Abstract
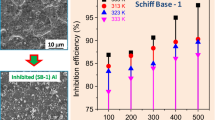
The present work describes the evaluation of anticorrosion property of 4-hydroxy-N′-[3-phenylprop-2-en-1-ylidene] benzohydrazide (HBH) on 6061 Al-15%(v) SiC(P) composite (Al-CM) in 0.5 M hydrochloric acid medium. The results of electrochemical measurement and specimen morphology study are discussed in this work. The adsorption of HBH over the Al-CM surface is through physisorption and obeys the Langmuir’s isotherm model. Potentiodynamic polarization study showed that the HBH acted as mixed inhibitor. The electrochemical impedance spectroscopy (EIS) study showed that the increase in the adsorption tendency of HBH on to the metal surface is due to increase in the polarization resistance. Density functional theory (DFT)-based calculations were carried out for both neutral and protonated HBH molecules which is well supported by the experimental observations.
















Similar content being viewed by others
Abbreviations
- Al-CM:
-
6061 Al-15%(v) SiC(P) composite
- HBH:
-
4-Hydroxy-N′-[3-phenylprop-2-en-1-ylidene] benzohydrazide
- HCl:
-
Hydrochloric acid medium
- EIS:
-
Electrochemical impedance spectroscopy
- PDP:
-
Potentiodynamic polarization
- OCP:
-
Open-circuit potential
- E corr :
-
Corrosion potential
- %IE:
-
Percentage Inhibition efficiency
- CR:
-
Corrosion rate
- i corr :
-
Corrosion current density
- DFT:
-
Density functional theory
- SEM:
-
Scanning electron microscope
- EDX:
-
Energy-dispersive X-ray
- AFM:
-
Atomic force microscope
References
Goulart CM, Esteves-Souza A, Martinez-Huitle CA, Rodrigues CJF, Maciel MAM, Echevarria A (2013) Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors. Corros Sci 67:281–291
Sharma CP (2004) Engineering materials: properties and applications of metals and alloys. Prentice-Hall of India, New Delhi
Sayed El, Sharif M (2011) Effects of graphite on the corrosion behavior of aluminum graphite composite in sodium chloride solutions. Int J Electrochem Sci 6:1085–1099
Pardo A, Merino MC, Merino S, Lopez MD, Viejo F, Carboneras M (2003) Influence of reinforcement grade and matrix composition on corrosion resistance of cast aluminium matrix composites (A3xx.x/SiCp) in a humid environment. Mater Corros 54:311–317
Monticelli C, Zucchi F, Brunoro G, Trabanelli G (1997) Corrosion and corrosion inhibition of alumina particulate/aluminium alloys metal matrix composites in neutral chloride solutions. J Appl Electrochem 27:325–334
Merino MC, Merino S, Viejo F, Carboneras M, Arrabal R (2005) Influence of reinforcement proportion and matrix composition on pitting corrosion behaviour of cast aluminium matrix composites (A3xx.x/SiCp). Corros Sci 47:1750–1764
Shahid M (1997) Mechanism of film growth during anodizing of Al-alloy-8090/SiC metal matrix composite in sulphuric acid electrolyte. J Mater Sci 32:3775–3781
Nayak J, Hebbar K (2008) Corrosion inhibition of T-6 treated 6061 Al–SiC(p) composite in hydrochloric acid. Trans Indian Inst Metals 61:221–224
Winkler SL, Ryan MP, Flower HM (2004) Pitting corrosion in cast 7XXX aluminium alloys and fibre reinforced MMCs. Corros Sci 46:893–902
Tang SW, Hu J, Zhao XH (2011) Corrosion behavior of a cerium-based conversion coating on alumina borate whisker-reinforced AA6061 composite pre-treated by hydrogen fluoride. Corros Sci 53:2636–2644
Patel AS, Panchal VA, Mudaliar GV, Shah NK (2013) Impedance spectroscopic study of corrosion inhibition of Al-Pure by organic Schiff base in hydrochloric acid. J Saud Chem Soc 17:53–59
Abdallah M (2004) Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution. Corros Sci 46:1981–1996
Branzoi V, Golgovici F, Branzoi F (2003) Aluminium corrosion in hydrochloric acid solutions and the effect of some organic inhibitors. Mater Chem Phys 78:122–131
Kumari P, Shetty P, Rao SA (2017) Electrochemical measurements for the corrosion inhibition of mild steel in 1M hydrochloric acid by using an aromatic hydrazide derivative. Arab J Chem 10:653–663
Kumari P, Shetty P, Rao SA (2015) Corrosion protection properties Of 4-hydroxy-N’-[1E,2E]-3-phenylprop-2-en-1-ylidene] benzohydrazideon mild steel in hydrochloric acid medium. Pro Met Phy Chem Surf 15:1034–1042
Kumari P, Shetty P, Rao SA, Sunil D (2017) Inhibition behaviour of 2-[(2-Methylquinoline-8-yl)Oxy] acetohydrazide on corrosion of mild steel in hydrochloric acid solution. Trans Indian Inst Met 70:1139–1150
Pinto GM, Nayak J, Shetty AN (2011) Corrosion inhibition of 6061 Al–15 vol. pct. SiC(p) composite and its base alloy in a mixture of sulphuric acid and hydrochloric acid by 4-(N, N-dimethyl amino) benzaldehyde thiosemicarbazone. Mater Chem Phys 125:628–640
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev Condense Mater B 37:785–789
Singh P, Ebenso EE, Olasunkanmi LO, Obot IB, Quraishi MA (2016) Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. J Phys Chem C 120:3408–3419
Verma C, Olasunkanmi LO, Ebenso EE, Quraishi MA, Obot IB (2016) Adsorption behavior of glucosamine-based, pyrimidinefused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C 120:11598–11611
Trowsdale AJ, Noble B, Haris SJ, Gibbins ISR, Thomson GE, Wood G (1996) The influence of silicon carbide reinforcement on the pitting behaviour of aluminium. Corros Sci 38:177–191
Yurt A, Ulutas S, Dal H (2006) Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl Surf Sci 253:19–925
Eduok UM, Umoren SA, Udoh AP (2012) Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions. Arab J Chem 5:325–337
Wang L, Shinohara T, Zhang B (2010) Influence of chloride, sulfate and bicarbonate anions on the corrosion behavior of AZ31 magnesium alloy. J Alloys Compd 496:500–507
Li WH, He Q, Zhang S, Pei S, Hou B (2007) Experimental and theoretical investigation of the adsorption behavior of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim Acta 52:6386–6394
Charitha BP, Rao P (2017) An ecofriendly approach for corrosion control of 6061 Al-15%(v) SiC(P) composite and its base alloy. Chin J Chem Eng 25:363–372
Aytac A, Ozmen U, Kabasakaloglu M (2005) Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater Chem Phys 89:176–181
Abdallah M, Fouda AS, El-Nagar DAM, Alfakeer M, Ghoneim MM (2019) Corrosion inhibition of two aluminium silicon alloys in 0.5 M HCl solution by some azole derivatives using electrochemical techniques. Surf Eng Appl Electrochem 55:172–182
Frers MM, Stefenel C, Mayer CT (2016) AC-impedance measurements on aluminium in chloride containing solutions and below the pitting potential. J Appl Electrochem 20:996–999
Mansfeld F, Lin S, Kim K, Shih H (1987) Pitting and surface modification of SiC/Al. Corros Sci 27:997–1000
Lebrini M, Robert F, Roos C (2010) Inhibition Effect of alkaloids extract from annona squamosa plant on the corrosion of C38 steel in normal hydrochloric acid medium. Int J Electrochem Sci 5:1698–1712
Xie YJ, Wu J, Che XZ, Chen Y, Huan HW, Deng GJ (2016) Efficient pyrido[1,2-a] benzimidazole formation from 2-aminopyridines and cyclohexanones under metal-free conditions. Green Chem 18:667–671
Niu L, Yin Y, Guo W, Lu M, Qin R, Chen S (2009) Application of scanning electrochemical microscope in the study of corrosion of metals. J Mater Sci 44:4511–4521
Larabi L, Harek Y, Benali O, Ghalem S (2005) Hydrazide derivatives as corrosion inhibitors for mild steel in 1 M HCl. Prog Org Coat 54:256–262
Yahalom J (1972) The significance of the energy of activation for the dissolution reaction of metal in acids. Corros Sci 12:867–868
Abdel Rehim SS, Magdy AM, Ibrahim KF (1999) 4-Aminoantipyrineas an inhibitor of mild steel corrosion in HCl solution. J Appl Electrochem 29:593–599
Li W, He Q, Zhang S, Pei C, Hou BJ (2008) Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem 38:289–295
Oguzie EE, Njoku VO, Enenebeaku CK, Akalezi CO, Obi C (2008) Effect of hexamethylpararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros Sci 50:3480–3486
Wang X, Yang H, Wang F (2010) A cationic gemini-surfactant as effective inhibitor for mild steel in HCl solutions. Corros Sci 52:1268–1276
Migahed MA, Mohammed HM, Al-Sabagh AM (2003) Corrosion inhibition of H-11 type carbon steel in 1 M hydrochloric acid solution by N-propyl amino lauryl amide and its ethoxylated derivatives. Mater Chem Phys 80:169–175
Ashassi-Sorkhabi H, Majidi MR, Seyyedi K (2004) Investigation of inhibition effect of some amino acids against steel corrosion in HCl solution. Appl Surf Sci 225:176–185
Bentiss F, Traisnel M, Lagrenee M (2001) Influence of 2,5-bis(4-dimethylaminophenyl)-1,3,4-thiadiazole on corrosion inhibition of mild steel in acidic media. J Appl Electrochem 31:41–48
Martinez S, Stern I (2002) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl Surf Sci 199:83–89
Aylor DM, Moran PJ (1985) Effect of reinforcement on the pitting behavior of aluminum-base metal matrix composites. J Electrochem Soc 132:1277–1281
Ekpe UJ, Ibok UJ, Ita BI, Offiong OE (1995) Inhibitory action of methyl and phenyl thiosemicarbazone derivatives on the corrosion of mild steel in hydrochloric acid. Mater Chem Phys 40:87–93
Pinto GM, Nayak J, Shetty AN (2011) Adsorption and inhibitor action of 4-(N, N-dimethylamino) benzaldehyde thiosemicarbazone on 6061 Al/SiC composite and its base alloy in sulfuric acid medium. Synth React Inorg Org Nano Met Chem 41:127–140
Senhaji O, Taouil R, Skalli MK, Bouachrine M, Hammouti B, Hamidi M (2011) Experimental and theoretical study for corrosion inhibition in normal hydrochloric acid solution by some new phosphonated compounds. Int J Electrochem Sci 6:6290–6299
Zhang W, Hui-Jing L, Wang M, Li-Juan W, Ai-Han Z, Yan-Chao W (2019) Highly effective inhibition of mild steel corrosion in HCl solution by using pyrido[1,2-a] benzimidazoles. New J Chem 43:413–426
Ansari K, Quraishi M, Singh A, Ramkumar S, Obote IB (2016) Corrosion inhibition of N80 steel in 15% HCl by pyrazolone derivatives: electrochemical, surface and quantum chemical studies. RSC Adv 6:24130–24141
Abdallah M, Gad EA, Sobhi M, Fahemi JH, Alfakeer MM (2019) Performance of tramadol drug as a safe inhibitor for aluminium corrosion in 1 M HCl solution and understanding mechanism of inhibition using DFT. Egypt J Petrol 28:173–181
Zhang K, Xu B, Yang W, Yin X, Liu Y, Chen Y (2015) Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 90:284–295
Obot IB, Gasem ZM (2014) Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros Sci 83:359–366
Dohare P, Chauhan D, Sorour A, Quraishi M (2017) DFT and experimental studies on the inhibition potentials of expired Tramadol drug on mild steel corrosion in hydrochloric acid. Mater Discov 9:30–41
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 10:31793–31874
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros Methods Mater 53:277–282
Carotenuto G, Gallo A, Nicolais L (1994) Degradation of SiC particles in aluminium-based composites. J Mater Sci 29:4967–4974
Charitha BP, Arunchandran C, Rao P (2017) Enhancement of surface coating characteristics of epoxy resin by dextran: an electrochemical approach. Ind Eng Chem Res 56:1137–1147
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shetty, D., Kumari, P.P., Rao, S.A. et al. Anticorrosion Behaviour of a Hydrazide Derivative on 6061 Al-15%(v) SiC(P) Composite in Acid Medium: Experimental and Theoretical Calculations. J Bio Tribo Corros 6, 59 (2020). https://doi.org/10.1007/s40735-020-00356-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00356-9