactus Communications) and Julie Wang, DPM, of Amgen Inc. provided editorial assistance, funded by Amgen Inc.
Author Contributions
JRC and HO designed the study. JRC, HO, KMF, and DC provided strategic input into the study design. JRC, HO, and KMF provided oversight and management of the study. CC, FX, and YS conducted the data analysis. All authors provided data interpretation and key input and review of the manuscript.
Disclosures
Jeffrey R. Curtis has received research grants and consulting fees from AbbVie, Amgen, Aqtual, Bendcare, Bristol Myers Squibb, CorEvitas, Eli Lilly, FASTER, GSK, IlluminationHealth, Janssen, Labcorp, Myriad, Novartis, Pfizer, Scipher Medicine, Setpoint, Tnacity Blue Ocean, UCB and United Rheumatology. David Collier is an employee and stockholder of Amgen Inc. Hafiz Oko-osi was an employee of Amgen Inc, at the time of conduct of this study. Kathleen M. Fox is an Amgen contract worker with no stock options. Fenglong Xie, Yujie Su, and Cassie Clinton have no conflicts to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Advarra Institutional Review Board (Pro00043329) and was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments, and Health Insurance Portability and Accountability Act compliance requirements.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://www.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Curtis, J.R., Fox, K.M., Xie, F. et al. The Economic Benefit of Remission for Patients with Rheumatoid Arthritis. Rheumatol Ther 9, 1329–1345 (2022). https://doi.org/10.1007/s40744-022-00473-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00473-6