Abstract
Introduction
Adhesive capsulitis (AC), which is characterised by shoulder pain and a limited range of motion (ROM), is usually diagnosed on the basis of clinical suspicion, with imaging only being used to exclude other causes of similar symptoms. The aim of this study was to identify and describe the typical ultrasound (US) features of AC in a group of patients with shoulder pain and stiffness.
Methods
This was a cross-sectional study of 1486 patients with AC in which two experienced US specialists examined the axillary pouch (AP), the coracohumeral ligament (CHL), the superior glenohumeral ligament (SGHL), and the long head of the biceps tendon (LHBT), and dynamically visualised the infraspinatus tendon during passive external rotation (PER) during a US evaluation of shoulder ROM.
Results
AC was confirmed in 106 patients (7.1%). Thickening of the AP of more than 4 mm was observed in 93.4% of the patients, whereas 6.6% showed AP thickening of less than 4 mm but more than 60% of the thickening in the contralateral shoulder. Effusion within the LHBT sheath was detected in 71% of the patients, and thickening of the CHL or SGHL in 88%. The dynamic study of the infraspinatus tendon showed reduced sliding with folding towards the joint capsule in 73% of cases, thus changing the tendon’s profile from flat to concave during PER. The reduced tendon sliding was associated with a bouncing movement that returned the tendon to its baseline resting position in 41.5% of cases.
Conclusions
We believe a sufficiently experienced US specialist can confirm a clinical diagnosis of AC by carrying out a comparative study of APs, evaluating the thickness of the CHL and SGHL, and detecting reduced sliding of the infraspinatus tendon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Adhesive capsulitis (also known as frozen shoulder) is a frequent disorder that causes pain and significantly limits shoulder function. |
Its diagnosis is generally based on the patient’s history and a physical examination. |
We studied the typical ultrasound features of adhesive capsulitis in patients referred to our outpatient clinic because of a painful shoulder. |
What was learned from the study? |
Axillary pouch thickening, reduced sliding of the infraspinatus tendon, and thickening of the shoulder pulleys are typical ultrasound findings of adhesive capsulitis. |
A sufficiently trained specialist can confirm a clinical diagnosis of adhesive capsulitis by carrying out a comparative ultrasound study of the shoulders. |
Digital Features
This article is published with digital features, including a video, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.17105714.
Introduction
Adhesive capsulitis (AC), also known as frozen shoulder, is a frequent pathological condition that causes pain and significantly limits the range of motion (ROM) of the shoulder. It was first described by Duplay in 1872, when it was given the name scapulohumeral periarthritis [1], and Codman called it frozen shoulder in 1934 [2]. However, it was not until 1945 that is was given its current name to describe capsular thickening of the glenohumeral joint after Neviaser had investigated the characteristics of the disorder [3].
AC is of uncertain aetiopathogenesis but characterised by significantly reduced active and passive movement in the absence of intrinsic shoulder disease [4]. It is most frequent in middle-aged women, usually in their fifth-seventh decade of life [5]. The prevalence of AC in the general population is about 2–5% but as high as 20% in people with diabetes [6, 7]. It often first affects one shoulder (usually the non-dominant shoulder) and then its contralateral after a period ranging from 6 months to 7 years [4, 8, 9]; however, it never affects the same shoulder more than once [4]. It can be divided into primary (idiopathic) and secondary AC, with the latter developing after stiffness and immobility caused by a previous shoulder trauma or surgery [10]. Other causes may be calcific tendinopathy of the rotator cuff tendons during the painful resorptive phase described by Uhthoff [11], or a systemic dysmetabolic, endocrinological, rheumatological, immunological, neurological, cardiac disorder, cancer, and sometimes Dupuytren’s disease [4, 9].
Only a few published papers have reliably and accurately described the ultrasound (US) features of AC, and the European Society of Musculoskeletal Radiology does not currently recommend its diagnostic use [12]. The diagnosis of AC is clinical, and based on the patient’s history and a physical examination. However, although imaging studies are not diagnostically necessary, they may help to exclude other causes of the symptoms [13].
The aim of this study was to identify and describe the typical US signs of AC in a group of patients affected by shoulder pain and stiffness.
Methods
Patients
Between April 2015 and May 2020, 1486 consecutive adult patients with shoulder pain and stiffness were examined by a specialist with 30 years of experience of musculoskeletal US to identify and evaluate the US features of AC. During the last 18 months of this period (from February 2019 to May 2020), he was joined by a second operator with 15 years of experience, and so 502 of the 1486 patients (about one-third) were examined by both, thus allowing an evaluation of the inter-rater reliability of the findings. The study period also included Italy’s COVID-19 lockdown from March to May 2020.
The exclusion criteria were (1) the presence of bilateral shoulder symptoms suggesting AC; (2) signs suggesting degenerative alterations of the shoulder (e.g. due to osteoarthritis); (3) the US detection of a lesion of the long head of the biceps tendon (LHBT) pulley that may limit ROM in external rotation; and (4) the US detection of an hourglass lesion of the LHBT [14, 15].
Fifty healthy volunteers (25 women and 25 men) with a mean age of 44.6 ± 13.2 years (34–69) were enrolled as a control group.
The study was approved by the Medical Research Ethics Committee of our institution (Comitato Etico Milano Area 2, approval no. 1080_2021bis), and informed consent was obtained from all of the patients. All of the study procedures were carried out in accordance with the ethical standards of our institution and/or the National Research Council, and the 1964 Declaration of Helsinki and its later amendments. In accordance with the current Italian legislation concerning privacy, all of the patients were guaranteed that their privacy would be protected and that their personal data would be properly used.
US Scanning Technique
The patients’ medical history was recorded to identify possible predisposing conditions, after which the patients underwent a preliminary clinical examination to evaluate any functional limitations in active and passive motion. They subsequently also underwent a US examination carried out using a Philips HD 15 with a 12-MHz multifrequency linear transducer and a GE EQ9 with a 6–15-MHz matrix linear probe in greyscale: gain and focus were adjusted to suit the different locations being scanned. A power Doppler signal was sought at a frequency of 7.5 MHz and a pulse repetition frequency of 0.8 kHz. The images were acquired and read at the same time. Dynamic flexion excursion (FE), adduction/internal rotation (IR) and external rotation (ER) were also assessed, the last of these to check the regular displacement of the subscapularis tendon insertion by at least 90° [16].
The rotator cuff tendons, subacromial-subdeltoid bursa (SAB), and the LHBT were carefully examined (focusing on signs of effusion within the tendon sheath, ELHBT), as well as the pulley ligaments (the coracohumeral ligament [CHL] and the superior glenohumeral ligament [SGHL]). The maximum thicknesses of the CHL and SGHL were compared with those in the unaffected contralateral shoulder, and axial measurements were made at the level of the pulley with the LHBT in short-axis view. The scanning position was adjusted to avoid anisotropic artefacts, and the probe was kept in the orthogonal scanning plane. The pulley ligaments were considered to be thickened if they were 60% thicker than those in the contralateral shoulder. The surface profile of the rotator interval, which normally has a concave contour, was also studied.
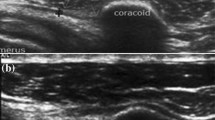
The axillary pouch (AP) was examined by means of oblique axial and coronal scans (Fig. 1a, b) following the humeral profile, which includes the anatomical neck and humeral head. The AP is the inferior part of the glenohumeral capsule located between the anterior and posterior bands of the inferior glenohumeral ligament (IGHL) [4] (Fig. 2). The presence of AP thickening was carefully investigated using the axillary approach and its maximum thickness was measured with the arm abducted 70°–90° in neutral rotation. It was considered thickened if it was more than 4 mm thick (the cut-off value reported in the current scientific literature) [6, 17, 18]; if it was less than 4 mm thick, thickening was defined as more than 60% of the thickness in the unaffected contralateral shoulder. Maximum AP thickness was measured in transversal and coronal scans. The axillary approach also allowed the exclusion of skeletal deformities of the humeral head due to osteoarthritis, which may be responsible for limited ROM in external and internal rotation. The presence of joint effusion was also investigated by studying the AP in slight abduction (30–40°).
Finally, a dynamic examination was used to assess the sliding of the infraspinatus tendon over the articular capsule during passive external rotation (PER) controlled from behind the patient. The infraspinatus tendon was studied by following its long axis using a posterior scapular approach to identify any altered backsliding [19]; the PER was carried out firmly to ensure that any alteration could be identified.
The results are described using the 2020 European League Against Rheumatism (EULAR) recommendations for reporting ultrasound studies in rheumatic and musculoskeletal diseases [20].
Clinical Confirmation of a US Diagnosis of Adhesive Capsulitis
After US imaging, all of the patients with suspected AC were examined by a specialist orthopedic consultant to make a definite diagnosis. The consultant, who was aware of the US findings, recorded each patient’s history of shoulder pain and stiffness and carried out a physical examination to investigate active and passive ROM, particularly in external but also in internal rotation, and compared the findings with those relating to the contralateral shoulder. According to the recent clinical practice guidelines issued by the Orthopedic Section of the American Physical Therapy Association, AC can be diagnosed in the presence of a greater than 25% loss of passive ROM in at least two planes combined with a 50% loss of passive external/internal rotation [21]. If the clinical evaluation suggested AC, no other investigations were made, and the diagnosis of AC was considered confirmed; the patients with an uncertain diagnosis were referred for a magnetic resonance imaging (MRI) examination.
Statistical Analysis
The Kolmogorov–Smirnov test was used to assess the normality of data distribution. The diagnostic accuracy of the US features was calculated on the basis of the sensitivity and specificity of the test in comparison with the current clinical criteria for AC. The Mann–Whitney test was used to compare quantitative parameters, and the intraclass correlation coefficient (ICC) was calculated to evaluate inter-rater reliability. The agreement of categorical data was assessed using Cohen’s kappa coefficient. According to Cicchetti [22], ICC and Cohen’s kappa values of greater than 0.75 are associated with excellent inter-rater agreement. The analyses were made using R statistical software, version 3.5.0, with p values of 0.05 or less being considered statistically significant.
Results
Patients’ Characteristics
Of the 1486 patients who were brought to our attention because of shoulder pain, 106 (7.2%) presented with clinical and instrumental evidence of AC: 71 women and 35 men with a mean age of 55.6 ± 15.3 years (28–67). These 106 patients underwent an orthopedic evaluation to exclude other disorders, and the 30 with an uncertain diagnosis also underwent supplementary instrumental investigations (X-ray and/or MRI) to exclude alternative disorders that may have been responsible for their shoulder stiffness, pain, and reduced ROM. After the clinical and instrumental evaluations 71 patients were diagnosed as having primary AC (67%), and 35 (33%) as having secondary AC due to concomitant predisposing conditions: 19 with rotator cuff calcific tendinopathy in Uhthoff’s resorptive phase [11]; seven with diabetes mellitus; two with thyroid diseases; two with dyslipidemia; one with Dupuytren’s disease; and four who had been recently immobilised.
The pre-COVID-19 prevalence of AC was 2–5% [4] but during Italy’s lockdown of March–May 2020, this dramatically increased to 43% of the subjects examined for shoulder impairment (13/30).
Shoulder ROM
Shoulder ROM was assessed during the course of dynamic US imaging, which revealed that the patients with AC had an active ROM that was as limited as or only slightly different from their passive ROM: 91% had limited FE; 98% limited IR; and 100% limited ER, defined as the altered displacement of the subscapularis tendon insertion with an ER excursion of less than 60–70°.
US Findings in Patients with AC
Study of the anatomical structures of the shoulder showed SAB thickening (more than 1.8 mm) in 12 of the 106 patients (11%); mean thickness 2.1 ± 0.21 mm (1.9–2.5) (Table 1). AP thickening of greater than 4 mm (mean 5.3 ± 1.15 mm, 4–9) (Fig. 3) was detected in 99 patients (sensitivity 93.4% and specificity 98%) in comparison with the controls (p < 0.0001) and also in comparison with the AP of the contralateral shoulder, which had similar thickness values to those of the controls (mean 1.9 ± 0.20 mm, 1.5–2.5; p < 0.0001). Seven patients (6.6%) showed AP thickening of less than 4 mm (mean 3.7 ± 0.16 mm, 3.4–3.9) but, in all cases, there was a significant, more than 60% difference from the thickness of the contralateral AP (p < 0.05) (Fig. 4). The sensitivity and specificity of the AP thickness were 100% and 98% respectively and the diagnostic accuracy was 98%. Fourteen patients (13%) showed thin anechoic joint effusion in the AP due to the entrapment of small amounts of fluid as a result of capsular contracture (Fig. 3). No power Doppler US signal was detected in the AP of any of the patients with AC.
a Oblique axial section: red arrows show the thickness of the axillary pouch in a patient with AC (AP thickening between the calipers). b Coronal section: red arrows show the thickness of the AP in another patient with AC. The dashed blue arrows indicate a small amount of joint effusion entrapped in the AP. HH humeral head, SN surgical neck
Twenty-three of the 1380 patients without AC presented with AP thickening of greater than 4 mm on both the affected and contralateral side. This was interpreted as a pathological finding not due to AC.
Mean AP thickness in the control group was 1.7 ± 0.7 mm (range 1.1–2.9), which was significantly different from that of the patients with AC with an AP thickening of greater than 4 mm (p < 0.0001) and those with an AP thickening of less than 4 mm (p < 0.05).
Seventy-five of the patients with AC (71%) showed ELHBT. Pulley thickening (CHL and/or SGHL) in comparison with the contralateral shoulder was detected in 93 patients (88%): mean thickness 2.3 ± 0.25 mm (1.6–2.7) vs 1 ± 0.11 mm (0.8–1.2); p < 0.0001. This sign had a sensitivity of 88%, a specificity of 52% and a diagnostic accuracy of 54.6%. The pulley was more than 60% thicker than that of the contralateral shoulder in all cases. Of the 93 patients with CHL or SGHL thickening, US showed that the normally concave rotator interval had a rounded and convex profile (Fig. 5) in 38, and a “pseudo-double” tendon appearance of the LHBT in the transverse section of the pulley in 43 (Fig. 6). The smaller false tendon was the CHL if it was lateral to the LHBT, and the SGHL if it was medial.
Axial section of the rotator interval of a right shoulder in a patient with AC. Coracohumeral ligament (CHL) thickness with a “pseudo-double” tendon appearance due to the smaller false tendon, which is the CHL lateral to the LHBT. CHL coracohumeral ligament, GT greater tuberosity, LHBT long head of the biceps tendon, LT lesser tubercle
The rotator cuff tendons appeared to be normal in 92 patients (87%), were affected by minor alterations (mild tendinopathy) in ten (9.4%), and showed a full thickness supraspinatus tendon tear in only four cases (3.7%): two Snyder type I and two Snyder type II lesions. The LHBT showed no intra-articular thickening [14, 15]. Dynamic visualisation of the infraspinatus tendon sliding backwards during a firm PER controlled by the specialist showed a characteristic change in shape from a flat to a concave profile: this sliding leads to the folding of the tendon internally towards the joint capsule (the tendon runs strictly adjacent to the capsule), and may be minimal or more pronounced (Figs. 7, 8). This feature was found in 77/106 (72.6% sensitivity, 100% specificity and 98% of diagnostic accuracy) but was only seen on the affected side and never on the contralateral side. In the seven patients with AP thickening of less than 4 mm but more than 60% of that on the contralateral side, the characteristic change from a flat to a concave profile of the infraspinatus tendon was seen during a firm PER of the shoulder. Furthermore, 44 patients with evident folding of the infraspinatus tendon during PER (44/106, i.e. 41.5% of the total population with AC and 44/77, i.e. 57% of patients with AC with evident folding of the infraspinatus tendon during PER) showed another distinctive feature that we called the bounce sign (sensitivity 41.5%, specificity 100%, diagnostic accuracy 95.8%). This is a sometimes slight, rapid and fleeting jolting of the infraspinatus tendon as it folds toward the articular capsule during ER that quickly returns to baseline resting position, something like a plucked guitar string (Video 1).
Video 1: Fifty-seven percent of the patients with AC whose infraspinatus tendon folded during PER showed another distinctive feature that we called the bounce sign: a sometimes slight, rapid and fleeting jolting of the infraspinatus tendon as it folds towards the articular capsule during ER that quickly returns to its baseline resting position—something like the movement of a plucked guitar string (MP4 1406 KB)
Dynamic study of the infraspinatus tendon (white arrows) sliding backwards during passive external rotation governed by the US specialist (T1–T2). Note the characteristic change in the tendon from a flat to a concave profile (white arrowhead). The sliding of the tendon folds it towards the joint capsule because they are closely contiguous. HH humeral head
Intraclass Correlation Coefficient (ICC)
The ICC of the inter-rater agreement of AP measurements in 42 patients with AC was 0.998 (p < 0.0001), which indicates an excellent level of agreement and therefore good inter-rater reliability. Cohen’s kappa for the qualitative measurement of the sliding of the infraspinatus tendon over the joint capsule during PER was 0.85, which also represents an excellent level of inter-rater agreement.
Discussion
AC is currently clinically diagnosed on the basis of the patient’s history and a physical examination: although not required, imaging is considered a useful means of excluding other causes of shoulder stiffness and pain [13]. However, a diagnosis of AC can be made simply on the basis of some typical US imaging features. The benefits of US in evaluation of shoulder problems are well known in scientific literature [23, 24]. There are only a few reports in the literature that reliably describe the specific US signs characterising AC [4, 6, 18], and so the European Society of Musculoskeletal Radiology does not recommend the diagnostic use of US in such cases [12].
About one-third of our patients presented with a positive history of triggering causes or predisposing factors such as calcific tendinopathy of the rotator cuff, diabetes mellitus, limb immobilisation, thyroid diseases, dyslipidemia, or Dupuytren’s disease.
Calcific tendinopathy of the rotator cuff in the phase of painful resorption (Uhthoff’s phase 3) [11] is characterised by the presence of non-solid calcifications that can penetrate the articular space, and often induce an acute reactive inflammatory response (chemical arthritis) and later AC. In our opinion, many of the so-called idiopathic cases of AC are actually secondary to this pathological condition that may not have been previously diagnosed because it may be asymptomatic or only mildly symptomatic.
Our data indicate that AC is not rare but has a prevalence of 7.1%. Published reports [16, 18] describe a characteristic discrepancy between the clinical presentation of severe pain and functional limitation and the very poor findings of a standard US study of the shoulder. The symptoms, together with US findings of thickening of the AP and pulley ligaments [25] and the abnormal sliding of the infraspinatus tendon revealed by dynamic US imaging during PER [19], are fundamental for a correct diagnosis [4, 18]. Maximum AP thickness is easily measured by means of US imaging, and may be caused by thickening of the anterior or posterior bundle of the IGHL or full AP thickening between the bundles caused by synovial inflammation [18]. In patients with AC, it is easier to use transversal scans because of the limited abduction of the arm but, in our experience, coronal AP scanning is also usually feasible even if it is more difficult.
ELHBT has frequently been described as an indirect and aspecific sign of AC due to capsular shrinkage [25], and was detected in 71% of our cases. Also CHL or SGHL thickening is often reported to be the only sign of AC [4, 13, 25], but is not specific as it is frequently associated with other conditions such as overuse syndrome, trauma, etc. An MRI study of patients with AC first reported the finding of AP thickening in 1995 [17], but only a few subsequent studies have confirmed this [4, 16, 26, 27]. However, since the US imaging study by Michelin et al. [18], there has been greater recognition of the importance of US imaging assessments of the AP in the diagnosis of AC [4, 6, 19]. In 2004 [28], we started a preliminary study of the features of the AP in patients with AC using early-generation US equipment and poor-definition 7.5-MHz probes that identified an apparent distension of the recess that was initially misinterpreted as hypoechoic joint effusion. However, subsequent use of more modern musculoskeletal US equipment and probes made it clear that the hypoechoic image was a sign of the thickening of the articular capsule [29]. The present study used the latest-generation US equipment and found a thin capsular effusion in 13% of our patients with AC that was due to the entrapment of very small amounts of fluid as a result of capsular contraction.
The typical folding of the infraspinatus tendon when it slides backwards during PER, as well as the bounce sign in some patients when it returns to its initial position, is caused by the close relationship between the infraspinatus tendon and the glenohumeral joint capsule. The “downward dragging” of the tendon during PER is probably caused by adhesions between the tendon and the capsule in full-blown disease. The posterior part of the joint capsule is also often affected by capsular contracture, but to a lesser extent than the anterior and inferior parts. The bounce sign further reveals that the fibrous branches enveloping the deep portion of the infraspinatus tendon that is in contact with the joint capsule block the tendon and hinder its sliding backward after PER. In some patients, the tendon’s final sliding movement releases the adhesions and leads to its bouncing return to a normal position.
During the COVID-19 lockdown in Italy (February to May 2020), there was an increase in the proportion of cases of AC as 13 of the 30 patients (43%) presented with shoulder pain during this period. Although this may have been a selection bias due to referrals to a shoulder US specialist by other physicians or physiotherapists, it is an interesting epidemiological finding because, in addition to seasonal factors, it was probably due to inactivity during the lockdown being followed by a return to work that may have favoured the onset of the disease.
Most patients with AC experience resolution with non-surgical treatment, and only a small percentage of patients eventually require surgical treatment [30]. Physical therapy is the pillar of AC treatment, regardless of the disease stage. However, early treatment of AC is warranted to accelerate recovery [31]. Both oral non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular corticosteroid injections are effective in the treatment of AC [32]. Intra-articular corticosteroid injections provided benefit lasting up to 12 weeks, but not in the long term [33]. Another option is ultrasonography-guided hydrodistension, which was demonstrated to decrease pain with an effect lasting up to 4 months [34]. However, when non-surgical management fails within 12 months, surgical intervention is indicated. Manipulation under anaesthesia alone or in addition to arthroscopic capsular release (limited or circumferential) can be performed. However, this procedure requires expertise because of its potential complications [35, 36].
AC prevention is possible only in the secondary forms due to orthopedic surgery and shoulder trauma by early mobilizing the limb, when possible. Follow-up by dynamic US may be useful to monitor the improvement of the ROM after treatment.
Study Limitations
One limitation is that the study did not include multiparametric assessments of AC (using a power Doppler US evaluation of the rotator interval as well as elastography and contrast-enhanced ultrasound, CEUS) that may have contributed more information about the disorder. Another is that the exclusion criteria were based on physical examination and US imaging findings, and may not have correctly identified borderline cases such as Neviaser’s stage IV resolution phase [37] or patients with mild symptoms.
Conclusions
US imaging of the shoulder is a rapid, inexpensive and non-invasive technique that allows the comparative study of both shoulders. If carried out by an adequately experienced specialist, it can detect the typical features of AC described in this paper (AP thickening, the typical folding of the infraspinatus tendon during PER, and thickening of the pulley) and can therefore be used to confirm a clinical diagnosis.
References
Duplay ES. De la peri-arthrite scapulo humerale et des raideurs de l’epaule qui en son la consequence. Arch Gen Med. 1872;20:31–42.
Codman E. The shoulder: rupture of the supraspinatus tendon and other lesions in or about the subcromial bursa. Boston: Todd; 1934.
Neviaser JS. Adhesive capsulitis of the shoulder: a study of the pathological findings in periarthritis of the shoulder. J Am Acad Orthop Surg. 1945;27:211–22.
Fields BK, Skalski MR, Patel DB, et al. Adhesive capsulitis: review of imaging findings, pathophysiology, clinical presentation, and treatment options. Skeletal Radiol. 2019;48:1171–84.
Binder A, Bulgen D, Hazleman B, Roberts S. Frozen shoulder: a long-term prospective study. Ann Rheum Dis. 1984;43:361–4.
Zappia M, Di Pietto F, Aliprandi A, et al. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging. 2016;7:365–71.
Le HV, Lee SJ, Nazarian A, Rodriguez EK. Adhesive capsulitis of the shoulder: review of pathophysiology and current clinical treatments. Shoulder Elbow. 2017;9:75–84.
Reeves B. The natural history of the frozen shoulder syndrome. Scand J Rheumatol. 1975;4:193–6.
Hsu JE, Anakwenze OA, Warrender WJ, Abboud JA. Current review of adhesive capsulitis. J Shoulder Elbow Surg. 2011;20:502–14.
Lundberg BJ. The frozen shoulder: clinical and radiographical observations the effect of manipulation under general anesthesia structure and glycosaminoglycan content of the joint capsule local bone metabolism. Acta Orthop Scand. 1969;40:1–59.
Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5:183–91.
Klauser AS, Tagliafico A, Allen GM, et al. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur Radiol. 2012;22:1140–8.
Le HV, Lee SJ, Nazarian A, Rodriguez EK. Adhesive capsulitis of the shoulder: review of pathophysiology and current clinical treatments. Should Elb. 2017;9:75–84.
Boileau P, Ahrens PM, Hatzidakis AM. Entrapment of the long head of the biceps tendon: the hourglass biceps—a cause of pain and locking of the shoulder. J Shoulder Elb Surg. 2004;13:249–57.
Ricci V, Becciolini M, Özçakar L. “Hourglass” biceps tendon: an ultrasound “mime” of frozen shoulder? J Ultrasound Med. 2020;39:1021–2.
Van Holsbeeck MT. Musculoskeletal ultrasound. Radiol Clin North Am. 1999;37:907–25.
Emig E, Schweitzer ME, Karasick D, Lubowitz J. Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am J Roentgenol. 1995;164:1457–9.
Michelin P, Delarue Y, Duparc F, Dacher JN. Thickening of the inferior glenohumeral capsule: an ultrasound sign for shoulder capsular contracture. Eur Radiol. 2013;23:2802–6.
Draghi F, Trentanni C, Stella SM, Ciampi B, Bortolotto C, Galletti S. Patologia tendinea, capsulite adesiva. In: Galletti S, editor. Ecografia patologica muscoloscheletrica. Testo e Atlante: Piccin Editore Padova; 2018. p. 355–9.
Costantino F, Carmona L, Boers M, et al. EULAR recommendations for the reporting of ultrasound studies in rheumatic and musculoskeletal diseases (RMDs). Ann Rheum Dis. 2021;80:840–7.
Kelley MJ, Shaffer MA, Kuhn JE, et al. Shoulder pain and mobility deficits: adhesive capsulitis: clinical practice guidelines linked to the international classification of functioning, disability, and health from the Orthopaedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2013;43:A1–31.
Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284.
Han DS, Wu WT, Hsu PC, Chang HC, Huang KC, Chang KV. Sarcopenia is associated with increased risks of rotator cuff tendon diseases among community-dwelling elders: a cross-sectional quantitative ultrasound study. Front Med. 2021;8:630009.
Wu WT, Chen LR, Chang HC, Chang KV, Özçakar L. Quantitative ultrasonographic analysis of changes of the suprascapular nerve in the aging population with shoulder pain. Front Bioeng Biotechnol. 2021;9:640747.
Homsi C, Bordalo-Rodrigues M, Da Silva JJ, Stump XM. Ultrasound in adhesive capsulitis of the shoulder: is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006;35:673–8.
Carrillon Y, Noel E, Fantino O, Perrin-Fayolle O, Tran-Minh VA. Magnetic resonance imaging findings in idiopathic adhesive capsulitis of the shoulder. Rev Rhum Engl Ed. 1999;66:201–6.
Manton GL, Schweitzer ME, Weishaupt D, Karasick D. Utility of MR arthrography in the diagnosis of adhesive capsulitis. Skeletal Radiol. 2001;30:326–30.
Stella S, Ciampi B, Orsitto E, Benedetti E, Lippolis PV. Uno studio clinico e di imaging ecografico nella capsulite adesiva. XX SIUMB National Congress. Amsterdam: Elsevier; 2008. p. 2.
Stella SM, Ciampi B, Orsitto E, Melchiorre D, Lippolis PV. L'ispessimento del pouch ascellare verificabile con ecografia nella capsulite adesiva. XXIV SIUMB National Congress. Amsterdam: Elsevier; 2013. p. 54.
Levine WN, Kashyap CP, Bak SF, Ahmad CS, Blaine TA, Bigliani LU. Nonoperative management of idiopathic adhesive capsulitis. J Shoulder Elbow Surg. 2007;16:569–73.
van den Hout WB, Vermeulen HM, Rozing PM, Vliet Vlieland TP. Impact of adhesive capsulitis and economic evaluation of high-grade and low-grade mobilisation techniques. Aust J Physiother. 2005;51:141–9.
Dehghan A, Pishgooei N, Salami MA, et al. Comparison between NSAID and intra-articular corticosteroid injection in frozen shoulder of diabetic patients; a randomized clinical trial. Exp Clin Endocrinol Diabetes. 2013;121:75–9.
Prestgaard T, Wormgoor MEA, Haugen S, Harstad H, Mowinckel P, Brox JI. Ultrasound-guided intra-articular and rotator interval corticosteroid injections in adhesive capsulitis of the shoulder: a double-blind, sham-controlled randomized study. Pain. 2015;156:1683–91.
Yoong P, Duffy S, McKean D, Hujairi NP, Mansour R, Teh JL. Targeted ultrasound-guided hydrodilatation via the rotator interval for adhesive capsulitis. Skeletal Radiol. 2015;44:703–8.
Song C, Song C, Li C. Outcome of manipulation under anesthesia with or without intra-articular steroid injection for treating frozen shoulder: a retrospective cohort study. Medicine. 2021;100:e23893.
Takahashi R, Iwahori Y, Kajita Y, et al. Clinical results and complications of shoulder manipulation under ultrasound-guided cervical nerve root block for frozen shoulder: a retrospective observational study. Pain Ther. 2019;8:111–20.
Neviaser RJ, Neviaser TJ. The frozen shoulder diagnosis and management. Clin Orthop Relat Res. 1987;223:59–64.
Acknowledgements
This study is dedicated to the memory of Giovanni Serafini, MD.
Funding
No funding was received for the study or the publication of this article.
Authorship
All of the named authors meet the authorship criteria of the International Committee of Medical Journal Editors (ICMJE), take responsibility for the integrity of the work carried, and have approved the publication of this report.
Authorship Contributions
All of the authors contributed to the study conception and design; data collection and statistical analysis were carried out by SMS, RG and BC; the first draft of the manuscript was written by SMS and reviewed by all of the other authors.
Disclosures
Salvatore Massimo Stella, Roberta Gualtierotti, Barbara Ciampi, Cesare Trentanni, Luca Maria Sconfienza, Andrea Del Chiaro, Patrizia Pacini, Mario Miccoli, Stefano Galletti have nothing to disclose
Compliance with Ethics Guidelines
The study was approved by the Medical Research Ethics Committee of our institution (Comitato Etico Milano Area 2, approval no. 1080_2021bis), it was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. All of the subjects gave their informed consent to the study.
Data Availability
The datasets generated and/or analysed during this study are not publicly available for privacy reasons.
Patient Involvement
The authors would like to thank all of the participants for their involvement in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Stella, S.M., Gualtierotti, R., Ciampi, B. et al. Ultrasound Features of Adhesive Capsulitis. Rheumatol Ther 9, 481–495 (2022). https://doi.org/10.1007/s40744-021-00413-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00413-w