Abstract
This research investigates the effect of Schiff base, namely, N'-[4-(dimethylamino) benzylidene]-4-hydroxybenzohydrazide (SB) on the corrosion mitigation of mild steel (MS) in hydrochloric acid medium (HCl) using potentiodynamic polarization (PDP) technique. The study highlights the effect of SB concentration, HCl concentration, and temperature on the corrosion current density (icorr) and inhibition efficiency (IE). The adsorption of SB onto the MS surface was justified with the support of kinetic and thermodynamic parameters. The morphological behavior of the MS surface was analyzed by scanning electron microscopy (SEM) and atomic force microscopy (AFM). A blend of statistical study and interaction plots has been applied to obtain a thorough understanding of the corrosion parameters. Experimental results obtained by PDP measurements revealed that tested compounds had a good anti-corrosion capacity. The main and interaction effects of the parameters on the response were analyzed using Box-Behnken Design. An empirical model equation from experimental results elucidates the relationship among the variables. The optimized parameters for the maximum output were recognized. The maximum IE of 81.5 was predicted by RSM with temperature (A = 30 °C), HCl concentration (B = 0.5 M), and Inhibitor concentration (C = 0.0001 M). Using RSM, further analyses regarding individual and interaction effects between the variables can be more comprehensive. Few more parameters such as pH and time can be included in the future study.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mild steel is one of the extensively used ferrous alloys for a wide range of applications. It is the commonly employed metal for ample engineering applications due to its easy availability, low cost, and high strength, in fact it is the most extensively used metal compared to all the other metallic materials [1, 2]. Nevertheless, the high surface electrochemical reactivity of these materials has led to severe corrosion further resulting in high-cost waste every year [3, 4]. The annual losses due to corrosion include 3.4% of the global GDP and hence it has attained more attention by the industrial and academic sectors to reduce the losses caused due to corrosion process [5]. Recently, various corrosion combating methods were also employed. Among the effective approaches to cope up with these problems is the use of corrosion inhibitors if the process conditions permit. The use of corrosion inhibitors is the most practical, simple, and cost-effective technique [6]. These inhibitors get adsorbed onto the substrate either by chemical or physical mode and thus form a barrier at the metal electrolyte interface and hence reducing the corrosion rate [7, 8]. The inhibitor used in this case is a Schiff base molecule derived from the combination of benzo hydrazide and an aromatic aldehyde. Basically, benzohydrazide and derivatives find wide applications in the biological field making them a potential group of organic compounds [9]. The chemistry of the –C=N– group takes an important role in the inhibition process due to the presence of a double bond and lone pair of electrons on the nitrogen atom in the same moiety [10,11,12].
Mathematical techniques can provide valuable qualitative and quantitative evidence, for an improved understanding of this corrosion alleviation phenomenon. The design of the experiment (DoE) is an effective technique for determining new processes and optimization for better performance. DoE is a statistical and mathematical tool applied to elucidate the significance of specific processes and optimize system behavior. It aids in determining the factors with significant contribution to the process and also their interaction effects [13, 14]. DoE produces a lower number of experiments as compared to the traditional methods and decreases the time and cost for running the experiments [15].
An explanation of the system in terms of mathematical language and concepts is nothing but a mathematical model. It is a method employed to simulate real-time circumstances with mathematical model equations to predict their future behavior. The development of an empirical model is possible with numerous techniques. One of those techniques is Response surface methodology (RSM) [16]. The corrosion inhibition effect of Gongronema latifolium on aluminum in HCl solution was investigated using Central Composite design and the optimum Inhibition Efficiency at this optimum condition was predicted [17]. A study was designed to obtain the functional relationship between inhibition efficiency and operating variables by applying rotatable central composite design (RCCD) [18]. Factorial design was applied to determine the characteristics of the aluminum alloy under erosion-corrosion conditions in sulfuric acid and hydrochloric acid slurry [19, 20].
In the present research, a mathematical model was developed using RSM and BBD, to evaluate the relationship between the input variable [temperature, media, and inhibitor concentration] over the response [% IE].
Since the corrosion inhibition mechanism is dependent on certain factors, the metal/solution interfacial behavior cannot be explained by a sole experimental study. Therefore, there arises a need to combine the experimental and statistical study for an improved understanding of the corrosion mitigation process. In the present study, an effort is made to generate a statistical model to comprehend the inhibition mechanism along with the effect of various parameters on it. There are rarely few publications focusing on the behavior of corrosion inhibitors under optimized conditions.
Most of the research articles address only the influence of temperature, acid, and inhibitor concentration on the corrosion rate and percentage inhibition efficiency (%IE), but relatively less discussion on the optimization parameter for the corrosion process. So the present investigation emphasizes the corrosion inhibition process as well as statistical study, which includes a proper design of experiments to acquire data about the optimization conditions.
This research is, therefore, designed for dual purposes: (i) to establish the effectiveness of Schiff base as a corrosion inhibitor (ii) the use of Schiff base as an anti-corrosion agent for mild steel in acidic media with an optimization approach.
2 Experimental Procedure
2.1 Material and Conducting Medium
Commercially available MS sample is employed as working specimen with the following element composition (wt%): 0.15 (C), 0.15 (Si), 0.49 (Mn), 0.05 (P), 0.062 (S), and 0.05 (Cr). The specimens were prepared in cylindrical form, molded using cold setting resin having an open surface area of 1 cm2, and exposed to the corrosive medium. Initially, the test coupons are abraded with different grades of sandpapers, and the final surface finish was done by disk polishing. Analar grade hydrochloric acid solution was used to prepare the different concentrations (0.5, 1 and 1.5 M) corrosive medium.
2.2 Inhibitor Preparation
The Schiff base, namely, N'-[4-(dimethylamino) benzylidene]-4-hydroxybenzohydrazide (SB) was synthesized by the simple condensation reaction between N, N-dimethyl benzaldehyde (0.01 mol), and o-hydroxyl benzohydrazide (0.01 mol) as reported [21]. The synthetic method for the preparation of SB and characterization spectrum (FTIR and 1H NMR) for the same is given in Supplementary Fig. 1 (SF.1) and (SF.2), respectively.
2.3 Electrochemical Technique
The potentiodynamic polarization (PDP) technique was done using an electrochemical work station of model CH600 D-series and a three-electrode cell system. It consists of platinum (counter) mercury-mercurous (calomel reference) and MS as working electrodes all together embedded in Pyrex glass system. The steady-state open circuit potential (OCP) was allowed to attain by retaining the electrochemical system in an unaltered state at the end of minutes. After attaining OCP, the potential is sweeped in the range of − 250 to + 250 mV at a scan rate of 1 mV/s in order to obtain PDP results.
2.4 Surface Morphology Examination
SEM (EVO 18-5-57 model) and AFM (Innova-1B342 model) techniques were used to examine the surface morphological characterization of MS specimen. A specimen each was dipped in 1 M HCl and in 1 M HCl containing SB (30 ppm) for 3 h and then SEM and AFM pictures were documented.
2.5 Experimental Design
DoE is a statistical practice that was followed concurrently to determine the influence of multiple variables [22]. In this work, the parameters affecting the corrosion inhibition of mild steel in HCl which include temperature and inhibitor concentrations are investigated. The Input parameters and their levels are depicted in Table 1. Varying these parameters cause a significant effect on the corrosion protection of mild steel [23].
The statistical second-order polynomial model was used to evaluate the influence of the input parameters on the response.
where Y is the response output, β0 is the intercept constant, βi, βii, and βi are the regression coefficients of linear, square, and interaction terms of the model [24]. The model validity was proven using ANOVA and the significance of the terms was determined from the t-test and p-values (P < 0.05). The experimental procedure is represented in the form a flowchart in Fig. 1.
3 Results and Discussion
3.1 Inhibitor Characterization
Characterization of SB: Crystalline solid (95%); C16H17N3O2; m.p: 258–260 °C; IR (KBr) [cm−1]]: 3471(OH str.), 3178 (NH str.), 3109 (Ar. CH str.), 1627 (C=O), 1596 (C=N str.), 1504 (Ar. C=C str.), Fig. 1a.
1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.35 (1H, OH), 10.09 (1H, NH), 8.29 (1H, CH=N), 7.80–6.75 (8H, Ar. H), 2.98 (6H, CH3), Fig. 1b.
3.2 PDP Measurements
PDP plots for the deterioration of MS in 1 M HCl containing various concentration ns of SB at 30 °C are shown in Fig. 2a. Similar plots were obtained in 0.5 and 1.5 M HCl also. According to the literature [25], if the shift in the corrosion potential (Ecorr) value was on an average of less than ± 85 mV concerning blank, it suggests the mixed-type behavior of inhibitor and it hold holds good in the present study also. The corrosion current density (icorr) values obtained by extrapolating the cathodic and anodic plots are used in Eq. (1) to calculate % IE [26].
where icorr and icorr(inh) is the corrosion current density in the absence and presence of SB.
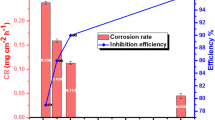
Results of the PDP on MS containing SB at different temperatures in various concentrations of HCl are given in Table 2. It is observed from Table 2 that the addition of SB resulted in a drastic decrease in the icorr and corrosion rate (CR). Further, the % IE increased with an increase in SB concentration. This is accredited to the increase in the surface coverage of the substrate by the adsorption of the SB [27].
3.3 Effect of Concentration of HCl, Variations in Temperature, and Kinetic Factors
To evaluate the effect of temperature on the % IE, the experiments were conducted at three different levels of temperatures (30, 40, and 50 °C) at three different levels of acid concentrations (0.5, 1.0, and 1.5 M) containing three levels of SB concentrations (5, 15 and 30 ppm). These results are then used to estimate the kinetic and thermodynamic parameters. It can be seen from Table 2 that the icorr values increased with an increase in temperature and acid concentration in the absence of SB. This may be due to an increase in the conductivity of the medium at a higher temperature, whereas % IE of SB is found to increase with increasing temperature, addressing the chemical adsorption of SB on the MS surface by transferring unpaired electrons to the unfilled d-orbital of iron [28]. On the other side, as the concentration of the medium increased, the % IE of SB is decreased. This can be accredited to the desorption of the SB molecules from the MS surface as a consequence of the increase in the aggressiveness of the medium [29]. The variation of the % IE (at 5 ppm of SB) at different temperatures and acid concentrations is given in Fig. 2b.
The % IE obtained at different temperatures is used to evaluate kinetic and thermodynamic parameters. The energy of activation (Ea) can be intended from the Arrhenius Eq. (2) and the enthalpy (ΔH#) and entropy (ΔS#) of activation from transition state Eq. (3), respectively [30].
where B is metal-dependent Arrhenius constant and R is the universal gas constant.
where h is Plank’s constant and N is Avagadro’s number. The Arrhenius plot of ln CR versus 1/T and plot of ln (CR/T) versus 1/T for the corrosion of MS in various concentrations of SB in 1 M HCl are illustrated in Fig. 3a and b correspondingly. The resultant data are tabulated in Table 3.
In Table 3, the lower values of Ea compared to the blank value attribute the chemical adsorption of SB molecule onto the MS surface because of the gradual rise in the adsorption at elevated temperature. Further, the decrease in Ea values in the presence of SB may be due to the swing in the net corrosion reaction from the unshielded part of the MS surface to the shielded one [31].
The surface coverage [ϴ = (% IE/100)] values were used to classify the best acceptable isotherm model and hence the adsorption type. Figure 4 showed a straight line with slope and correlation coefficient R2 values nearly equal to one, which specified that the adsorption of SB onto the MS surface obeyed Langmuir's isotherm model [32] as given by Eq. 4. [33].
where K is the adsorption equilibrium constant and Cinh is SB concentration (in ppm). The standard free energy of adsorption (ΔG°ads) was obtained from K using Eq. (5) [33].
where R is the universal gas constant and T is the absolute temperature.
The standard was thermodynamic expressions (6) and (7) that are used to obtain enthalpy of adsorption (ΔHoads) and the entropy of adsorption (ΔSoads) correspondingly [34]. The obtained adsorption parameters are tabulated in Table 4.
For the SB, the obtained ΔG°ads values lay between − 20 and − 40 kJ mol−1 indicating the probability of mixed adsorption [35]. The increase in the ∆G°ads values with the rise in temperature approaches the threshold value (− 40 kJ mol−1) by confirming the chemical adsorption of SB molecules onto the MS surface. The positive value of ΔHοads specifies the endothermic process due to the chemisorption of SB and the ΔS°ads value being highly negative demonstrated the reduction in disorderness from the metal to the inhibitor [36].
3.4 Surface Analysis
The difference in the surface morphology MS test coupons dipped in 1 M HCl with and without SB for 3 h was examined using SEM and AFM and the corresponding pictures are depicted in Fig. 5. SEM photographs of the MS specimen exposed to 1 M HCl without SB exhibited a rough and damaged surface with numerous pits caused due to the adsorption of chloride ions as shown in Fig. 5a. The smooth surface with a reduction in the number pits for the inhibited MS specimen at 5 and 30 ppm are shown in Fig. 5b and c, respectively. The microstructure of MS specimen immersed in 5 ppm of SB concentration showed (Fig. 5b) less smooth surface when compared to microstructure obtained at 30 ppm. It contains tiny pits on its surface but very less compared to microstructure of MS in the absence of inhibitor. This confirms formation of protective layer of SB molecules and the protection efficiency increased with increase in SB concentration from 5 to 30 ppm.
The surface unevenness of the unprotected (Fig. 6a) and protected (Fig. 6b) MS specimens was detected from the AFM images. The average surface roughness (Ra) and root-mean-square roughness (Rq) values obtained in the absence and presence of SB are 120.5, 144.8, 68.2,and 83.4 nm, respectively. The obtained roughness values of the substrate with SB were considerably lower than the degraded specimen surface which also confirms the reduction in the corrosion rate due to its adsorption on the MS surface.
3.5 Inhibition Mechanism
The inhibitive nature of any molecule and its adsorption onto the metal surface depends on its chemical structure, surface charge distribution, the morphology of specimen surface, type of corrosive media, operating conditions such as temperature, concentration/pH of medium etc. [37]. The obtained ΔGºads values confirm the mixed adsorption of SB molecule with predominant chemisorption and hence SB molecules can interact by both physical as well as a chemical mode of adsorption onto the MS surface. Physical adsorption is due to the electrostatic interactions between the protonated SB molecules and the charged MS surface. Generally, SB molecule protonated in HCl solution and the metal gets positive charge due to metal dissolution at anodic region, whereas H+ ions from the solution get reduced at the cathodic region. This positively charged metal surface attracts the negatively charged Cl− ion from HCl resulting in the formation of electrical double layer at the metal solution interface. This double layer facilitates the adsorption of protonated SB molecules onto it by electrostatic interaction, whereas chemical adsorption is due to donor–acceptor interactions [38]. The electron density at the potential centers of the SB molecule influences the inhibition efficiency. The π electrons of the two aromatic rings, imine group, and unbound lone pair electrons available on the nitrogen and oxygen atoms can be contributed to the empty d-orbitals leading to chemisorption [39]. The schematic representation for electrostatic and electron pair interaction is shown in Fig. 7.
3.6 Quantum Chemical Studies
The computational study enables density functional theory (DFT) calculations with Schrodinger material science suite with 6-31G basic set and B3LYP function. The highest frontier occupied (EHOMO) and lowest frontier vacant (ELUMO) molecular orbital energies were assessed. These parameters were then used to compute other quantum chemical parameters such as energy gap between then (ΔE), absolute electronegativity (χ), chemical hardness (ɳ), softness (σ), and the fraction of electron transfer (ΔN). The main advantage of this computational work is to provide a theoretical framework of effective inhibition mechanism based on the inhibitor structure and to predict the adsorption mode onto the material surface. The optimized structure of SB obtained using DFT, and the interactions between the structure of SB and its activity were also conferred. The optimized structures of SB and with HOMO and LUMO configuration are depicted in Fig. 8.
The greater EHOMO values of SB signify its better electron giving a tendency to the empty d-orbitals of Fe, whereas the lower ELUMO values denote stronger electron receiving tendencies from the metal [40, 41]. The difference between the HOMO and LUMO molecular orbitals (ΔE) displayed a lower value for SB, indicating lesser energy is required for the removal of an electron from the last occupied orbital and hence exhibiting higher inhibition efficiency [42]. According to the Koopmans theorem [43, 44], the electron affinity (A) and ionization potential (I) are obtained simply from A = − EHOMO and I = − ELUMO. The lesser the value of ionization potential, the easier the elimination of an electron from the inhibitor, and the higher will be its inhibition efficiency. The electronegativity (χ) and electrophilicity (ω) of SB were 1.1403 and 0.8769 which signifies the steadiness and reactivity of the inhibitor molecule [45].
Chemical hardness (η) and softness (σ) constraints are related according to the acid–base theory. Normally, a hard molecule has the minimum affinity, while a soft molecule has a high tendency to react. In the current case, the smaller values of chemical softness for SB indicate the evidence for its maximum inhibition efficiency [46, 47]. The possibility of the corrosion inhibitor to contribute the electrons to the material surface is explained depending on the fraction of electrons that are transferred (ΔN). According to the literature [48], ΔN value must be greater than zero and less than 3.6 for a proficient inhibitor. The ΔN value of SB being 0.464 (ΔN > 0) clearly describes that the electron donation occurs from the inhibitor to the metal surface.
Mulliken charge for SB is depicted in Fig. 8b. The values of Mulliken charges and DFT parameters are illustrated in Table 5. These values deliver useful information in locating the high electron density area of the inhibitor [49]. The greater the electron density on the heteroatom, the stronger will be the coordinate bond between inhibitor and metal surface [50]. From the table, it is observed that the atoms such as O and N possess excessive negative charge thereby facilitating the stronger adsorption of SB molecules onto the metal surface and hence reducing the corrosion rate.
3.7 Optimization
3.7.1 Box-Behnken Design (BBD) Analysis
Three independent variables, including temperature (A), HCl concentration (B), and inhibitor concentration (C), at three levels were chosen for the study.
The following model was found from the empirical data of the present experiment (in terms of coded units) and is depicted in Eq. 8
This function delineates the interaction of experimental variables on the IE. The result evaluation showed that the variable with the maximum effect on IE was inhibitor concentration (C), while the effect of temperature (A) and HCl concentration (B) was significantly smaller.
The model coefficients with positive sign represent synergistic effect, while negative sign represents antagonistic effect. The coefficients of model factors C, A2, B2, C2, AB, and BC positively contribute to the model equation, while A, B, and AC have a negative impact on the developed model [51].
3.8 Statistical Analysis of the Corrosion Inhibition Process
3.8.1 Analysis of Variance (ANOVA)
The F-test and p-value confirmed the statistical significance of the regression model. The ANOVA data for the response surface model are presented in Table 6. Mostly, the p-value can be utilized to reveal the significance of a coefficient. The smaller the p-value, the higher is the significance of the corresponding coefficient. Usually, a p-value less than 0.05 demonstrates the significance of the model terms [52]. As seen in Table 6, the p-value of the regression model shows a single star which indicates the least significance, suggesting the significance of the model terms. Moreover, the linear coefficients (A,B,C) and the interaction coefficients (A2, B2, C2) are significant since p-value is less than 0.05. The concentration of HCl and temperature is correlated with the IE. Besides, the incompetence of a model to exemplify the experimental data can be reflected by the lack of fit [53].
Table 6 shows the adequacy of the model which is accomplished by the determination of the regression coefficient value and the F-test. The determination coefficient (R2) value of 0.9702 demonstrates more than 97% response variability being predicted by the current model. Correspondingly, it validates the model since an R2 value of more than 0.6 is desirable. Higher R2 values illustrate that the predicted values are in good accuracy with the experimental data. From the predicted R2 and adjusted R2 (91.67%) values, it may be observed that they are in reasonably good agreement with each other. For a reasonable agreement, the variation between the two values should be less 20% [54].
3.9 Surface and Contour Plots
Response surface plots such as 2-dimensional contour and 3-dimensional surface plots are suitable for creating appropriate response values and operating conditions. In a contour plot, the response surface is considered in a 2D plane where all points that have a similar response are connected to acquire contour lines of constant responses. A surface plot usually exhibits a 3D view that aids in providing a better picture of the response [55].
Figure 9 demonstrates the interaction effect of the 3D response surface plot of temperature, media concentration, and inhibitor concentration on corrosion IE. It confirms that the corrosion IE moderately decreases with increasing media concentration and decreasing temperature.
The IE was found to be maximum at the highest temperature of 50 °C and the lowest concentration of HCl concentrations as depicted in Fig. 9. The results obtained were in line with those found in the literature [56].
3.10 Main Effects
Figure 10 demonstrates the influence of each parameter under study. It is worth mentioning that the values considered by each point relate to the average of the corrosion IE obtained at this level, independent of other parameter levels, and the overall mean is plotted across each panel.
The increase in corrosion IE with the rise in temperature, as witnessed in this study (Fig. 10), is suggestive of chemisorption mechanism usually attributed to charge transfer from the SB molecules to the MS surface to form a coordinate bond. With the increase in the HCl concentration as expected, the IE decreased due to an increase in the aggressiveness of the medium.
The % IE increased with an increase in SB concentration. The increase in the surface coverage enhances the availability/adsorption of the active inhibitor components onto the corroding metal surface [57].
The highest IE’s are obtained when MS is in 0.0001 M SB concentration with 0.5 M HCl at 50 °C temperature and the least IE’ was obtained at 0.00001 M SB concentration with 1.5 M HCl at 30 °C temperature.
3.11 Normal Probability Plot
The normality of the data was done by means of a normal probability plot. The normal probability plot of IE of SB is shown in Fig. 11. The normal probability plot for IE unveils residuals falling on the straight line. This means that the errors are disseminated normally [51]. There is nothing uncommon with this graph as the residual is equally distributed along the mean line and there is no probable outlier that exposes any non-normality in the distribution. The residual plot for IE of SB shows no predictable pattern.
3.12 Interaction Plot
Interaction plot aids in illustrating the connection between a continuous response and one categorical factor on the second categorical factor [12]. Contrasting the main effect graph which demonstrates the effect of each study parameter on the response, the interaction plot delivers the information of the influence of two or more parameters on the response (IE). Figure 12 illustrates the interaction effect of means of corrosion IE at various combinations of factor levels.
Three variable interactions result in a total of six interaction plots. Each of these plots illustrate dissimilar interactions, which can be examined by the slope and the distance between the minimum and maximum values. Since the slope of the lines displayed in Fig. 12 is not horizontal, it may be stated that there is significant interaction between the parameters representing those lines. The deviations in the response mean (IE) of MS in HCl media from the low to high levels of the input variable depend on the level of the second study variable. ANOVA displayed in Table 5 also endorses the significance of interaction A*B. This indicates that a combination of temperature and media (HCl concentration) has a substantial influence on the rate of MS corrosion.
The MINITAB optimizer exhibited the maximum IE of 83.92% at a temperature of 30 °C, 0.5 M HCl concentration, and 0.0001 M inhibitor concentration. Experimental runs were performed and the PDP studies yielded a protection efficiency of 81.5 under the optimized conditions.
3.13 Significance of This Study
The present work explores the optimum conditions for the highest IE by considering temperature, medium, and inhibitor concentration. The study utilized the design of the experiment. The optimum process conditions have a significant influence in subsiding the corrosion rate. Thus, the study can benefit the industries employing mild steel equipment. It is imperative to develop the study further to establish optimized values with a broader range of factors and experiments.
4 Conclusions
-
PDP studies validated SB as a mixed-type corrosion inhibitor for MS corrosion in HCl medium, obeying Langmuir’s adsorption isotherm.
-
Kinetic and thermodynamic parameters established chemisorption of SB molecules onto the MS surface.
-
SEM and AFM images prove the protective film formation of SB on MS surface which acts as a barrier at the metal solution interface.
-
The RSM optimization used in this study has meticulously predicted the process parameters for maximum corrosion inhibition efficiency. This technique could also provide vital information for the understanding of any application from laboratory scale to industrial processes.
-
The statistical model for corrosion IE formed using RSM was found to be favorable. It was shown that this equation could successfully explain the experimental data, at a 95% confidence level.
References
Ahmed S, Ali WB, Khadom AA (2019) Synthesis and investigations of heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid. Int J Ind Chem 10:159–173
Shukla SK, Ebenso EE (2011) Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium. Int J Electrochem Sci 6:3277–3291
Umoren SA, Eduok UM, Oguzie EE (2008) Corrosion inhibition of mild steel in 1 M H2SO4 by polyvinyl pyrrolidone and synergisticiodide additives. Portugaliae Electrochim Acta 26:533–546
El-Etre AY (2008) Inhibition of C-steel corrosion in acidic solution using the aqueous extract of zallouh root. Mater Chem Phys 108:278–282
Angst UM (2018) Challenges and opportunities in corrosion of steel in concrete. Mater Struct 5:1–20
Hemapriya V, Prabakaran M, Chitra S, Swathika M, Kim SH, Chung IM (2020) Utilization of biowaste as an eco-friendly biodegradable corrosion inhibitor for mild steel in 1 mol/L HCl solution. Arab J Chem Article in Press
Abd El–Maksoud SA, (2008) The efect of organic compounds on the electrochemical behaviour of steel in acidic media. Int J Electrochem Sci 3:528–555
Ashassi-Sorkhabia H, Shaabanib B, Seifzadeh D (2005) Corrosion inhibition of mild steel by some Schif base compounds in hydrochloric acid. Appl Surf Sci 239:154–164
Quraishi MA, Sardar R, Jamal D (2001) Corrosion inhibition of mild steel in hydrochloric acid by some aromatic hydrazides. Mater Chem Phys 71:309–313
Hassan N, Ramadan AM, Khalil S, Ghany NAA, Asiri AM, El-Shishtawy RM (2020) Experimental and computational investigations of a novel quinoline derivative as a corrosion inhibitor for mild steel in salty water. Colloids Surf A Physicochem Eng Asp 607:125454
Ahamad I, Prasad R, Quraishi MA (2010) Thermodynamic, electrochemical and quantum chemical investigation of some Schif bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros Sci 52:933–942
Soltani N, Behpour M, Ghoreishi SM, Naeimi H (2010) Corrosion inhibition of mild steel in hydrochloric acid solution by some double Schif bases. Corros Sci 52:1351–1361
Lavanya M, Rao P, Murthy VR, Selvaraj S (2020) Parametric study of aluminium alloy fouling in marine environment using RSM technique. Tribol-Mater Surf Interface 14:110–118
Necchi F, Carducci M, Pisoni I, Rossi O, Saul A, Rondini S (2019) Development of FAcE (Formulated Alhydrogel competitive ELISA) method for direct quantification of OAg present in Shigella sonnei GMMA-based vaccine and its optimization using Design of Experiments approach. J Immunol Methods 471:11–17
Akbarzadeh E, Ibrahim MNM, Rahim AA (2012) Monomers of lignin as corrosion inhibitors for mild steel: study of their behaviour by factorial experimental design. Corros Eng Sci Technol 47:302–311
Vimalraj S, Varahamoorthi R, Bala AU, Karthikeyan R (2020) Modeling and optimizing the laser parameters for corrosion resistance in 316 SS laser hard faced surface using tungsten carbide. Mater Today 26:2485–2490
Njoku Chigoziri N, Onyelucheya OE (2015) Response surface optimization of the inhibition efficiency of Gongronema latifolium as an inhibitor for aluminium corrosion in HCl solutions. Int Mater Chem 1:4–13
Afzalkhah M, Masoum S, Behpour M, Naeimi H, Reisi-Vanani A (2017) Experimental and theoretical investigation of inhibition efficiency of 2-(2-hydroxyphenyl)-benzothiazole using impedance spectroscopy, experimental design, and quantum chemical calculations. Ind Eng Chem Res 56:9035–9044
Das S, Saraswathi YL, Mondal DP (2006) Erosive–corrosive wear of aluminum alloy composites: influence of slurry composition and speed. Wear 261:180–190
Khajuria A, Akhtar M, Pandey MK, Singh MP, Raina A, Bedi R, Singh B (2019) Influence of ceramic Al2O3 particulates on performance measures and surface characteristics during sinker EDM of stir cast AMMCs. World J Eng 16:526
Renata BO, Elaine MSE, Rodrigo PPS, Anderso AA, Carlos UK (2008) Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur J Med Chem 43:1983–1988
Deresse ND, Deshpande V, Taifa IWR (2020) Experimental investigation of the effects of process parameters on material removal rate using Taguchi method in external cylindrical grinding operation. Int J Eng Sci Technol 23:405–420
Bouhlal F, Mazkour A, Labjar H, Benmessaoud M, Serghini-Idrissi M, El-Mahi S, El-Hajjaji M, Labjar N (2020) Combination effect of hydro-alcoholic extract of spent coffee grounds (HECG) and potassium Iodide (KI) on the C38 steel corrosion inhibition in 1M HCl medium: Experimental design by response surface methodology. Chem Data Collect 29:100499
Iqbal MMA, Bakar WA, Toemen S, Razak FIA, Azelee NIW (2020) Optimization study by Box-Behnken design (BBD) and mechanistic insight of CO2 methanation over Ru-Fe-Ce/γ-Al2O3 catalyst by in-situ FTIR technique. Arab J Chem 13:4170–4179
Rbaa M, Gala M, Benhiba F, Obot IB, Oudda H, EbnTouhami M, Lakhrissi B, Zarrouk A (2019) Synthesis and investigation of quinazoline derivatives based on 8-hydroxyquinoline as corrosion inhibitors for mild steel in acidic environment: experimental and theoretical studies. Ionics 25:3473–3491
Mistry BM, Jauhari S (2015) Studies on the inhibitive effect of (Z)-4-chloro-N-((2-chloroquinolin-3-yl)methylene)aniline Schiff base on the corrosion of mild steel in 1 N HCl solution. Res Chem Intermed 41:6289–6307
Ehteshamzadeh M, Jafari AH, Naderi E, Hosseini MG (2009) Effect of carbon steel microstructure and molecular structure of two new Schiff base compounds on inhibition performance in 1 N HCl solution by EIS. Mater Chem Phys 113:986–993
Kumari P, Shetty P, Rao SA, Sunil D (2020) Synthesis, characterization and anticorrosion behaviour of a novel hydrazide derivative on mild steel in hydrochloric acid medium. Bull Mater Sci 43:46
Prithvi KP, Rao SA (2019) Inhibitive effect of 2-[4-(dimethylamino) benzylidene] hydrazinecarbothioamide on corrosion of mild steel in acidic solution. Surf Eng Appl Electrochem 55:481–491
Schorr M, Yahalom J (1972) The significance of the energy of activation for the dissolution reaction of metal in acids. Corros Sci 12:867–868
Shetty D, Kumari P, Rao SA, Shetty P (2020) Anticorrosion behaviour of a hydrazide derivative on 6061 Al-15%(v) SiC(P) composite in acid medium: experimental and theoretical calculations. J Bio- Tribo-Corros 6:59
Kumari P, Shetty P, Rao SA, Sunil D (2017) Inhibition behaviour of 2-[(2-methylquinolin-8-yl) oxy] acetohydrazide on the corrosion of mild steel in hydrochloric acid solution. Trans Indian Inst Met 70:1139–1150
Rao Y, Kumari P, Sunil D, Shetty P, Rao SA (2019) Attenuation of acid corrosion of mild steel using a novel organic dye: electrochemical and surface measurements. Surf Eng Appl Electrochem 55:443–454
Saliyan VR, Adhikari AV (2008) Quinolin-5-ylmethylene-3-{[8-(trifluoromethyl)quinolin-4-yl]thio} propanohydrazide as an effective inhibitor of mild steel corrosion in HCl solution. Corros Sci 50:55–61
Shivakumar SS, Mohana KN (2013) Studies on the inhibitive performance of Cinnamomumzeylanicum extracts on the corrosion of mild steel in hydrochloric acid and sulphuric acid media. J Mater Environ Sci 4:448–459
Wang X, Yang H, Wang F (2010) A cationic gemini-surfactant as effective inhibitor for mild steel in HCl solutions. Corros Sci 52:1268–1276
Kagatikar S, Sunil D, Kumari P, Shetty P (2020) Investigation of anticorrosive property of carbazolecarbaldehydeazine on mild steel using electrochemical, morphological and theoretical studies. J Bio- Tribo-Corros 6:136
Kumari P, Shetty P, Nagalaxmi SD (2020) Effect of cysteine as environmentally friendly inhibitor on AA6061-T6 corrosion in 0.5 M HCl: electrochemical and surface studies. Surf Eng Appl Electrochem 56:624–634
Awad MK, Mustafa MR, Elnga MMA (2010) Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. J Mol Struct 959:66–74
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50:2981–2992
Arukalam IO (2014) Durability and synergistic effects of KI on the acid corrosion inhibition of mild steel by hydroxypropylmethylcellulose. Carbohydr Polym 112:291–299
Geerlings P, Proft FD, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873
Obot IB, Macdonald DD, Gasem ZM (2015) Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: part 1: an overview. Corros Sci 99:1–30
ElWanees SA, Seda SH (2019) Corrosion inhibition of zinc in aqueous acidic media using a novel synthesized Schiff base-an experimental and theoretical study. J Dispers Sci Technol 40:1813–1826
Abdallah M, Atwa ST, Salem AS (2013) Synergistic effect of some halide ions on the inhibition of zinc corrosion inhydrochloric acid by tetrahydrocarbazole derivatives compounds. Int J Electrochem Sci 8:10001–10021
Peme T, Olasunkanmi LO, Bahadur I, Adekunle AS, Kabanda MM, Ebenso EE (2015) Adsorption and corrosion inhibition studies of some selected dyes as corrosion inhibitors for mild steel in acidic medium: gravimetric, electrochemical, quantum chemical studies and synergistic effect with iodide ions. J Bio- Tribo-Corros 20:16004–16029
Samardzija KB, Khaled KF, Hackerman N (2005) The investigation of the inhibiting action of O-, S- and N- dithiocarbamato (1,4,8,11-tetraazacyclotetra decane)cobalt(III) complexes on the corrosion of iron in HClO4 acid. Appl Surf Sci 240:327–340
Gao G, Liang C (2007) Electrochemical and DFT studies of β-aminoalcohols as corrosion inhibitors for brass. Electrochim Acta 52:4554–4559
Hasanov R, Sadikoǧlu M, Bilgiç S (2007) Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution. Appl Surf Sci 253:3913–3921
Uo X, Bai R, Zhen D, Yang Z, Huang D, Mao H, Li X, Zou H, Xiang Y, Liu K, Wen Z (2019) Response surface optimization of the enzyme-based ultrasound-assisted extraction of acorn tannins and their corrosion inhibition properties. Ind Crops Prod 129:405–413
Salam KK, Agarry SE, Arinkoola AO, Shoremekun IO (2015) Optimization of operating conditions affecting microbiologically influenced corrosion of mild steel exposed to crude oil environments using response surface methodology. Biotechnol J Int 7:68–78
Tansuğ G, Tüken T, Kıcır N, Erbil M (2014) Investigation of 2-aminoethanethiol as corrosion inhibitor for steel using response surface methodology (RSM). Ionics 20(2):287–294
Dada M, Popoola P, Aramide O, Mathe N, Pityana S (2021) Optimization of the corrosion property of a high entropy alloy using response surface methodology. Mater Today Proc 38:1024–1030
Antony J (2014) A systematic methodology for design of experiments. In: Antony J (ed) Design of experiments for engineers and scientists, 2nd edn. Elsevier, Amsterdam, pp 33–50
Anadebe VC, Onukwuli OD, Omotioma M, Okafor NA (2018) Optimization and electrochemical study on the control of mild steel corrosion in hydrochloric acid solution with bitter kola leaf extract as inhibitor. S Afr J Chem 71:51–61
Odejobi Oludare J, Akinbulumo Olatunde A (2019) Modeling and optimization of the inhibition efficiency of Euphorbia heterophylla extracts based corrosion inhibitor of mild steel corrosion in HCL media using a response surface methodology. J Chem Technol Metal 54:217–232
Hamzat AK, Adediran IA, Alhems LM, Riaz M (2020) Investigation of corrosion rate of mild steel in fruit juice environment using factorial experimental design. Int J Corros. https://doi.org/10.1155/2020/5060817
Acknowledgements
Authors are grateful to Manipal Institute of Technology, MAHE for providing laboratory facilities.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This research is not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of Interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumari, P., Lavanya, M. Optimization of Inhibition Efficiency of a Schiff Base on Mild Steel in Acid Medium: Electrochemical and RSM Approach. J Bio Tribo Corros 7, 110 (2021). https://doi.org/10.1007/s40735-021-00542-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-021-00542-3