Abstract
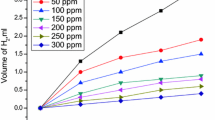
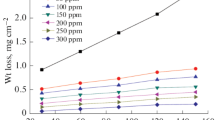
Herein, Begonia obliqua (BGN) plant extract was assessed as a low-cost, green and efficient corrosion inhibitor for low-carbon steel (LCS) in 1 M HCl solution using mass loss, hydrogen evolution, electrochemical impedance spectroscopy, Tafel polarization (TP), electrochemical frequency modulation as well as surface studies by X-ray photoelectron spectroscopy (XPS), scanning electronic microscope and atomic force microscope. Increasing BGN extract dose increases the charge transfer resistance (R ct) and decreases the double-layer capacitance (C dl). The TP results showed mixed-type inhibition behavior of the studied extract with inhibition efficiency (IE) of 87.7% achieved with the addition of 300 ppm of BGN. The adsorption isotherm model of BGN extract on LCS surface followed Temkin adsorption isotherm. The inhibition mechanism of BGN was explained according to the increasing the IE with temperature and the activation parameters which suggest significant chemisorption of the BGN extract on the LCS surface. The XPS studies confirmed the formation of protective layer of BGN extract on LCS surface. Results obtained by different techniques used showed good agreement confirming the possible use of the investigated inhibitor in the industrial application.












Similar content being viewed by others
References
Li LF, Caenen P, Celis JP (2008) Effect of hydrochloric acid on pickling of hot-rolled 304 stainless steel in iron chloride-based electrolytes. Corros Sci 50:804–810
Abdel Nazeer A, El-Abbasy HM, Fouda AS (2013) Adsorption and corrosion inhibition behavior of carbon steel by cefoperazone as eco-friendly inhibitor in HCl. J Mater Eng Perform 22:2314–2322
Quraishi MA, Ahamad I, Singh AK, Shukla SK, Lal B, Singh V (2008) N-(Piperidinomethyl)-3-[(pyridylidene) amino] isatin: A new and effective acid corrosion inhibitor for mild steel. Mater Chem Phys 112:1035–1039
Verma C, Ebenso EE, Olasunkanmi LO, Quraishi MA, Obot IB (2016) Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C 120:11598–11611
Roberge PR (2008) Corrosion engineering principles and practice. McGrawHill, New York, p 19
Quraishi MA, Sardar R (2002) Aromatic triazoles as corrosion inhibitors for carbon steel in acidic environments. Corrosion 58:748–755
Karakus M, Sahin M, Bilgic S (2005) An investigation on the inhibition effects of some new dithiophosphonic acid monoesthers on the corrosion of the steel in 1 M HCl medium. Mater Chem Phys 92:565–571
Sherif EM, Park SM (2006) Inhibition of copper corrosion in acidic pickling solutions by N-phenyl-1,4-phenylenediamine. Electrochim Acta 51:4665–4673
Al-Otaibi MS, Al-Mayouf AM, Khan M, Mousa AA, Al-Mazroa SA, Alkhathlan HZ (2014) Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab J Chem 7:340–346
Zucchi F, Omar IH (1985) Plant extracts as corrosion inhibitors of mild steel in HCl solutions. Surf Technol 24(4):391–399
Gunasekaran G, Chauhan LR (2004) Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. Electrochim Acta 49:4387–4395
Li Y, Zhao P, Liaqng Q, Hou B (2005) Berberine as a natural source inhibitor for mild steel in 1 M H2SO4. Appl Surf Sci 252:1245–1253
El-Etre AY, Abdallah M, El-Tantawy ZE (2005) Corrosion inhibition of some metals using lawsonia extract. Corros Sci 47:385–395
Fouda AS, Abdel Nazeer A, Ibrahim M, Fakih M (2013) Ginger extract as green corrosion inhibitor for steel in sulfide polluted salt water. J Korean Chem Soc 57(2):272–278
El-Etre AY (2006) Khillah extract as inhibitor for acid corrosion of SX 316 steel. Appl Surf Sci 252:8521–8525
Fouda AS, Megahed HE, Fouad N, Elbahrawi NM (2016) Corrosion inhibition of carbon steel in 1 M hydrochloric acid solution by aqueous extract of thevetia peruviana. J Bio Tribo Corros 2:1–16
Oguzie EE, Adindu CB, Enenebeaku CK, Ogukwe CE, Chidiebere MA, Oguzie KL (2012) Natural products for materials protection: mechanism of corrosion inhibition of mild steel by acid extracts of Piper guineense. J Phys Chem C 116:13603–13615
Souza FS, Giacomelli C, Goncalves RS, Spinelli A (2012) Adsorption behavior of caffeine as a green corrosion inhibitor for copper. Mat Sci Eng 32:2436–2444
Abdel Nazeer A, Shalabi K, Fouda AS (2015) Corrosion inhibition of carbon steel by Roselle extract in hydrochloric acid solution: electrochemical and surface study. Res Chem Intermed 41:4833–4850
Rahal C, Masmoudi M, Abdelhedi R, Sabot R, Jeannin M, Bouaziz M, Refait P (2016) Olive leaf extract as natural corrosion inhibitor for pure copper in 0.5 M NaCl solution: a study by voltammetry around OCP. J Mol Liq 215:47–57
Njoku DI, Ukaga I, Ikenna OB, Oguzie EE, Oguzie KL, Ibisi N (2016) Natural products for materials protection: corrosion protection of aluminium in hydrochloric acid by Kola nitida extract. J Mol Liq 219:417–424
Khadraoui A, Khelifa A, Hachama K, Mehdaoui R (2016) Thymus algeriensis extract as a new eco-friendly corrosion inhibitor for 2024 aluminium alloy in 1 M HCl medium. J Mol Liq 214:293–297
Kalaiselvi P, Chellammal S, Palanichamy S, Subramanian G (2010) Artemisia pallens as corrosion inhibitor for mild steel in HCl medium. Mater Chem Phys 120:643–648
Abdallah YM, Hassan HM, Shalabi K, Fouda AS (2014) Effects of arctostaphylos uva-ursi extract as green corrosion inhibitor for Cu10Ni Alloy in 1 M HNO3. Int J Electrochem Sci 9:5073–5091
Shalabi K, Fouda AS, Elewady GY, El-Askalany A (2014) Adsorption and inhibitive properties of Phoenix dactylifera L. Extract as a green inhibitor for aluminum and aluminum–silicon alloy in HCl. Prot Met Phys Chem Surf 50(3):420–431
Fouda AS, Tawfik H, Badr AH (2012) Corrosion inhibition of mild steel by Camellia Sinensis extract as green inhibitor. Adv Mater Corros 1:1–7
Shalabi K, Abdel Nazeer A (2015) Adsorption and inhibitive effect of Schinus Terebinthifolius extract as a green corrosion inhibitor for carbon steel in acidic solution. Prot Met Phys Chem Surf 51(5):908–917
Abd-El-Nabey BA, Abdel-Gaber AM, Ali ME, Khamis E, El-Housseiny S (2013) Inhibitive action of Cannabis plant extract on the corrosion of copper in 0.5 M H2SO4. Int J Electrochem Sci 8:5851–5865
Al-Turkustani AM, Arab ST (2010) Aloe plant extract as environmentally friendly inhibitor on the corrosion of aluminum in hydrochloric acid in absence and presence of iodide ions. Mod Appl Sci 4(5):105–124
Fouda AS, Abdallah YM, Elawady GY, Ahmed RM (2015) Electrochemical study on the effectively of Hyoscyamus Muticus extract as a green inhibitor for corrosion of copper in 1 M HNO3. J Mater Environ Sci 5(6):1519–1531
Abdel Nazeer A, Allam NK, Youssef GI, Ashour EA (2011) Effect of glycine on the electrochemical and stress corrosion cracking behavior of Cu10Ni alloy in sulfide polluted salt water. Ind Eng Chem Res 50(14):8796–8802
Ogbonna N, Matthew CM, Okechukwu DO (2011) Inhibition of copper corrosion by acid extracts of Gnetum africana and Musa acuminate Peel. Int J Multidiscip Sci Eng 2(5):5–9
Fouda AS, Mohamed AE, Khalid MA (2016) Trigonella stellate extract as corrosion inhibitor for copper in 1 M nitric acid solution. J Chem Pharm Res 8(2):86–98
Pasupathy A, Nirmala S, Abirami G, Satish A, Milton RP (2014) Inhibitive action of Phyllanthus amarus extract on the corrosion of zinc in 0.5 N H2SO4 medium. Int J Sci Res 4(3):1–3
Krishnegowda PM, Venkatesha VT, Krishnegowda PKM, Shivayogiraju SB (2013) Acalypha torta leaf extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Ind Eng Chem Res 52:722–728
Fouda AS, Ibrahim AA, El-behairy WT (2014) Thiophene derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solutions. Der Pharma Chemica 6(5):144–157
Bockris JOM, Drazic D (1962) The kinetics of deposition and dissolution of iron: effect of alloying impurities. Electrochim Acta 7:293–313
Frumkin A (1925) Die Kapillarkurve der höheren Fettsäuren und die Zustandsgleichung der Oberflächenschicht. Zeitschrift für Physikalische Chemie 116:466
Yurt A, Bereket G, Kivrak A, Balaban A, Erk B (2005) Effect of schiff bases containing pyridyl group as corrosion inhibitors for low carbon steel in 0.1 M HCl. J Appl Electrochem 35:1025–1032
Ivanov ES (1986) Inhibitors for metal corrosion in acid media. Metallurgy 34:1990–1997
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum Arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti Corros Meth Mater 53:277–282
El-Etre AY (2003) Inhibition of aluminum corrosion using Opuntia extract. Corros Sci 45:2485–2495
Abdallah M (2004) Antibacterial drugs as corrosion inhibitors for corrosion of aluminum in hydrochloric solution. Corros Sci 46:1981–1996
Putilova I, Balezin S, Barannik V (1960) Metallic corrosion inhibitors. Pergamon, Oxford
Canay S, Hersek N, Culha A, Bilgic S (1998) Evaluation of titanium in oral conditions and its electrochemical corrosion behavior. J Oral Rehabil 25(10):759–764
Kus E, Mansfeld F (2006) An evaluation of the electrochemical frequency modulation (EFM) technique. Corros Sci 48:965–979
Caigman GA, Metcalf SK, Holt EM (2000) Thiophene substituted dihydropyridines. J Chem Cryst 30:415–422
Abdel Nazeer A, Allam NK, Fouda AS, Ashour EA (2012) Effect of cysteine on the electrochemical behavior of Cu10Ni alloy in sulfide polluted environments: experimental and theoretical aspects. Mater Chem Phys 136:1–9
El Achouri M, Kertit S, Gouttaya HM, Nciri B, Bensouda Y, Perez L, Infante MR, Elkacemi K (2001) Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α, ω-bis-(dimethyl tetradecyl ammonium bromide). Prog Org Coat 43:267–273
Abdel Nazeer A, El-Abbasy HM, Fouda AS (2013) Antibacterial drugs as environmentally-friendly corrosion inhibitors for carbon steel in acid medium. Res Chem Intermed 39:921–939
Macdonald JR, Johanson WB (1987) In: Macdonald JR (ed) Theory in impedance spectroscopy. Wiley, New York
Mertens SF, Xhoffer C, Decooman BC, Temmerman E (1997) Short-term deterioration of polymer-coated 55% Al–Zn. 1. Behavior of thin polymer films. Corrosion 53:381–388
Reis FM, de Melo HG, Costa I (2006) EIS investigation on Al 5052 alloy surface preparation for self-assembling monolayer. Electrochim Acta 51:1780–1788
Zerga B, Sfaira M, Rais Z, Ebn Touhami M, Taleb M, Hammouti B, Imelouane B, Elbachiri A (2009) Lavender oil as an ecofriendly inhibitor for mild steel in 1 M HCl. Mater Tech 97:297–305
Lagrenee M, Mernari B, Bouanis M, Traisnel M, Bentiss F (2002) Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media. Corros Sci 44:573–588
McCafferty E, Hackerman N (1972) Kinetics of iron corrosion in concentrated acidic chloride solutions. J Electrochem Soc 119:146–154
Ma H, Chen S, Niu L, Zhao S, Li S, Li D (2002) Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. J Appl Electrochem 32:65–72
El Faydy M, Galai M, El Assyry A, Tazouti A, Touir R, Lakhrissi B, Ebn-Touhami M, Zarrouk A (2016) Experimental investigation on the corrosion inhibition of carbon steel by 5-(chloromethyl)-8-quinolinol hydrochloride in hydrochloric acid solution. J Mol Liq 219:396–404
Khaled KF (2008) Molecular simulation, quantum chemical calculations and electrochemical studies for inhibition of mild steel by triazoles. Electrochim Acta 53:3484–3492
Boumhara K, Tabyaoui M, Jama C, Bentiss F (2015) Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1M HCl solution: Electrochemical and XPS investigations. J Ind Eng Chem 29:146–155
Sastri VS, Elboujdaini M, Brown JR, Perumareddi JR (1996) Surface analysis of inhibitor films formed in hydrogen sulfide medium. Corrosion (Houston) 52:447–452
El Hamdani N, Fdil R, Tourabi M, Jama C, Bentiss F (2015) Alkaloids extract of Retama monosperma (L.) Boiss. Seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and surface studies. Appl Surf Sci 357:1294–1305
Gao X, Liu S, Lu H, Gao F, Ma H (2015) Corrosion inhibition of iron in acidic solutions by monoalkyl phosphate esters with different chain lengths. Ind Eng Chem Res 54:1941–1952
Droulas JL, Minhduc T, Jugnet Y (1991) Etude des propiétés interfaciales des dépôts par évaporation et pulvérisation d’ aluminium sur polyéthylène téréphtalate. Le Vide. les Couches Minces 88:39–41
Goldberg MJ, Clabes JG, Kovac CA (1988) Metal-polymer chemistry. II. Chromium-polyimide interface reactions and related organometallic chemistry. J Vac Sci Technol A 6:991–996
Gu TB, Chen ZJ, Jiang XH, Zhou LM, Liao YW, Duan M, Wang H, Pu Q (2015) Synthesis and inhibition of N-alkyl-2-(4-hydroxybut-2-ynyl) pyridinium bromide for mild steel in acid solution: box-Behnken design optimization and mechanism probe. Corros Sci 90:118–132
Chevalier M, Robert F, Amusant N, Traisnel M, Roos C, Lebrini M (2014) Enhanced corrosion resistance of mild steel in 1 M hydrochloric acid solution by alkaloids extract from Aniba rosaeodora plant: electrochemical, phytochemical and XPS studies. Electrochim Acta 131:96–105
Mourya P, Singh P, Tewari AK, Rastogi RB, Singh MM (2015) Relationship between structure and inhibition behavior of quinolinium salts for mild steel corrosion: experimental and theoretical approach. Corros Sci 95:71–87
Luo H, Guan YC, Khan KN (1998) Corrosion inhibition of a mild steel by aniline and alkylamines in acidic solutions. Corrosion 54:721–731
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouda, A.S., Abdel Nazeer, A. & El behairy, W.T. Assessment of Begonia Extract as New Eco-friendly Inhibitor for Low-Carbon-Steel Corrosion in Acidic Environment. J Bio Tribo Corros 4, 7 (2018). https://doi.org/10.1007/s40735-017-0122-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-017-0122-z