Abstract
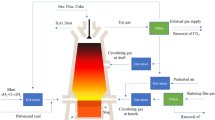
The blast furnace gas, a low-calorific value fuel, is worthy of recycling. However, the sulfuric contents in blast furnace gases would cause pipeline corrosion and air pollution that are adverse to reutilization of blast furnace gases. Therefore, desulfurization technologies of blast furnace gas are necessary, which have been recently attracting much attention. This article clarifies main sources and forms of sulfur in blast furnace gases. Then, COS/H2S removal methods that are commonly used are introduced. In fact, blast furnace gases usually exist in low-temperature and low-pressure conditions, whose major components are N2/CO/CO2/H2 and minor components are sulfide/chloride. When blast furnace gases are used as a fuel, the sulfuric component will be converted into SO2 that causes severe air pollution to atmospheric environments. However, according to recent findings, once sulfides are removed ahead of the utilization process of blast furnace gases, other air-polluting components (e.g., hydrogen chloride) could be also removed via synergistic effects. In light of these characteristics, this article discusses the feasibility of various desulfurization methods suitable for blast furnace gases with the aim to provide useful information for the development of blast furnace gas desulfurization technologies.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Guo Y-H. Current station and tendency of purification and upgrading of blast furnace gas. J Iron Steel Res. 2020;32(7):525–531. This reference firstly introduces many purification methods of blast furnace gas used in industries for now. Then, this reference speculates some possible development tendency of blast furnace gas purification.
Wang Y, Lei X, Deng L, et al. A review on utilization of combustible waste gas (I): blast furnace gas, converter gas and coke oven gas. Therm Power Gen. 2014;43(07):1–9+14.
• Deng B, Yan X, Peng B, et al. Analysis of corrosion in blast furnace gas pipes and anticorrosion measures. Metallurgical Power. 2018;2018(09):13–16+21. This paper in detail theoretically explains how blast furnace gas gets pipes in steel plants corroded, which is exactly the most crucial background of this manuscript. In the meantime, some thoughts of this paper provides some other possible methods of removing sulfur as references.
Hui C, Chang-shui W, Jian-long W, et al. Discussion on evaluation of dechlorination effect for alkali liquor spray scrubber after BF dry dust-catch. Iron Steel. 2019;54(02):31–4.
Zhang X-S, Xu M, Wu J-L, et al. Sulfur analysis and measures for reducing sulfur in ironmaking process of Shougang Jingtang. Iron Steel. 2018;54(03):18–22.
Zhang W, Zhang X, Zheng M. Sulfur form of metallurgical coke influence on sulfur in blast furnace gas. Energy Metall Ind. 2019;38(2):55–59.
Meng Y, Zhongyi A, Zhifeng L, et al. Research and application of fine desulfurization system of blast furnace gas in iron and steel enterprises. Energy Metall Ind. 2021;40(01):61–4.
Liu JH. Advances of carbonyl sulfide removal at lower and ordinary temperature. Chin J Chem Eng. 2013;41(19):22–24+48.
Ewing SP, Lockshon D, Jencks WP. Mechanism of cleavage of carbamate anions. J Am Chem Soc. 1980;102(9):3072–84.
Littel RJ, Versteeg GF, Swaaij W. Kinetics of COS with primary and secondary amines in aqueous solutions. AIChE J. 1992;38(2):244–50.
Littel RJ, Versteeg GF, Swaaij WV. Kinetic study of COS with tertiary alkanolamine solutions. 1. Experiments in an intensely stirred batch reactor. Ind Eng Chem Res. 1992;31(5):1262–1269.
Hani AA, Gabriel RI, Orville CS. Absorption of carbonyl sulfide in aqueous methyl diethanolamine. Chem Eng Sci. 1989;44(3):631–9.
Lee SC, Snodgrass MJ, Park MK, et al. Kinetics of removal of carbonyl sulfide by aqueous monoethanolamine. Environ Sci Technol. 2001;35(11):2352–7.
Seagraves J. Sulfur removal in amino plants. Hydrocarbon Eng. 2001;12(4):47–52.
Ming KE, Dong CHEN, Qi FENG, et al. Research progress on the reaction mechanism and reaction kinetics of carbonyl sulfide and alcohol amine solution. Sci Tech Chem Ind. 2014;22(06):71–4.
Niu G, Huang Y, Wang J. Application of the cool methanol absorption technology to the natural gas purification process. Nat Gas Cheml Ind. 2003(2):26–29.
Hongyan W, Hong-hong Y, Xiaolong T, et al. Development of Carbonyl Sulfide Removal. Chem Ind Eng. 2010;27(01):67–72.
Zhang J, Li X, Wang S, et al. The research of removing COS at low temperature by modified active carbon. Liaoning Chem Ind. 1998;3:103–104.
Fang L, Zhang Y, Zhou J, et al. Study on simultaneous removal of COS and H2S from carbon dioxide by activated carbon fiber. Chin J Chem Eng. 2008;33(2):25–31
Zhengxi LI. Removal of organic sulfur from refinery LPG. Petroleum Proces Petrochem. 1996;11:27–30.
Wang X, Ding L, Zhao Z. Novel hydrode sulfurization nano-catalyzers derived from Co3O4 nanocrystals with different shapes. Catal Today. 2011;175(1):509–14.
Xinxue L, Yingxin L, Xionghui W. Technology for carbonyl sulfide removal. Modern Chem Ind. 2004;08:19–22.
Caixia D. Application techniques of organic sulfur hydroconversion catalysts. Ind Catal. 2003;09:13–7.
Bachelier J, Aboulayt A, Lavalley JC, et al. Activity of different metal oxides towards COS hydrolysis. Effect of SO2 and sulfation. Catal Today. 1993;17:55–62
He E, Huang G, Fan H, et al. Macroporous alumina- and titania-based catalyst for carbonyl sulfide hydrolysis at ambient temperature. Fuel. 2019;246:277–84.
Fiedorow R, Léauté R, Lana IGD. A study of the kinetics and mechanism of COS hydrolysis over alumina. J Catal. 1984;85(2):339–48.
Li K, Wang C, Ning P, et al. Surface characterization of metal oxides-supported activated carbon fiber catalysts for simultaneous catalytic hydrolysis of carbonyl sulfide and carbon disulfide. Res J Environ Sci. 2020;10.
Ping NING, Lili YU, Honghong YI, et al. Effect of Fe/Cu/Ce loading on the coal-based activated carbons for hydrolysis of carbonyl sulfide. J Rare Earths. 2010;2:205–10.
Yi H, Zhao S, Tang X, et al. Influence of calcination temperature on the hydrolysis of carbonyl sulfide over hydrotalcite-derived Zn–Ni–Al catalyst. Catal Commun. 2011;12(15):1492–5.
Hongyan W, Honghong Y, Xiaolong T, et al. Catalytic hydrolysis of COS over calcined CoNiAl hydrotalcite-like compounds modified by cerium. Appl Clay Sci. 2012;70:8–13.
Williams BP, Young NC, West J, et al. Carbonyl sulphide hydrolysis using alumina catalysts. Catal Today. 1999;49(1–3):99–104.
Akimoto M, Lana I. Role of reduction sites in vapor-phase hydrolysis of carbonyl sulfide over alumina catalysts. J Catal. 1980;62(1):84–93.
Hoggan PE, Aboulayt A, Pieplu A, et al. Mechanism of COS hydrolysis on alumina. J Catal. 1994;149(2):300–6.
Liu J, Liu Y, Xue L, et al. Oxygen poisoning mechanism of catalytic hydrolysis of OCS over Al2O3 at room temperature. Acta Phys Chim Sin. 2007;23(7):997–1002.
Rhodes C, Riddel SA, West J, et al. The low-temperature hydrolysis of carbonyl sulfide and carbon disulfide: a review. Catal Today. 2000;59(3–4):443–64.
Kailasa SK, Koduru JR, Vikrant K, et al. Recent progress on solution and materials chemistry for the removal of hydrogen sulfide from various gas plants. J Mol Liq. 2020;297:111886.
Jun W. Study on sulfur recovery technology by Claus process. Fuel Chem Process. 2020;51(04):48–51.
Wang R, Shi G, Wei W, et al. Methods of removing hydrogen sulfide from industrial gaspresent situation and prospects. Nat Gas Ind. 199;03:97–103+12–13
Zheng S, Zhuang G, Wu Z. Studies on bacterial desulfurization of the h2s-containing industrial gases. Acta Microbiol Sin. 1993;33(03):192–198.
Zhang J, Yi H, Ning P, et al. Advances of the study on absorption technology of hydrogen sulfide. Tech Eq Environ Pol Cont. 2002;06:47–52.
Xiang LI, Xueqian WANG, Pengfei LI, et al. Characteristic components analysis of blast furnace gas and its influence on desulfurization process. Chem Ind Eng Prog. 2021;40:6629–39.
• Zhang B, Xue Q, Niu D, et al. Current situation of blast furnace gas utilization and new technologies for energy saving and emission reduction. Ironmaking. 2018;37(2):51–55. This reference introduces how to simultaneously achieve energy saving and emission reduction, which is the next development tendency of blast furnace gas utilization. What is more, some technologies of this reference is the ongoing research core of blast furnace gas for steel plants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no known competing interest (personal or institutional) to declare.
Human and Animal Rights and Informed Consent
It is solemnly stated that this study does not involve anything about human subjects (or related to animals) nor allude to anything of inhumanity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Air Pollution
Rights and permissions
About this article
Cite this article
Li, P., Wang, G., Dong, Y. et al. A Review on Desulfurization Technologies of Blast Furnace Gases. Curr Pollution Rep 8, 189–200 (2022). https://doi.org/10.1007/s40726-022-00212-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-022-00212-z