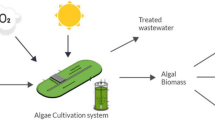
Abstract
Purpose of Review
Growing algae in wastewater offers carbon-neutral biomass production and pollutant removal. However, practical applications of wastewater-grown algal biomass have social acceptability issues in the food and feed industries due to unexpected threats (such as human/animal pathogens and toxins) associated with the wastewater-grown biomass. Therefore, considering the substantial pollutant removal potential of microalgae and the abundance of wastewater as a growth media, alternative bioprocessing routes of the wastewater-grown biomass should be developed. This review highlights some non-food and non-feed applications of wastewater-grown algae biomass.
Recent Findings
Wastewater-grown algal biomass contains high amounts of carbohydrates, proteins, and lipids depending upon the composition of wastewater and algal species grown. These three significant metabolites are precursors to bioenergy and biomaterial products such as bioethanol, biogas, and bioplastics. Hydrolysis of the wastewater-grown algal biomass can be easily improved to enhance the microbial fermentation yields to produce bioethanol and biobutanol. Fresh algal biomass, residual biomass, or both can be used as feedstocks in anaerobic digestion/co-digestion to produce biogas. Depending upon the selected species, wastewater-grown algal biomass can also produce biopolymers whose productivity depends on growth conditions, wastewater composition, and biopolymer synthesis method. Enzymatic, eco-friendly chemicals and mechanical approaches used to prepare biopolymers from algal biomass should be optimized for higher yields of biopolymers.
Summary
Although wastewater-grown biomass has acceptability issues, it offers certain environmental benefits, including atmospheric carbon capture, phycoremediation of pollutants, and water recycling. This manuscript highlights the recent progress and emerging trends of wastewater-grown algal biomass as a feedstock with potential applications for fermentation, anaerobic digestion, and bioprocessing to produce clean energy and bioplastics.
Graphical Abstract





Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of Importance •• Of more importance
• Khan AZ, Malik S, Mehmood MA, Shahid A, Shahzad T, Zhao X-Q, et al. Two-stage algal cultivation for the biotransformation of urban wastewater’s pollutants into multiple bioproducts in a circular bioeconomy paradigm. Energy Convers Manage. 2022;273:116400. https://doi.org/10.1016/j.enconman.2022.116400. This paper highlights the two-stage cultivation of cyanobacterium in urban wastewater for biomass grown and their biomass cascade processing for pigments, lipids, and enzyme production. Other stage biomass is used as biofertilizer in the soil.
• Malik S, Shahid A, Betenbaugh MJ, Liu C-G, Mehmood MA. A novel wastewater-derived cascading algal biorefinery route for complete valorization of the biomass to biodiesel and value-added bioproducts. Energy Convers Manage. 2022;256:115360. https://doi.org/10.1016/j.enconman.2022.115360. This paper highlights biomass production in urban wastewater and how biomass was processed in a cascade way for pigments and lipids extraction and enzyme production. Biomass was harvested using low-cost flocculants.
Haider MN, Liu C-G, Tabish TA, Balakrishnan D, Show P-L, Qattan SYA, et al. Resource recovery of the wastewater-derived nutrients into algal biomass followed by its cascading processing to multiple products in a circular bioeconomy paradigm. Fermentation. 2022;8(11):650. https://doi.org/10.3390/fermentation8110650.
• Shahid A, Khan AZ, Malik S, Liu C-G, Mehmood MA, Syafiuddin A, et al. Advances in green technologies for the removal of effluent organic matter from the urban wastewater. Current pollution reports. 2021;7(4):463–75. https://doi.org/10.1007/s40726-021-00203-6. The effects of effluent organic matter on wastewater treatment have been examined. The pros and cons of conventional, sophisticated, and biological effluent organic matter treatment methods have been discussed. Hybrid systems have been proposed to alleviate the drawbacks of standalone technology and enhance process efficiency. Future trends may include microalgae and cyanobacteria-based effluent organic matter removal and soil amendment.
Shahid A, Khan AZ, Jabeen F, Liu CG, Asif M, Mehmood MA. Cyanobacteria-Based Biorefineries for a Sustainable Future of Bioindustry. In: Oncel SS, editor. A Sustainable Green Future. Cham: Springer; 2023. https://doi.org/10.1007/978-3-031-24942-6_24.
Usman M, Amin M, Kamal I, Shahid A, Xu J, Alam MA, et al. Algae-mediated resource recovery from urban wastewater. Current pollution reports. 2023;9(2):243–58. https://doi.org/10.1007/s40726-023-00254-x.
Lee S-A, Kim M, Kim H-S, Ahn C-Y. Extra benefit of microalgae in raw piggery wastewater treatment: pathogen reduction. Microbiome. 2022;10(1):142. https://doi.org/10.1186/s40168-022-01339-3.
Karpagam R, Abinaya N, Gnanam R. Assortment of native microalgae for improved biomass and lipid production on employing vegetable waste as a frugal cultivation approach for biodiesel application. Curr Microbiol. 2021;78(10):3770–81. https://doi.org/10.1007/s00284-021-02643-1.
Lee J-C, Moon K, Lee N, Ryu S, Song SH, Kim YJ, et al. Biodiesel production and simultaneous treatment of domestic and livestock wastewater using indigenous microalgae, Chlorella sorokiniana JD1-1. Sci Rep. 2023;13(1):15190. https://doi.org/10.1038/s41598-023-42453-y.
Kurniawan SB, Ahmad A, Imron MF, Abdullah SRS, Othman AR, Hasan HA. Potential of microalgae cultivation using nutrient-rich wastewater and harvesting performance by biocoagulants/bioflocculants: mechanism, multi-conversion of biomass into valuable products, and future challenges. J Clean Prod. 2022;365:132806. https://doi.org/10.1016/j.jclepro.2022.132806.
Tsolcha ON, Tekerlekopoulou AG, Akratos CS, Aggelis G, Genitsaris S, Moustaka-Gouni M, et al. Agroindustrial wastewater treatment with simultaneous biodiesel production in attached growth systems using a mixed microbial culture. Water. 2018;10(11):1693. https://doi.org/10.3390/w10111693.
Hawrot-Paw M, Koniuszy A, Gałczyńska M, Zając G, Szyszlak-Bargłowicz J. Production of microalgal biomass using aquaculture wastewater as growth medium. Water. 2020;12(1):106. https://doi.org/10.3390/w12010106.
Chan SS, Khoo KS, Chew KW, Ling TC, Show PL. Recent advances biodegradation and biosorption of organic compounds from wastewater: microalgae-bacteria consortium - a review. Biores Technol. 2022;344:126159. https://doi.org/10.1016/j.biortech.2021.126159.
Ye Y, Ngo HH, Guo W, Chang SW, Nguyen DD, Zhang X, et al. Nutrient recovery from wastewater: from technology to economy. Bioresource technology reports. 2020;11:100425. https://doi.org/10.1016/j.biteb.2020.100425.
Robles Á, Aguado D, Barat R, Borrás L, Bouzas A, Giménez JB, et al. New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the circular economy. Biores Technol. 2020;300:122673. https://doi.org/10.1016/j.biortech.2019.122673.
Karpagam R, Jawaharraj K, Gnanam R. Review on integrated biofuel production from microalgal biomass through the outset of transesterification route: a cascade approach for sustainable bioenergy. Sci Total Environ. 2021;766:144236. https://doi.org/10.1016/j.scitotenv.2020.144236.
•• Show P-L, Park Y-K, Al-Zuhair S, Ashokkumar V. Guest editorial: special issue on “microalgae biorefinery: current bottlenecks, challenges, and future directions.” Phytochem Rev. 2023;22(4):829–31. https://doi.org/10.1007/s11101-023-09881-0. No-sterile heterotrophic conditions are unsustainable for microalgae. Yeast removed nutrients well from non-sterile municipal effluent. Municipal wastewater treatment using crabtree-positive yeast-generated ethanol.
Zafar Khan A, Haider MN, Zhao X-Q, Bai F-W, Musharraf SG, Ahmad N, et al. Evaluation of resource recovery potential of the Pseudoscillatoria coralii BERC01 under variable compositions of wastewater to produce biomass for cyanobacterium biorefinery. Sustainable Energy Technol Assess. 2022;54:102804. https://doi.org/10.1016/j.seta.2022.102804.
•• Idris SN, Amelia TSM, Bhubalan K, Lazim AMM, Zakwan NAMA, Jamaluddin MI, et al. The degradation of single-use plastics and commercially viable bioplastics in the environment: a review. Environ Res. 2023;231:115988. https://doi.org/10.1016/j.envres.2023.115988. This work proposes an innovative microalgal integrated system to efficiently clean swine wastewater and create biofuels.
Alias NH, Abdullah N, Othman NH, Marpani F, Zainol MM, Shayuti MSM. Sustainability Challenges and Future Perspectives of Biopolymer. In: Nadda AK, Sharma S, Bhat R, editors. Biopolymers. Springer, Cham: Springer Series on Polymer and Composite Materials; 2022. https://doi.org/10.1007/978-3-030-98392-5_17.
Machado G, Santos F, Lourega R, Mattia J, Faria D, Eichler P, Auler A. Biopolymers from Lignocellulosic Biomass. In Lignocellulosic Biorefining Technologies (eds A.P. Ingle, A.K. Chandel and S.S. Silva). 2020. https://doi.org/10.1002/9781119568858.ch7.
Bolaji I, Nejad B, Billham M, Mehta N, Smyth B, Cunningham E. Multi-criteria decision analysis of agri-food waste as a feedstock for biopolymer production. Resour Conserv Recycl. 2021;172:105671. https://doi.org/10.1016/j.resconrec.2021.105671.
El-Dalatony MM, Sharma P, Hussein EE, Elnaggar AY, Salama E-S. Pig- and vegetable-cooked waste oils as feedstock for biodiesel, biogas, and biopolymer production. Biomass conversion and biorefinery. 2022. https://doi.org/10.1007/s13399-021-02281-4.
Devadas VV, Khoo KS, Chia WY, Chew KW, Munawaroh HSH, Lam M-K, et al. Algae biopolymer towards sustainable circular economy. Biores Technol. 2021;325:124702. https://doi.org/10.1016/j.biortech.2021.124702.
Arora Y, Sharma S, Sharma V. Microalgae in bioplastic production: a comprehensive review. Arab J Sci Eng. 2023;48(6):7225–41. https://doi.org/10.1007/s13369-023-07871-0.
Shukla A, Kumar D, Girdhar M, Kumar A, Goyal A, Malik T, et al. Strategies of pretreatment of feedstocks for optimized bioethanol production: distinct and integrated approaches. Biotechnology for biofuels and bioproducts. 2023;16(1):44. https://doi.org/10.1186/s13068-023-02295-2.
Narayanan M. Promising biorefinery products from marine macro and microalgal biomass: a review. Renew Sustain Energy Rev. 2024;190:114081. https://doi.org/10.1016/j.rser.2023.114081.
Yirgu Z, Leta S, Hussen A, Khan MM. Pretreatment and optimization of reducing sugar extraction from indigenous microalgae grown on brewery wastewater for bioethanol production. Biomass conversion and biorefinery. 2023;13(8):6831–45. https://doi.org/10.1007/s13399-021-01779-1.
Walls LE, Velasquez-Orta SB, Romero-Frasca E, Leary P, Yáñez Noguez I, Orta Ledesma MT. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem Eng J. 2019;151:107319. https://doi.org/10.1016/j.bej.2019.107319.
Romero-Frasca E, Velasquez-Orta SB, Escobar-Sánchez V, Tinoco-Valencia R, Orta Ledesma MT. Bioprospecting of wild type ethanologenic yeast for ethanol fuel production from wastewater-grown microalgae. Biotechnol Biofuels. 2021;14(1):93. https://doi.org/10.1186/s13068-021-01925-x.
Qu W, Loke Show P, Hasunuma T, Ho S-H. Optimizing real swine wastewater treatment efficiency and carbohydrate productivity of newly microalga Chlamydomonas sp QWY37 used for cell-displayed bioethanol production. Biores Technol. 2020;305:123072. https://doi.org/10.1016/j.biortech.2020.123072.
Bhuyar P, Trejo M, Dussadee N, Unpaprom Y, Ramaraj R, Whangchai K. Microalgae cultivation in wastewater effluent from tilapia culture pond for enhanced bioethanol production. Water Sci Technol. 2021;84(10–11):2686–94. https://doi.org/10.2166/wst.2021.194.
Tsolcha ON, Patrinou V, Economou CN, Dourou M, Aggelis G, Tekerlekopoulou AG. Utilization of biomass derived from cyanobacteria-based agro-industrial wastewater treatment and raisin residue extract for bioethanol production. Water. 2021;13(4):486. https://doi.org/10.3390/w10111693.
Dhandayuthapani K, Sarumathi V, Selvakumar P, Temesgen T, Asaithambi P, Sivashanmugam P. Study on the ethanol production from hydrolysate derived by ultrasonic pretreated defatted biomass of Chlorella sorokiniana NITTS3. Chemical data collections. 2021;31:100641. https://doi.org/10.1016/j.cdc.2020.100641.
Guo Y, Liu Y, Guan M, Tang H, Wang Z, Lin L, et al. Production of butanol from lignocellulosic biomass: recent advances, challenges, and prospects. RSC Adv. 2022;12(29):18848–63. https://doi.org/10.1039/D1RA09396G.
Wang Y, Ho S-H, Yen H-W, Nagarajan D, Ren N-Q, Li S, et al. Current advances on fermentative biobutanol production using third generation feedstock. Biotechnol Adv. 2017;35(8):1049–59. https://doi.org/10.1016/j.biotechadv.2017.06.001.
Kallarakkal KP, Muthukumar K, Alagarsamy A, Pugazhendhi A, Naina Mohamed S. Enhancement of biobutanol production using mixotrophic culture of Oscillatoria sp in cheese whey water. Fuel. 2021;284:119008. https://doi.org/10.1016/j.fuel.2020.119008.
Tekin Nazlıhan, Karatay Sevgi Ertuğrul, Dönmez Gönül. Third generation biobutanol production by Clostridium beijerinckii in a medium containing mixotrophically cultivated Dunaliella salina biomass. Preparative Biochemistry & Biotechnology. 2023. https://doi.org/10.1080/10826068.2023.2248298.
Wang Y, Ho S-H, Cheng C-L, Nagarajan D, Guo W-Q, Lin C, et al. Nutrients and COD removal of swine wastewater with an isolated microalgal strain Neochloris aquatica CL-M1 accumulating high carbohydrate content used for biobutanol production. Biores Technol. 2017;242:7–14. https://doi.org/10.1016/j.biortech.2017.03.122.
•• Onay M. The effects of indole-3-acetic acid and hydrogen peroxide on Chlorella zofingiensis CCALA 944 for bio-butanol production. Fuel. 2020;273:117795. https://doi.org/10.1016/j.fuel.2020.117795. Chlorella zofingiensis grown in varied concentrations of municipal wastewater and Indole-3-acetic acid with hydrogen peroxide pre-treatment: bio-butanol content, yield, carbohydrate, protein, biomass concentration, biomass productivity, and specific growth curve.
Solé-Bundó M, Passos F, Romero-Güiza MS, Ferrer I, Astals S. Co-digestion strategies to enhance microalgae anaerobic digestion: a review. Renew Sustain Energy Rev. 2019;112:471–82. https://doi.org/10.1016/j.rser.2019.05.036.
Peng S, Colosi LM. Anaerobic Digestion of algae biomass to produce energy during wastewater treatment. Water Environ Res. 2016;88(1):29–39. https://doi.org/10.2175/106143014X14062131179195.
Markou G, Ilkiv B, Brulé M, Antonopoulos D, Chakalis L, Arapoglou D, et al. Methane production through anaerobic digestion of residual microalgal biomass after the extraction of valuable compounds. Biomass Convers Biorefin. 2022;12(2):419–26. https://doi.org/10.1007/s13399-020-00703-3.
Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact. 2018;17(1):36. https://doi.org/10.1186/s12934-018-0879-x.
Kant Bhatia S, Ahuja V, Chandel N, Gurav R, Kant Bhatia R, Govarthanan M, et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Biores Technol. 2022;358:127437. https://doi.org/10.1016/j.biortech.2022.127437.
Hansen BB, Spittle S, Chen B, Poe D, Zhang Y, Klein JM, et al. Deep eutectic solvents: a review of fundamentals and applications. Chem Rev. 2021;121(3):1232–85. https://doi.org/10.1021/acs.chemrev.0c00385.
Raj T, Morya R, Chandrasekhar K, Kumar D, Soam S, Kumar R, et al. Microalgae biomass deconstruction using green solvents: challenges and future opportunities. Biores Technol. 2023;369:128429. https://doi.org/10.1016/j.biortech.2022.128429.
Mehariya S, Fratini F, Lavecchia R, Zuorro A. Green extraction of value-added compounds form microalgae: a short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J Environ Chem Eng. 2021;9(5):105989. https://doi.org/10.1016/j.jece.2021.105989.
El Achkar T, Greige-Gerges H, Fourmentin S. Basics and properties of deep eutectic solvents: a review. Environ Chem Lett. 2021;19(4):3397–408. https://doi.org/10.1007/s10311-021-01225-8.
Ramos-Suárez JL, Carreras N. Use of microalgae residues for biogas production. Chem Eng J. 2014;242:86–95. https://doi.org/10.1016/j.cej.2013.12.053.
Ganesh Saratale R, Kumar G, Banu R, Xia A, Periyasamy S, Dattatraya SG. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Biores Technol. 2018;262:319–32. https://doi.org/10.1016/j.biortech.2018.03.030.
Santos-Ballardo DU, Font-Segura X, Ferrer AS, Barrena R, Rossi S, Valdez-Ortiz A. Valorisation of biodiesel production wastes: anaerobic digestion of residual Tetraselmis suecica biomass and co-digestion with glycerol. Waste Manage Res. 2015;33(3):250–7. https://doi.org/10.1177/0734242x15572182.
•• González-González LM, Astals S, Pratt S, Jensen PD, Schenk PM. Osmotic shock pre-treatment of Chaetoceros muelleri wet biomass enhanced solvent-free lipid extraction and biogas production. Algal Res. 2021;54:102177. https://doi.org/10.1016/j.algal.2020.102177. Wet microalgal biomass was pre-treated with osmotic shock to determine its potential for lipid extraction and biogas generation in an integrated system for Chaetoceros muelleri biodiesel and biogas production.
de Oliveira MC, Bassin ID, Cammarota MC. Microalgae and cyanobacteria biomass pretreatment methods: a comparative analysis of chemical and thermochemical pretreatment methods aimed at methane production. Fermentation. 2022;8(10):497. https://doi.org/10.3390/fermentation8100497.
Zhao B, Ma J, Zhao Q, Laurens L, Jarvis E, Chen S, et al. Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production. Biores Technol. 2014;161:423–30. https://doi.org/10.1016/j.biortech.2014.03.079.
Capson-Tojo G, Torres A, Muñoz R, Bartacek J, Jeison D. Mesophilic and thermophilic anaerobic digestion of lipid-extracted microalgae N. gaditana for methane production. Renewable Energy. 2017;105:539–46. https://doi.org/10.1016/j.renene.2016.12.052.
Ahmad A, Ashraf SS. Sustainable food and feed sources from microalgae: food security and the circular bioeconomy. Algal Res. 2023;74:103185. https://doi.org/10.1016/j.algal.2023.103185.
Parimi NS, Singh M, Kastner JR, Das KC. Biomethane and biocrude oil production from protein extracted residual Spirulina platensis. Energy. 2015;93:697–704. https://doi.org/10.1016/j.energy.2015.09.041.
Yellezuome D, Zhu X, Wang Z, Liu R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: a review. Renew Sustain Energy Rev. 2022;157:112043. https://doi.org/10.1016/j.rser.2021.112043.
Yenigün O, Demirel B. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 2013;48(5):901–11. https://doi.org/10.1016/j.procbio.2013.04.012.
Yukesh Kannah R, Kavitha S, Parthiba Karthikeyan O, Rene ER, Kumar G, Rajesh BJ. A review on anaerobic digestion of energy and cost effective microalgae pretreatment for biogas production. Biores Technol. 2021;332:125055. https://doi.org/10.1016/j.biortech.2021.125055.
Nghiem LD, Koch K, Bolzonella D, Drewes JE. Full scale co-digestion of wastewater sludge and food waste: Bottlenecks and possibilities. Renew Sustain Energy Rev. 2017;72:354–62. https://doi.org/10.1016/j.rser.2017.01.062.
Azarmanesh R, Zarghami Qaretapeh M, Hasani Zonoozi M, Ghiasinejad H, Zhang Y. Anaerobic co-digestion of sewage sludge with other organic wastes: a comprehensive review focusing on selection criteria, operational conditions, and microbiology. Chem Eng J Adv. 2023;14:100453. https://doi.org/10.1016/j.ceja.2023.100453.
Meneses-Jácome A, Diaz-Chavez R, Velásquez-Arredondo HI, Cárdenas-Chávez DL, Parra R, Ruiz-Colorado AA. Sustainable energy from agro-industrial wastewaters in Latin-America. Renew Sustain Energy Rev. 2016;56:1249–62. https://doi.org/10.1016/j.rser.2015.12.036.
Romero-Güiza MS, Vila J, Mata-Alvarez J, Chimenos JM, Astals S. The role of additives on anaerobic digestion: a review. Renew Sustain Energy Rev. 2016;58:1486–99. https://doi.org/10.1016/j.rser.2015.12.094.
Schwede S, Kowalczyk A, Gerber M, Span R. Anaerobic co-digestion of the marine microalga Nannochloropsis salina with energy crops. Biores Technol. 2013;148:428–35. https://doi.org/10.1016/j.biortech.2013.08.157.
Ramos-Suárez JL, Martínez A, Carreras N. Optimization of the digestion process of Scenedesmus sp. and Opuntia maxima for biogas production. Energy Convers Manag. 2014;88:1263–70. https://doi.org/10.1016/j.enconman.2014.02.064.
El-Mashad HM. Kinetics of methane production from the codigestion of switchgrass and Spirulina platensis algae. Biores Technol. 2013;132:305–12. https://doi.org/10.1016/j.biortech.2012.12.183.
Carminati P, Gusmini D, Pizzera A, Catenacci A, Parati K, Ficara E. Biogas from mono- and co-digestion of microalgal biomass grown on piggery wastewater. Water Sci Technol. 2018;78(1):103–13. https://doi.org/10.2166/wst.2018.134.
Wang M, Lee E, Zhang Q, Ergas SJ. Anaerobic co-digestion of swine manure and microalgae Chlorella sp.: experimental studies and energy analysis. BioEnergy research. 2016;9(4):1204–15. https://doi.org/10.1007/s12155-016-9769-4.
Mahdy A, Fotidis IA, Mancini E, Ballesteros M, González-Fernández C, Angelidaki I. Ammonia tolerant inocula provide a good base for anaerobic digestion of microalgae in third generation biogas process. Biores Technol. 2017;225:272–8. https://doi.org/10.1016/j.biortech.2016.11.086.
Ameen F, Ranjitha J, Ahsan N, Shankar V. Co-digestion of microbial biomass with animal manure in three-stage anaerobic digestion. Fuel. 2021;306:121746. https://doi.org/10.1016/j.fuel.2021.121746.
•• Astals S, Musenze RS, Bai X, Tannock S, Tait S, Pratt S, et al. Anaerobic co-digestion of pig manure and algae: Impact of intracellular algal products recovery on co-digestion performance. Biores Technol. 2015;181:97–104. https://doi.org/10.1016/j.biortech.2015.01.039. Experimental findings were utilized to build a high-level concept for an integrated biorefinery processing pig manure and onsite farmed algae, assessing methane output and co-product recovery per mass of manure.
Fernández-Rodríguez MJ, Rincón B, Fermoso FG, Jiménez AM, Borja R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Biores Technol. 2014;157:263–9. https://doi.org/10.1016/j.biortech.2014.01.096.
Passos F, Cordeiro PHM, Baeta BEL, de Aquino SF, Perez-Elvira SI. Anaerobic co-digestion of coffee husks and microalgal biomass after thermal hydrolysis. Biores Technol. 2018;253:49–54. https://doi.org/10.1016/j.biortech.2017.12.071.
Arias DM, Solé-Bundó M, Garfí M, Ferrer I, García J, Uggetti E. Integrating microalgae tertiary treatment into activated sludge systems for energy and nutrients recovery from wastewater. Biores Technol. 2018;247:513–9. https://doi.org/10.1016/j.biortech.2017.09.123.
Wang M, Sahu AK, Rusten B, Park C. Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Biores Technol. 2013;142:585–90. https://doi.org/10.1016/j.biortech.2013.05.096.
•• Avila R, Justo Á, Carrero E, Crivillés E, Vicent T, Blánquez P. Water resource recovery coupling microalgae wastewater treatment and sludge co-digestion for bio-wastes valorisation at industrial pilot-scale. Biores Technol. 2022;343:126080. https://doi.org/10.1016/j.biortech.2021.126080. A winery firm is integrating a microalgae-based system into its WWTP facilities to collect nutrients and convert them into value goods and bioenergy as part of a circular bioeconomy initiative.
Chen B, Wan C, Mehmood MA, Chang J-S, Bai F, Zhao X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products–a review. Biores Technol. 2017;244:1198–206. https://doi.org/10.1016/j.biortech.2017.05.170.
•• Costa SS, Miranda AL, Andrade BB, de Jesus AD, Souza CO, de Morais MG, et al. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int J Biol Macromol. 2018;116:552–62. https://doi.org/10.1016/j.ijbiomac.2018.05.064. This research examined how nitrogen availability affected cell growth, biomass composition, production, and polyhydroxyalkanoate characteristics in three microalgal strains.
Rahman A, Putman RJ, Inan K, Sal FA, Sathish A, Smith T, et al. Polyhydroxybutyrate production using a wastewater microalgae based media. Algal Res. 2015;8:95–8. https://doi.org/10.1016/j.algal.2015.01.009.
Mallick N, Gupta S, Panda B, Sen R. Process optimization for poly (3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production by Nostoc muscorum. Biochem Eng J. 2007;37(2):125–30. https://doi.org/10.1016/j.bej.2007.04.002.
Kavitha G, Kurinjimalar C, Sivakumar K, Kaarthik M, Aravind R, Palani P, et al. Optimization of polyhydroxybutyrate production utilizing waste water as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int J Biol Macromol. 2016;93:534–42. https://doi.org/10.1016/j.ijbiomac.2016.09.019.
Mendhulkar VD, Shetye LA. Synthesis of biodegradable polymer polyhydroxyalkanoate (PHA) in cyanobacteria Synechococcus elongates under mixotrophic nitrogen-and phosphate-mediated stress conditions. Ind Biotechnol. 2017;13(2):85–93. https://doi.org/10.1089/ind.2016.0021.
Koch M, Forchhammer K. Polyhydroxybutyrate: a useful product of chlorotic cyanobacteria. Microb Physiol. 2021;31(2):67–77. https://doi.org/10.1159/000515617.
•• Koch M, Berendzen KW, Forchhammer K. On the Role and Production of Polyhydroxybutyrate (PHB) in the Cyanobacterium Synechocystis sp. PCC 6803. Life. 2020;10(4):47. https://doi.org/10.3390/life10040047. PHB formation factors are described in this paper. The circumstances under which PHB is favorable during nitrogen deprivation are unknown. This research can assist generate strains with more PHB.
Rueda E, Gonzalez-Flo E, Roca L, Carretero J, García J. Accumulation of polyhydroxybutyrate in Synechocystis sp isolated from wastewaters: effect of salinity, light, and P content in the biomass. J Environ Chem Eng. 2022;10(3):107952. https://doi.org/10.1016/j.jece.2022.107952.
Singhon P, Phoraksa O, Incharoensakdi A, Monshupanee T. Increased bioproduction of glycogen, lipids, and poly(3-hydroxybutyrate) under partial supply of nitrogen and phosphorus by photoautotrophic cyanobacterium Synechocystis sp PCC 6803. J Appl Phycol. 2021;33(5):2833–43. https://doi.org/10.1007/s10811-021-02494-0.
Pezzolesi L, Samorì C, Zoffoli G, Xamin G, Simonazzi M, Pistocchi R. Semi-continuous production of polyhydroxybutyrate (PHB) in the chlorophyta Desmodesmus communis. Algal Res. 2023;74:103196. https://doi.org/10.1016/j.algal.2023.103196.
Grivalský T, Lakatos GE, Štěrbová K, Manoel JAC, Beloša R, Divoká P, et al. Poly-β-hydroxybutyrate production by Synechocystis MT_a24 in a raceway pond using urban wastewater. Appl Microbiol Biotechnol. 2024;108(1):44. https://doi.org/10.1007/s00253-023-12924-3.
Rodríguez-Contreras A, Koller M, Miranda-de Sousa Dias M, Calafell-Monfort M, Braunegg G, Marqués-Calvo MS. High production of poly (3-hydroxybutyrate) from a wild Bacillus megaterium Bolivian strain. J Appl Microbiol. 2013;114(5):1378–87. https://doi.org/10.1111/jam.12151.
• Kurian NS, Das B. Comparative analysis of various extraction processes based on economy, eco-friendly, purity and recovery of polyhydroxyalkanoate: a review. Int J Biol Macromol. 2021;183:1881–90. https://doi.org/10.1016/j.ijbiomac.2021.06.007. Nutrient-deficient medium with abundant carbon creates PHA. Bioplastic’s commercial usage relies on manufacturing costs. Non-PHA cell mass reduction is crucial to PHA extraction. Non-PHA cell removal by protons and biological digestion is an excellent PHA extraction procedure. PHA production may be increased via genetic engineering.
Jiang G, Johnston B, Townrow DE, Radecka I, Koller M, Chaber P, et al. Biomass extraction using non-chlorinated solvents for biocompatibility improvement of polyhydroxyalkanoates. Polymers. 2018;10(7):731. https://doi.org/10.3390/polym10070731.
Koller M. Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. The EuroBiotech Journal. 2020;4(3):113–26. https://doi.org/10.2478/ebtj-2020-0013.
Umesh M, Mamatha C. Comparitive study on efficacy of alkaline and acid extraction for PHA recovery from Bacillus subtilis NCDC0671. Int J Recent Sci Res. 2018;9:29467–71.
Costa SS, Miranda AL, de Morais MG, Costa JAV, Druzian JI. Microalgae as source of polyhydroxyalkanoates (PHAs) — a review. Int J Biol Macromol. 2019;131:536–47. https://doi.org/10.1016/j.ijbiomac.2019.03.099.
Suzuki DV, Carter JM, Rodrigues MFA, da Silva ES, Maiorano AE. Purification of polyhydroxybutyrate produced by Burkholderia cepacia IPT64 through a chemical and enzymatic route. World J Microbiol Biotechnol. 2008;24(6):771–5. https://doi.org/10.1007/s11274-007-9537-x.
Pérez-Rivero C, López-Gómez JP, Roy I. A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: state-of-the-art and latest developments. Biochem Eng J. 2019;150:107283. https://doi.org/10.1016/j.bej.2019.107283.
Macagnan KL, Alves MI, Moreira ADS. Approaches for Enhancing Extraction of Bacterial Polyhydroxyalkanoates for Industrial Applications. In: Kalia V, editor. Biotechnological Applications of Polyhydroxyalkanoates. Singapore: Springer; 2019. https://doi.org/10.1007/978-981-13-3759-8_15.
Wilawan B, Chan S, Ling T, Show P-L, Ng E-P, Jonglertjunya W, et al. Advancement of carotenogenesis of astaxanthin from Haematococcus pluvialis: recent insight and way forward. Mol Biotechnol. 2023. https://doi.org/10.1007/s12033-023-00768-1.
Samarasinghe N, Fernando S, Faulkner WB. Effect of high pressure homogenization on aqueous phase solvent extraction of lipids from Nannochloris oculata microalgae.. J Energy Nat Resour. 2012;1(1):1–7. https://doi.org/10.11648/j.jenr.20120101.11.
Osorio-Arias JC, Vega-Castro O, Martínez-Monteagudo SI. Fundamentals of high-pressure homogenization of foods. In Reference Module in Food Science. Amsterdam, The Netherlands: Elsevier BV; 2020.
Koller M, Niebelschütz H, Braunegg G. Strategies for recovery and purification of poly [(R)-3-hydroxyalkanoates](PHA) biopolyesters from surrounding biomass. Eng Life Sci. 2013;13(6):549–62. https://doi.org/10.1002/elsc.201300021.
Jacquel N, Lo C-W, Wei Y-H, Wu H-S, Wang SS. Isolation and purification of bacterial poly (3-hydroxyalkanoates). Biochem Eng J. 2008;39(1):15–27. https://doi.org/10.1016/j.bej.2007.11.029.
Dhankhar P. Homogenization fundamentals. IOSR. Journal of Engineering. 2014;4(5):8.
Dong T, Knoshaug EP, Pienkos PT, Laurens LML. Lipid recovery from wet oleaginous microbial biomass for biofuel production: a critical review. Appl Energy. 2016;177:879–95. https://doi.org/10.1016/j.apenergy.2016.06.002.
Grimi N, Dubois A, Marchal L, Jubeau S, Lebovka NI, Vorobiev E. Selective extraction from microalgae Nannochloropsis sp using different methods of cell disruption. Biores Technol. 2014;153:254–9. https://doi.org/10.1016/j.biortech.2013.12.011.
Xu K, Zou W, Peng B, Guo C, Zou X. Lipid droplets from plants and microalgae: characteristics, extractions, and applications. Biology. 2023;12(4):594. https://doi.org/10.3390/biology12040594.
Funding
The authors are thankful to the Higher Education Commission of Pakistan for supporting their various research projects.
Author information
Authors and Affiliations
Contributions
M.A.M., M.Amin. M. N. H. prepared the first draft of the manuscript. S.M. and H. A. M. prepared tables and figures. M. A.A., J.X., and A.H.A. reviewed and edited the manuscript. M.A.M., A.Z.K, and R.B. conceptualized the idea, and edited the final version of the manuscript. All authors reviewed and agreed for the submission.
Corresponding authors
Ethics declarations
Conflict of Interest
There are no competing interests among the authors.
Human and Animal Rights and Informed Consent
This article did not involve any human or animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Algae cultivation in wastewater offers biomass production, pollutant removal, and wastewater recycling.
• Industrial utilization of wastewater-grown biomass remains questionable and indeterminate.
• Alternative bioprocessing routes of wastewater-grown biomass should be developed.
• Fermentation of wastewater-grown biomass could be a promising option to produce fuel alcohols.
• Co-digestion of wastewater-grown biomass to biogas and biotransformation to biopolymers needs detailed studies.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehmood, M.A., Amin, M., Haider, M.N. et al. Wastewater-Grown Algal Biomass as Carbon-neutral, Renewable, and Low Water Footprint Feedstock for Clean Energy and Bioplastics. Curr Pollution Rep 10, 172–188 (2024). https://doi.org/10.1007/s40726-024-00294-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-024-00294-x