Abstract
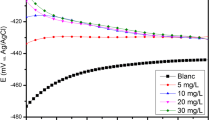
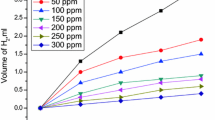
The aim of this study is to examine Achillea santolina extract (ASE) as a green inhibitor for carbon steel (CS) in corrosive medium (1.0 M HCl) under different experimental conditions. This investigation occurs by using mass loss (ML), open-circuit potential, potentiodynamic polarization (PP), electrochemical frequency modulation (EFM), AC impedance spectroscopy (EIS), energy-dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), and atomic force microscopy (AFM). The inhibition efficiency (%IE) enhanced by rising of ASE dose and also, with temperature increasing. The addition of 0.01 M KI to 1.0 M HCl solution containing ASE decreases the corrosion of CS and improves the performance of ASE by a synergistic effect. The presence of the iodide ions improves the surface film formed on the surface of CS. The inhibition effect of the ASE on the CS surface was confirmed by EDX, SEM, and AFM analysis. This work represents the first use of ASE as an effective green corrosion inhibitor.
















Similar content being viewed by others
References
Khamis E (1990) The effect of temperature on the acidic dissolution of steel in the presence of inhibitors. Corrosion 46(6):476–484
Quraishi MA, Sardar R (2003) Corrosion inhibition of mild steel in acid solutions by some aromatic oxadiazoles. Mater Chem Phys 78(2):425–431
Machnikova E, Whitmire KH, Hackerman N (2008) Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives. Electrochim Acta 53(20):6024–6032
Saadawy M (2015) An important world crop-barley—as a new green inhibitor for acid corrosion of steel. Anti Corros Methods Mater 62(4):220–228
El Hosary AA, Saleh RM (1993) Effect of molasses on the corrosion of steel, aluminium and copper in acidic media. Prog Underst Prev Corros 2:911–915
Saleh RM, Ismall AA, El Hosary AA (1982) Corrosion Inhibition by Naturally Occurring Substances: VII. The effect of aqueous extracts of some leaves and fruit-peels on the corrosion of steel, Al, Zn and Cu in acids. Br Corros J 17(3):131–135
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48(9):2765–2779
Avwiri GO, Igho FO (2003) Inhibitive action of Vernonia amygdalina on the corrosion of aluminium alloys in acidic media. Mater Lett 57(22–23):3705–3711
Abdel-Gaber AM, Abd-El-Nabey BA, Khamis E, Abd-El-Khalek DE (2008) Investigation of fig leaf extract as a novel environmentally friendly antiscalent for CaCO3 calcareous deposits. Desalination 230(1–3):314–328
Umoren SA, Eduok UM, Solomon MM, Udoh AP (2016) Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab J Chem 9:S209–S224
Al-Otaibi MS, Al-Mayouf AM, Khan M, Mousa AA, Al-Mazroa SA, Alkhathlan HZ (2014) Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab J Chem 7:340–346
Saxena Akhil, Prasad Dwarika, Haldhar Rajesh, Singh Gurmeet, Kumar Akshay (2018) Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mol Liq 258:89–97
Al-Snafi AE (2013) Chemical constituents and pharmacological activities of milfoil (Achillea santolina). A review. Int J Pharm Tech Res 5(3):1373–1377
Khan MA (1998) Chemical constituents of Centaurea Iberica and Achillea Santouna and synthesis of myoglobin and insulin. Doctoral dissertation, University of Karachi, Karachi
Tabanca N, Demirci B, Gurbuez I, Demirci F, Becnel JJ, Wedge DE, Baser KH (2011) Essential oil composition of five collections of Achillea biebersteinii from central Turkey and their antifungal and insecticidal activity. Agricultural Research Service University MS Natural Products Utilization Research Unit
El-Shazly AM, Hafez SS, Wink M (2004) Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L.(Asteraceae) from Egypt. Die Pharmazie Int J Pharm Sci 59(3):226–230
Murakawa T, Kato T, Nagaura S, Hackerman N (1968) A contribution to the understanding of the synergistic effect of anions for the corrosion inhibition of Fe by amines. Corros Sci 8(7):483–489
Umoren SA, Solomon MM (2015) Effect of halide ions on the corrosion inhibition efficiency of different organic species—a review. J Ind Eng Chem 21:81–100
Fouda AS, Al-Zehry HH, Elsayed M (2018) Synergistic effect of potassium iodide with Cassia italica extract on the corrosion inhibition of carbon steel used in cooling water systems in 0.5 MH2SO4. J Bio Tribo Corros 4(2):23
Oguzie EE, Wang SG, Li Y, Wang FH (2008) Corrosion and corrosion inhibition characteristics of bulk nanocrystalline ingot iron in sulphuric acid. J Solid State Electrochem 12(6):721–728
Motawea MM, El-Hossiany A, Fouda AS (2019) Corrosion control of copper in nitric acid solution using chenopodium extract. Int J Electrochem Sci 14(1):1372–1387
Fouda AS, Nazeer AA (2018) Assessment of begonia extract as new eco-friendly inhibitor for low-carbon-steel corrosion in acidic environment. J Bio Tribo Corros 4(1):7
Fouda AS, El-Awady GY, El Behairy WT (2018) Prosopis juliflora plant extract as potential corrosion inhibitor for low-carbon steel in 1 M HCl solution. J Bio Tribo Corros 4(1):8
Fouda AS, El-Abbasy HM, El-Sherbini AA (2018) Inhibitive effect of artemisia judaica herbs extract on the corrosion of carbon steel in hydrochloric acid solutions. Int J Corros Scale Inhib 7(2):213–235
Ammar N, Jabnoun-Khiareddine H, Mejdoub-Trabelsi B, Nefzi A, Mahjoub MA, Daami-Remadi M (2017) Pythium leak control in potato using aqueous and organic extracts from the brown alga Sargassum vulgare (C. Agardh, 1820). Postharvest Biol Technol 130:81–93
Bosch RW, Hubrecht J, Bogaerts WF, Syrett BC (2001) Electrochemical frequency modulation: a new electrochemical technique for online corrosion monitoring. Corrosion 57(1):60–70
Abdel-Rehim SS, Khaled KF, Abd-Elshafi NS (2006) Electrochemical frequency modulation as a new technique for monitoring corrosion inhibition of iron in acid media by new thiourea derivative. Electrochim Acta 51(16):3269–3277
Quraishi MA, Sardar R (2002) Aromatic triazoles as corrosion inhibitors for mild steel in acidic environments. Corrosion 58(9):748–755
Zhang DQ, Cai QR, He XM, Gao LX, Kim GS (2009) Corrosion inhibition and adsorption behavior of methionine on copper in HCl and synergistic effect of zinc ions. Mater Chem Phys 114(2–3):612–617
Rosliza R, Nik WW, Senin HB (2008) The effect of inhibitor on the corrosion of aluminum alloys in acidic solutions. Mater Chem Phys 107(2–3):281–288
Reis FM, De Melo HG, Costa I (2006) EIS investigation on Al 5052 alloy surface preparation for self-assembling monolayer. Electrochim Acta 51(8–9):1780–1788
Hsu CH, Mansfeld F (2001) Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 57(9):747–748
Ostovari A, Hoseinieh SM, Peikari M, Shadizadeh SR, Hashemi SJ (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros Sci 51(9):1935–1949
Quraishi MA, Farooqi IH, Saini PA (1999) Investigation of some green compounds as corrosion and scale inhibitors for cooling systems. Corrosion 55(5):493–497
Tao Z, Zhang S, Li W, Hou B (2009) Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros Sci 51(11):2588–2595
Ferreira ES, Giacomelli C, Giacomelli FC, Spinelli A (2004) Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater Chem Phys 83(1):129–134
de Souza FS, Spinelli A (2009) Caffeic acid as a green corrosion inhibitor for mild steel. Corros Sci 51(3):642–649
Fuchs-Godec R, Pavlović MG (2012) Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainless-steel X4Cr13 in sulphuric acid. Corros Sci 58:192–201
Okafor PC, Zheng Y (2009) Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros Sci 51(4):850–859
Solmaz R, Kardaş G, Culha M, Yazıcı B, Erbil M (2008) Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim Acta 53(20):5941–5952
Nnaji NJN, Obi-Egbedi NO, Ani JU (2011) Leaf extract of anthodeista djalonensis as corrosion inhibitor of aluminium in HCL solution. J Sci Ind Res 9:26–32
Khaled KF, El-Maghraby A (2014) Experimental, Monte Carlo and molecular dynamics simulations to investigate corrosion inhibition of mild steel in hydrochloric acid solutions. Arab J Chem 7(3):319–326
Hosseini M, Mertens SF, Arshadi MR (2003) Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzenesulphonate and hexamethylenetetramine. Corros Sci 45(7):1473–1489
Gomma GK, Wahdan MH (1995) Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater Chem Phys 39(3):209–213
Khaled KF (2011) Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements. Mater Chem Phys 125(3):427–433
Umoren SA, Obot IB, Obi-Egbedi NO (2009) Raphia hookeri gum as a potential eco-friendly inhibitor for mild steel in sulfuric acid. J Mater Sci 44(1):274–279
Arab ST, Noor EAA (1993) Inhibition of acid corrosion of steel by some S-alkylisothiouronium iodides. Corrosion 49(2):122–129
El-Rehim SA, Ibrahim MA, Khaled KF (1999) 4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution. J Appl Electrochem 29(5):593–599
Yu G, Zhang XY, Du YL (2001) Mobile hydrogen monitoring in the wall of hydrogenation reactors. Corrosion 57(1):71–77
Şahin M, Bilgic S, Yılmaz H (2002) The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl Surf Sci 195(1–4):1–7
Tang L, Li X, Si Y, Mu G, Liu G (2006) The synergistic inhibition between 8-hydroxyquinoline and chloride ion for the corrosion of cold rolled steel in 0.5 M sulfuric acid. Mater Chem Phys 95(1):29–38
Maayta AK, Al-Rawashdeh NAF (2004) Inhibition of acidic corrosion of pure aluminum by some organic compounds. Corros Sci 46(5):1129–1140
Abboud Y, Abourriche A, Saffaj T, Berrada M, Charrouf M, Bennamara A, Hannache H (2009) A novel azo dye, 8-quinolinol-5-azoantipyrine as corrosion inhibitor for mild steel in acidic media. Desalination 237(1–3):175–189
Benabdellah M, Aouniti A, Dafali A, Hammouti B, Benkaddour M, Yahyi A, Ettouhami A (2006) Investigation of the inhibitive effect of triphenyltin 2-thiophene carboxylate on corrosion of steel in 2 M H3PO4 solutions. Appl Surf Sci 252(23):8341–8347
Li X, Deng S, Fu H (2009) Synergism between red tetrazolium and uracil on the corrosion of cold rolled steel in H2SO4 solution. Corros Sci 51:1344–1355
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fouda, A.S., Shalabi, K. & Shaaban, M.S. Synergistic Effect of Potassium Iodide on Corrosion Inhibition of Carbon Steel by Achillea santolina Extract in Hydrochloric Acid Solution. J Bio Tribo Corros 5, 71 (2019). https://doi.org/10.1007/s40735-019-0260-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0260-6